Diet in Irritable Bowel Syndrome (IBS): Interaction with Gut Microbiota and Gut Hormones
Abstract
1. Introduction
2. Diet in IBS
3. Gut Microbiota
4. Gut Hormones
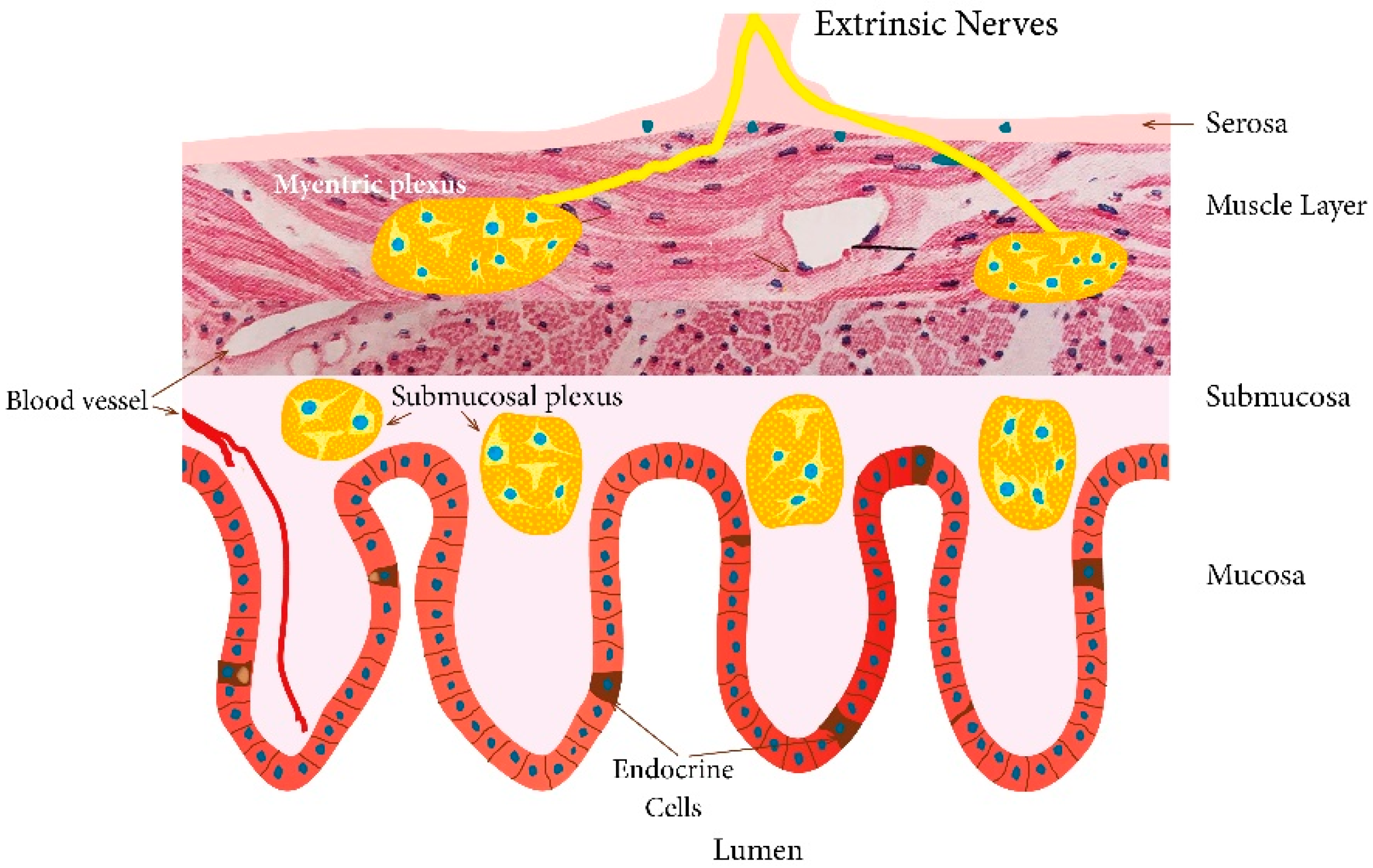
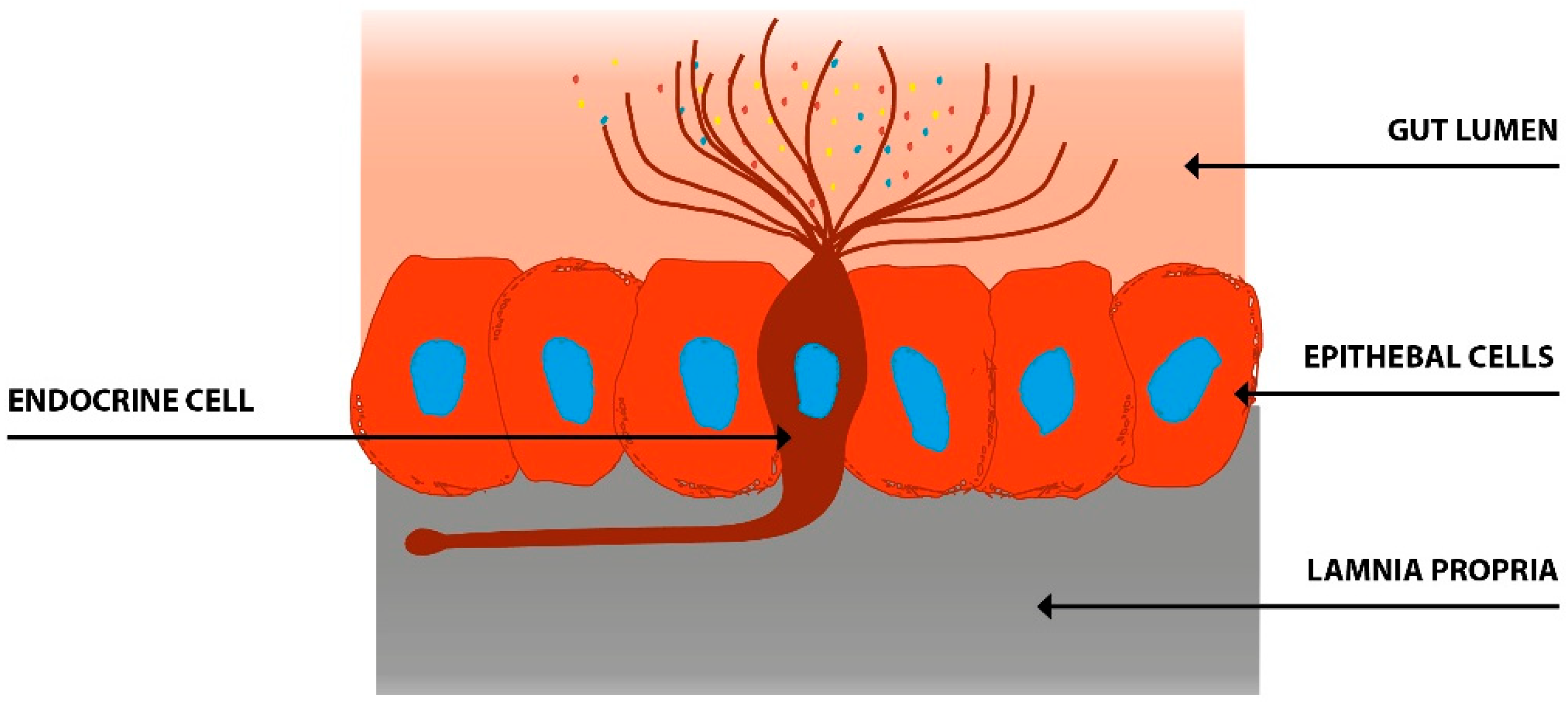
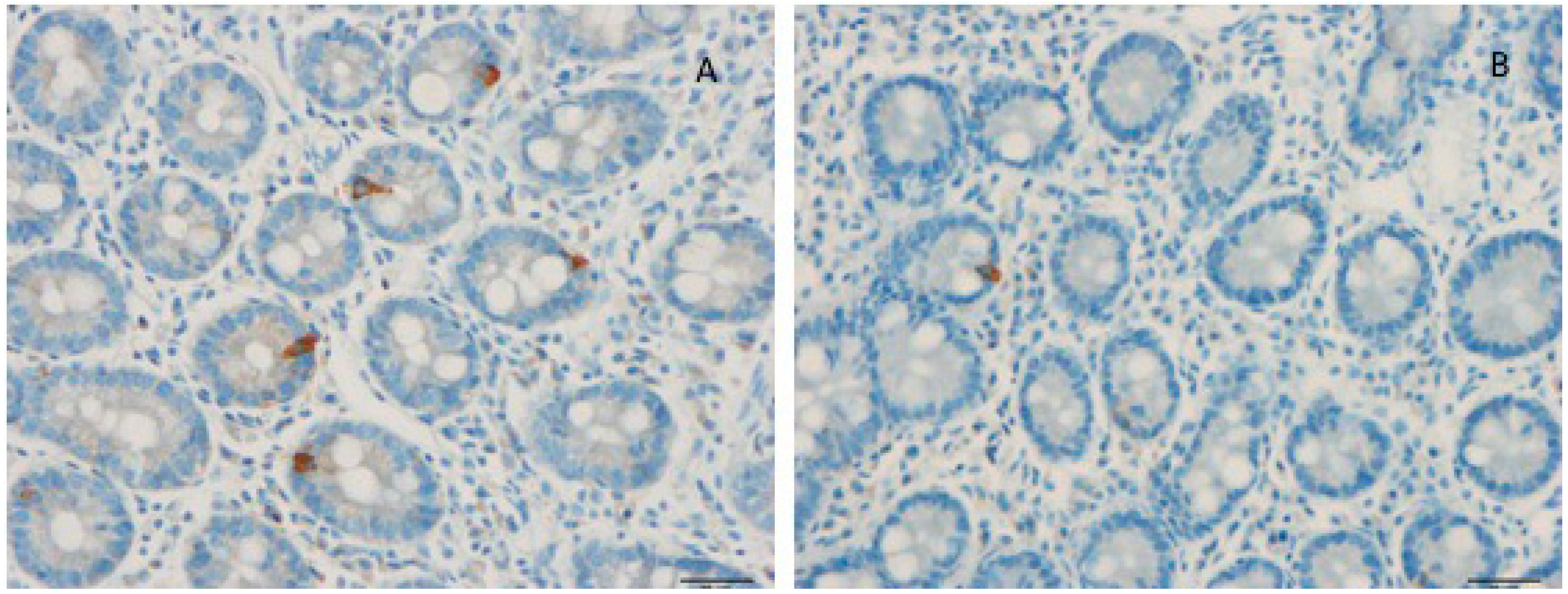
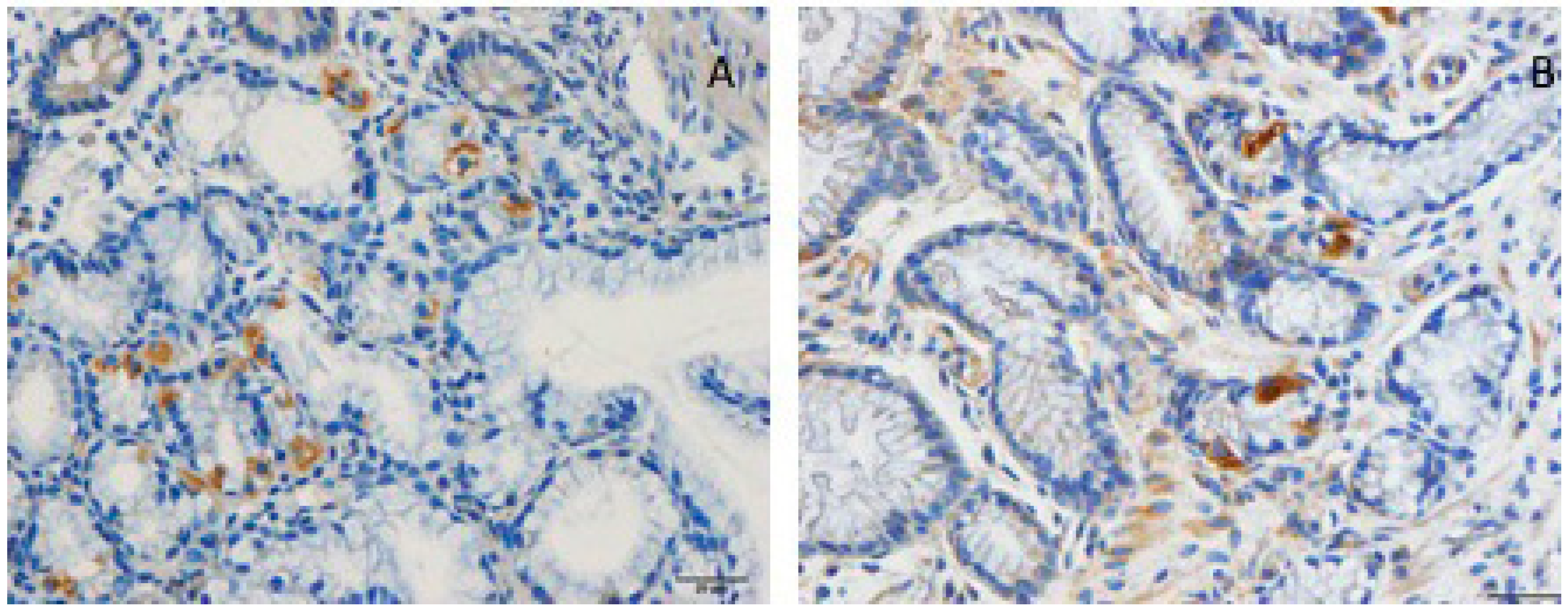
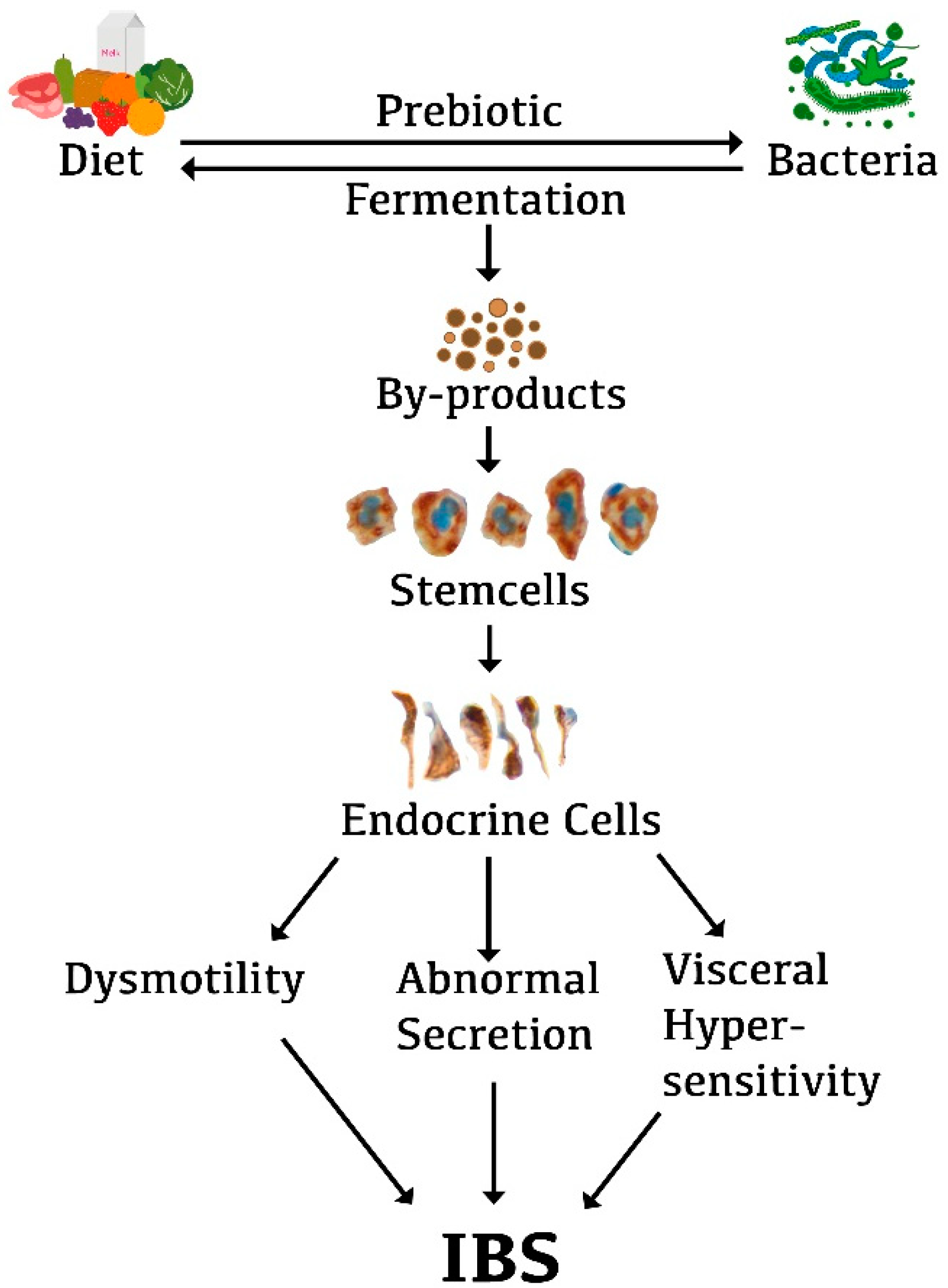
5. Interaction between Diet, Microbiota, and Endocrine Cells in the Guts of Patients with IBS
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El-Salhy, M. Recent developments in the pathophysiology of irritable bowel syndrome. World J. Gastroenterol. 2015, 21, 7621–7636. [Google Scholar] [CrossRef] [PubMed]
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [CrossRef]
- El-Salhy, M. Irritable bowel syndrome: Diagnosis and pathogenesis. World J. Gastroenterol. 2012, 18, 5151–5163. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Gilja, O.H.; Hatlebakk, J.G. Overlapping of irritable bowel syndrome with erosive esophagitis and the performance of Rome criteria in diagnosing IBS in a clinical setting. Mol. Med. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R.; Aziz, Q.; Creed, F.; Emmanuel, A.; Houghton, L.; Hungin, P.; Jones, R.; Kumar, D.; Rubin, G.; Trudgill, N.; et al. Guidelines on the irritable bowel syndrome: Mechanisms and practical management. Gut 2007, 56, 1770–1798. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.G.; Irvine, E.J.; Pare, P.; Ferrazzi, S.; Rance, L. Functional gastrointestinal disorders in Canada: First population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig. Dis. Sci. 2002, 47, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.G.; Heaton, K.W. Functional bowel disorders in apparently healthy people. Gastroenterology 1980, 79, 283–288. [Google Scholar] [CrossRef]
- Agreus, L.; Svardsudd, K.; Nyren, O.; Tibblin, G. Irritable bowel syndrome and dyspepsia in the general population: Overlap and lack of stability over time. Gastroenterology 1995, 109, 671–680. [Google Scholar] [CrossRef]
- Kennedy, T.M.; Jones, R.H.; Hungin, A.P.; O’Flanagan, H.; Kelly, P. Irritable bowel syndrome, gastro-oesophageal reflux, and bronchial hyper-responsiveness in the general population. Gut 1998, 43, 770–774. [Google Scholar] [CrossRef]
- Drossman, D.A.; Li, Z.; Andruzzi, E.; Temple, R.D.; Talley, N.J.; Thompson, W.G.; Whitehead, W.E.; Janssens, J.; Funch-Jensen, P.; Corazziari, E.; et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig. Dis. Sci. 1993, 38, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Hungin, A.P.; Whorwell, P.J.; Tack, J.; Mearin, F. The prevalence, patterns and impact of irritable bowel syndrome: An international survey of 40,000 subjects. Aliment. Pharmacol. Ther. 2003, 17, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Lydeard, S. Irritable bowel syndrome in the general population. BMJ 1992, 304, 87–90. [Google Scholar] [CrossRef]
- O’Keefe, E.A.; Talley, N.J.; Zinsmeister, A.R.; Jacobsen, S.J. Bowel disorders impair functional status and quality of life in the elderly: A population-based study. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, M184–M189. [Google Scholar] [CrossRef] [PubMed]
- Everhart, J.E.; Renault, P.F. Irritable bowel syndrome in office-based practice in the United States. Gastroenterology 1991, 100, 998–1005. [Google Scholar] [CrossRef]
- Harvey, R.F.; Salih, S.Y.; Read, A.E. Organic and Functional Disorders in 2000 Gastroenterology Outpatients. Lancet 1980, 1, 632–634. [Google Scholar] [CrossRef]
- Quigley, E.M.; Locke, G.R.; Mueller-Lissner, S.; Paulo, L.G.; Tytgat, G.N.; Helfrich, I.; Schaefer, E. Prevalence and management of abdominal cramping and pain: A multinational survey. Aliment. Pharmacol. Ther. 2006, 24, 411–419. [Google Scholar] [CrossRef]
- Miller, V.; Whitaker, K.; Morris, J.A.; Whorwell, P.J. Gender and irritable bowel syndrome: The male connection. J. Clin. Gastroenterol. 2004, 38, 558–560. [Google Scholar] [CrossRef][Green Version]
- El-Salhy, M.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Irritable Bowel Syndrome: Diagnosis, Pathogenesis and Treatment Options; Nova Science Publishers Inc.: New York, NY, USA, 2012; pp. 873–878. [Google Scholar] [CrossRef]
- Whitehead, W.E.; Burnett, C.K.; Cook, E.W., 3rd; Taub, E. Impact of irritable bowel syndrome on quality of life. Dig. Dis. Sci. 1996, 41, 2248–2253. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Hays, R.D.; Kilbourne, A.; Naliboff, B.; Mayer, E.A. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000, 119, 654–660. [Google Scholar] [CrossRef]
- Talley, N.J.; Gabriel, S.E.; Harmsen, W.S.; Zinsmeister, A.R.; Evans, R.W. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology 1995, 109, 1736–1741. [Google Scholar] [CrossRef]
- Schuster, M.M. Defining and diagnosing irritable bowel syndrome. Am. J. Manag. Care 2001, 7, S246–S251. [Google Scholar]
- Mitchell, C.M.; Drossman, D.A. Survey of the AGA membership relating to patients with functional gastrointestinal disorders. Gastroenterology 1987, 92, 1282–1284. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Hawkey, C.J.; Mayer, E.A.; Jones, R.H.; Naesdal, J.; Wilson, I.K.; Peacock, R.A.; Wiklund, I.K. Characteristics of patients with irritable bowel syndrome recruited from three sources: Implications for clinical trials. Aliment. Pharmacol. Ther. 2001, 15, 959–964. [Google Scholar] [CrossRef]
- El-Salhy, M. Recent advances in the diagnosis of irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1161–1174. [Google Scholar] [CrossRef]
- Drossman, D.A.; Morris, C.B.; Schneck, S.; Hu, Y.J.; Norton, N.J.; Norton, W.F.; Weinland, S.R.; Dalton, C.; Leserman, J.; Bangdiwala, S.I. International survey of patients with IBS: Symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J. Clin. Gastroenterol. 2009, 43, 541–550. [Google Scholar] [CrossRef]
- Locke, G.R., 3rd; Zinsmeister, A.R.; Talley, N.J.; Fett, S.L.; Melton, L.J., 3rd. Familial association in adults with functional gastrointestinal disorders. Mayo. Clin. Proc. 2000, 75, 907–912. [Google Scholar] [CrossRef]
- Kalantar, J.S.; Locke, G.R., 3rd; Zinsmeister, A.R.; Beighley, C.M.; Talley, N.J. Familial aggregation of irritable bowel syndrome: A prospective study. Gut 2003, 52, 1703–1707. [Google Scholar] [CrossRef]
- Kanazawa, M.; Endo, Y.; Whitehead, W.E.; Kano, M.; Hongo, M.; Fukudo, S. Patients and nonconsulters with irritable bowel syndrome reporting a parental history of bowel problems have more impaired psychological distress. Dig. Dis. Sci. 2004, 49, 1046–1053. [Google Scholar] [CrossRef]
- Morris-Yates, A.; Talley, N.J.; Boyce, P.M.; Nandurkar, S.; Andrews, G. Evidence of a genetic contribution to functional bowel disorder. Am. J. Gastroenterol. 1998, 93, 1311–1317. [Google Scholar] [CrossRef]
- Levy, R.L.; Jones, K.R.; Whitehead, W.E.; Feld, S.I.; Talley, N.J.; Corey, L.A. Irritable bowel syndrome in twins: Heredity and social learning both contribute to etiology. Gastroenterology 2001, 121, 799–804. [Google Scholar] [CrossRef]
- Lembo, A.; Zaman, M.; Jones, M.; Talley, N.J. Influence of genetics on irritable bowel syndrome, gastro-oesophageal reflux and dyspepsia: A twin study. Aliment. Pharmacol. Ther. 2007, 25, 1343–1350. [Google Scholar] [CrossRef]
- Wojczynski, M.K.; North, K.E.; Pedersen, N.L.; Sullivan, P.F. Irritable bowel syndrome: A co-twin control analysis. Am. J. Gastroenterol. 2007, 102, 2220–2229. [Google Scholar] [CrossRef]
- Bengtson, M.B.; Ronning, T.; Vatn, M.H.; Harris, J.R. Irritable bowel syndrome in twins: Genes and environment. Gut 2006, 55, 1754–1759. [Google Scholar] [CrossRef]
- D’Amato, M. Genes and functional GI disorders: From casual to causal relationship. Neurogastroenterol. Motil. 2013, 25, 638–649. [Google Scholar] [CrossRef]
- El-Salhy, M.; Ostgaard, H.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. The role of diet in the pathogenesis and management of irritable bowel syndrome (Review). Int. J. Mol. Med. 2012, 29, 723–731. [Google Scholar] [CrossRef]
- Ostgaard, H.; Hausken, T.; Gundersen, D.; El-Salhy, M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol. Med. Rep. 2012, 5, 1382–1390. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gilja, O.H.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Interaction between ingested nutrients and gut endocrine cells in patients with irritable bowel syndrome (Review). Int. J. Mol. Med. 2014, 34, 363–371. [Google Scholar] [CrossRef]
- El-Salhy, M.; Mazzawi, T.; Hausken, T.; Hatlebakk, J.G. Interaction between diet and gastrointestinal endocrine cells. Biomed. Rep. 2016, 4, 651–656. [Google Scholar] [CrossRef]
- El-Salhy, M.; Lilbo, E.; Reinemo, A.; Salmeøid, L.; Hausken, T. Effects of a health program comprising reassurance, diet management, probiotic administration and regular exercise on symptoms and quality of life in patients with irritable bowel syndrome. Gastroenterol. Insights 2010, 2, 21–26. [Google Scholar] [CrossRef]
- Jarrett, M.; Heitkemper, M.M.; Bond, E.F.; Georges, J. Comparison of diet composition in women with and without functional bowel disorder. Gastroenterol. Nurs. 1994, 16, 253–258. [Google Scholar] [CrossRef]
- Saito, Y.A.; Locke, G.R., 3rd; Weaver, A.L.; Zinsmeister, A.R.; Talley, N.J. Diet and functional gastrointestinal disorders: A population-based case-control study. Am. J. Gastroenterol. 2005, 100, 2743–2748. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gundersen, D. Diet in irritable bowel syndrome. Nutr. J. 2015, 14, 36. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Hausken, T. The relation between celiac disease, nonceliac gluten sensitivity and irritable bowel syndrome. Nutr. J. 2015, 14, 92. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Diet and irritable bowel syndrome, with a focus on appetite-regulating hormones. In Nutrition in the Prevention and Treatment of Abdominal Obesity; Watson, R.R., Ed.; Elsevier: San Diego, CA, USA, 2014; pp. 5–16. [Google Scholar]
- El-Salhy, M. Diet in the pathophysiology and management of irritable bowel syndrome. Clevel. Clin. J. Med. 2016, 83, 663–664. [Google Scholar] [CrossRef][Green Version]
- Staudacher, H.M.; Whelan, K.; Irving, P.M.; Lomer, M.C. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J. Hum. Nutr. Diet. 2011, 24, 487–495. [Google Scholar] [CrossRef]
- Eswaran, S.L.; Chey, W.D.; Han-Markey, T.; Ball, S.; Jackson, K. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. Am. J. Gastroenterol. 2016, 111, 1824–1832. [Google Scholar] [CrossRef]
- Bohn, L.; Storsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Tornblom, H.; Simren, M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015, 149, 1399–1407.e2. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef]
- Catassi, G.; Lionetti, E.; Gatti, S.; Catassi, C. The Low FODMAP Diet: Many Question Marks for a Catchy Acronym. Nutrients 2017, 9, 292. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef]
- McKenzie, Y.A.; Alder, A.; Anderson, W.; Wills, A.; Goddard, L.; Gulia, P.; Jankovich, E.; Mutch, P.; Reeves, L.B.; Singer, A.; et al. British Dietetic Association evidence-based guidelines for the dietary management of irritable bowel syndrome in adults. J. Hum. Nutr. Diet. 2012, 25, 260–274. [Google Scholar] [CrossRef]
- McKenzie, Y.A.; Thompson, J.; Gulia, P.; Lomer, M.C. British Dietetic Association systematic review of systematic reviews and evidence-based practice guidelines for the use of probiotics in the management of irritable bowel syndrome in adults (2016 update). J. Hum. Nutr. Diet. 2016, 29, 576–592. [Google Scholar] [CrossRef]
- Simren, M.; Mansson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Bjornsson, E.S. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef]
- Monsbakken, K.W.; Vandvik, P.O.; Farup, P.G. Perceived food intolerance in subjects with irritable bowel syndrome—Etiology, prevalence and consequences. Eur. J. Clin. Nutr. 2006, 60, 667–672. [Google Scholar] [CrossRef]
- Bohn, L.; Storsrud, S.; Simren, M. Nutrient intake in patients with irritable bowel syndrome compared with the general population. Neurogastroenterol. Motil. 2013, 25, 23e1. [Google Scholar] [CrossRef]
- Bagyanszki, M.; Bodi, N. Gut region-dependent alterations of nitrergic myenteric neurons after chronic alcohol consumption. World J. Gastrointest. Pathophysiol. 2015, 6, 51–57. [Google Scholar] [CrossRef][Green Version]
- Bagyanszki, M.; Krecsmarik, M.; De Winter, B.Y.; De Man, J.G.; Fekete, E.; Pelckmans, P.A.; Adriaensen, D.; Kroese, A.B.; Van Nassauw, L.; Timmermans, J.P. Chronic alcohol consumption affects gastrointestinal motility and reduces the proportion of neuronal NOS-immunoreactive myenteric neurons in the murine jejunum. Anat. Rec. 2010, 293, 1536–1542. [Google Scholar] [CrossRef]
- Bagyanszki, M.; Torfs, P.; Krecsmarik, M.; Fekete, E.; Adriaensen, D.; Van Nassauw, L.; Timmermans, J.P.; Kroese, A.B. Chronic alcohol consumption induces an overproduction of NO by nNOS- and iNOS-expressing myenteric neurons in the murine small intestine. Neurogastroenterol. Motil. 2011, 23, e237–e248. [Google Scholar] [CrossRef]
- Bodi, N.; Jancso, Z.; Talapka, P.; Pal, A.; Poles, M.Z.; Bagyanszki, M.; Hermesz, E.; Fekete, E. Gut region-specific rearrangement of the cellular and subcellular compartments of nitric oxide synthase isoforms after chronic ethanol consumption in rats. Histol. Histopathol. 2014, 29, 1547–1555. [Google Scholar]
- Krecsmarik, M.; Izbeki, F.; Bagyanszki, M.; Linke, N.; Bodi, N.; Kaszaki, J.; Katarova, Z.; Szabo, A.; Fekete, E.; Wittmann, T. Chronic ethanol exposure impairs neuronal nitric oxide synthase in the rat intestine. Alcohol Clin. Exp. Res. 2006, 30, 967–973. [Google Scholar] [CrossRef]
- Nazer, H.; Wright, R.A. The effect of alcohol on the human alimentary tract: A review. J. Clin. Gastroenterol. 1983, 5, 361–365. [Google Scholar]
- Hayes, P.; Corish, C.; O’Mahony, E.; Quigley, E.M. A dietary survey of patients with irritable bowel syndrome. J. Hum. Nutr. Diet. 2014, 27 (Suppl. S2), 36–47. [Google Scholar] [CrossRef]
- Bohn, L.; Storsrud, S.; Tornblom, H.; Bengtsson, U.; Simren, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef]
- Faresjo, A.; Johansson, S.; Faresjo, T.; Roos, S.; Hallert, C. Sex differences in dietary coping with gastrointestinal symptoms. Eur. J. Gastroenterol. Hepatol. 2010, 22, 327–333. [Google Scholar] [CrossRef]
- Reding, K.W.; Cain, K.C.; Jarrett, M.E.; Eugenio, M.D.; Heitkemper, M.M. Relationship between patterns of alcohol consumption and gastrointestinal symptoms among patients with irritable bowel syndrome. Am. J. Gastroenterol. 2013, 108, 270–276. [Google Scholar] [CrossRef]
- McKenzie, Y.A.; Bowyer, R.K.; Leach, H.; Gulia, P.; Horobin, J.; O’Sullivan, N.A.; Pettitt, C.; Reeves, L.B.; Seamark, L.; Williams, M.; et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J. Hum. Nutr. Diet. 2016, 29, 549–575. [Google Scholar] [CrossRef]
- Gonlachanvit, S.; Mahayosnond, A.; Kullavanijaya, P. Effects of chili on postprandial gastrointestinal symptoms in diarrhoea predominant irritable bowel syndrome: Evidence for capsaicin-sensitive visceral nociception hypersensitivity. Neurogastroenterol. Motil. 2009, 21, 23–32. [Google Scholar] [CrossRef]
- Schmulson, M.J.; Valdovinos, M.A.; Milke, P. Chili pepper and rectal hyperalgesia in irritable bowel syndrome. Am. J. Gastroenterol. 2003, 98, 1214–1215. [Google Scholar] [CrossRef]
- Aniwan, S.; Gonlachanvit, S. Effects of Chili Treatment on Gastrointestinal and Rectal Sensation in Diarrhea-predominant Irritable Bowel Syndrome: A Randomized, Double-blinded, Crossover Study. J. Neurogastroenterol. Motil. 2014, 20, 400–406. [Google Scholar] [CrossRef]
- Bortolotti, M.; Porta, S. Effect of red pepper on symptoms of irritable bowel syndrome: Preliminary study. Dig. Dis. Sci. 2011, 56, 3288–3295. [Google Scholar] [CrossRef]
- Patcharatrakul, T.; Gonlachanvit, S. Chili Peppers, Curcumins, and Prebiotics in Gastrointestinal Health and Disease. Curr. Gastroenterol. Rep. 2016, 18, 19. [Google Scholar] [CrossRef]
- Ferrucci, L.M.; Daniel, C.R.; Kapur, K.; Chadha, P.; Shetty, H.; Graubard, B.I.; George, P.S.; Osborne, W.; Yurgalevitch, S.; Devasenapathy, N.; et al. Measurement of spices and seasonings in India: opportunities for cancer epidemiology and prevention. Asian Pac. J. Cancer Prev. APJCP 2010, 11, 1621–1629. [Google Scholar]
- Govindarajan, V.S.; Rajalakshmi, D.; Chand, N. Capsicum—Production, technology, chemistry, and quality. Part IV. Evaluation of quality. Crit. Rev. Food Sci. Nutr. 1987, 25, 185–282. [Google Scholar] [CrossRef]
- Govindarajan, V.S.; Sathyanarayana, M.N. Capsicum—Production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. Crit. Rev. Food Sci. Nutr. 1991, 29, 435–474. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Shukla, R.; Ghoshal, U.; Gwee, K.A.; Ng, S.C.; Quigley, E.M. The gut microbiota and irritable bowel syndrome: Friend or foe? Int. J. Inflam. 2012, 2012, 151085. [Google Scholar] [CrossRef]
- Gwee, K.A. Irritable bowel syndrome in developing countries—A disorder of civilization or colonization? Neurogastroenterol. Motil. 2005, 17, 317–324. [Google Scholar] [CrossRef]
- Gwee, K.A.; Lu, C.L.; Ghoshal, U.C. Epidemiology of irritable bowel syndrome in Asia: Something old, something new, something borrowed. J. Gastroenterol. Hepatol. 2009, 24, 1601–1607. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Abraham, P.; Bhatt, C.; Choudhuri, G.; Bhatia, S.J.; Shenoy, K.T.; Banka, N.H.; Bose, K.; Bohidar, N.P.; Chakravartty, K.; et al. Epidemiological and clinical profile of irritable bowel syndrome in India: Report of the Indian Society of Gastroenterology Task Force. Indian J. Gastroenterol. 2008, 27, 22–28. [Google Scholar]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Hugon, P.; Dufour, J.C.; Colson, P.; Fournier, P.E.; Sallah, K.; Raoult, D. A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infects Dis. 2015, 15, 1211–1219. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Kriss, M.; Hazleton, K.Z.; Nusbacher, N.M.; Martin, C.G.; Lozupone, C.A. Low diversity gut microbiota dysbiosis: Drivers, functional implications and recovery. Curr. Opin. Microbiol. 2018, 44, 34–40. [Google Scholar] [CrossRef]
- Wilson, B.C.; Vatanen, T.; Cutfield, W.S.; O’Sullivan, J.M. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front. Cell Infect. Microbiol. 2019, 9, 2. [Google Scholar] [CrossRef]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef]
- El-Salhy, M.; Mazzawi, T. Fecal microbiota transplantation for managing irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 439–445. [Google Scholar] [CrossRef]
- Casen, C.; Vebo, H.C.; Sekelja, M.; Hegge, F.T.; Karlsson, M.K.; Ciemniejewska, E.; Dzankovic, S.; Froyland, C.; Nestestog, R.; Engstrand, L.; et al. Deviations in human gut microbiota: A novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment. Pharmacol. Ther. 2015, 42, 71–83. [Google Scholar] [CrossRef]
- Enck, P.; Mazurak, N. Dysbiosis in Functional Bowel Disorders. Ann. Nutr. Metab. 2018, 72, 296–306. [Google Scholar] [CrossRef]
- Pozuelo, M.; Panda, S.; Santiago, A.; Mendez, S.; Accarino, A.; Santos, J.; Guarner, F.; Azpiroz, F.; Manichanh, C. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci. Rep. 2015, 5, 12693. [Google Scholar] [CrossRef]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The Microbiome and Irritable Bowel Syndrome—A Review on the Pathophysiology, Current Research and Future Therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Collins, S.M. A role for the gut microbiota in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 497–505. [Google Scholar] [CrossRef]
- El-Salhy, M.; Seim, I.; Chopin, L.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Irritable bowel syndrome: The role of gut neuroendocrine peptides. Front. Biosci. 2012, 4, 2783–2800. [Google Scholar] [CrossRef]
- Mawe, G.M.; Coates, M.D.; Moses, P.L. Review article: Intestinal serotonin signalling in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2006, 23, 1067–1076. [Google Scholar] [CrossRef]
- Wade, P.R.; Chen, J.; Jaffe, B.; Kassem, I.S.; Blakely, R.D.; Gershon, M.D. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J. Neurosci. 1996, 16, 2352–2364. [Google Scholar] [CrossRef]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef]
- Gershon, M.D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 14–21. [Google Scholar] [CrossRef]
- Gershon, M.D. Serotonin is a sword and a shield of the bowel: Serotonin plays offense and defense. Trans. Am. Clin. Climatol. Assoc. 2012, 123, 268–280, discussion 280. [Google Scholar]
- El-Salhy, M.; Mazzawi, T.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. The role of peptide YY in gastrointestinal diseases and disorders (Review). Int. J. Mol. Med. 2013, 31, 275–282. [Google Scholar] [CrossRef]
- Dubrasquet, M.; Bataille, D.; Gespach, C. Oxyntomodulin (glucagon-37 or bioactive enteroglucagon): A potent inhibitor of pentagastrin-stimulated acid secretion in rats. Biosci. Rep. 1982, 2, 391–395. [Google Scholar] [CrossRef]
- Schjoldager, B.T.; Baldissera, F.G.; Mortensen, P.E.; Holst, J.J.; Christiansen, J. Oxyntomodulin: A potential hormone from the distal gut. Pharmacokinetics and effects on gastric acid and insulin secretion in man. Eur. J. Clin. Investig. 1988, 18, 499–503. [Google Scholar] [CrossRef]
- Schjoldager, B.; Mortensen, P.E.; Myhre, J.; Christiansen, J.; Holst, J.J. Oxyntomodulin from distal gut. Role in regulation of gastric and pancreatic functions. Dig. Dis. Sci. 1989, 34, 1411–1419. [Google Scholar] [CrossRef]
- Dakin, C.L.; Small, C.J.; Batterham, R.L.; Neary, N.M.; Cohen, M.A.; Patterson, M.; Ghatei, M.A.; Bloom, S.R. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology 2004, 145, 2687–2695. [Google Scholar] [CrossRef]
- Wynne, K.; Park, A.J.; Small, C.J.; Patterson, M.; Ellis, S.M.; Murphy, K.G.; Wren, A.M.; Frost, G.S.; Meeran, K.; Ghatei, M.A.; et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: A double-blind, randomized, controlled trial. Diabetes 2005, 54, 2390–2395. [Google Scholar] [CrossRef]
- Camilleri, M. Peripheral mechanisms in irritable bowel syndrome. N. Engl. J. Med. 2012, 367, 1626–1635. [Google Scholar] [CrossRef]
- Jianu, C.S.; Fossmark, R.; Syversen, U.; Hauso, O.; Waldum, H.L. A meal test improves the specificity of chromogranin A as a marker of neuroendocrine neoplasia. Tumour Biol. 2010, 31, 373–380. [Google Scholar] [CrossRef]
- Gunawardene, A.R.; Corfe, B.M.; Staton, C.A. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int. J. Exp. Pathol. 2011, 92, 219–231. [Google Scholar] [CrossRef]
- May, C.L.; Kaestner, K.H. Gut endocrine cell development. Mol. Cell Endocrinol. 2010, 323, 70–75. [Google Scholar] [CrossRef]
- Sandstrom, O.; El-Salhy, M. Ageing and endocrine cells of human duodenum. Mech. Ageing Dev. 1999, 108, 39–48. [Google Scholar] [CrossRef]
- El-Salhy, M. Ghrelin in gastrointestinal diseases and disorders: A possible role in the pathophysiology and clinical implications (review). Int. J. Mol. Med. 2009, 24, 727–732. [Google Scholar] [CrossRef][Green Version]
- Tolhurst, G.; Reimann, F.; Gribble, F.M. Intestinal sensing of nutrients. Handb. Exp. Pharmacol. 2012, 309–335. [Google Scholar] [CrossRef]
- Lee, J.; Cummings, B.P.; Martin, E.; Sharp, J.W.; Graham, J.L.; Stanhope, K.L.; Havel, P.J.; Raybould, H.E. Glucose sensing by gut endocrine cells and activation of the vagal afferent pathway is impaired in a rodent model of type 2 diabetes mellitus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R657–R666. [Google Scholar] [CrossRef]
- Parker, H.E.; Reimann, F.; Gribble, F.M. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev. Mol. Med. 2010, 12, e1. [Google Scholar] [CrossRef]
- Raybould, H.E. Nutrient sensing in the gastrointestinal tract: Possible role for nutrient transporters. J. Physiol. Biochem. 2008, 64, 349–356. [Google Scholar] [CrossRef]
- San Gabriel, A.; Nakamura, E.; Uneyama, H.; Torii, K. Taste, visceral information and exocrine reflexes with glutamate through umami receptors. J. Med. Investig. 2009, 56, 209–217. [Google Scholar] [CrossRef][Green Version]
- Rudholm, T.; Wallin, B.; Theodorsson, E.; Naslund, E.; Hellstrom, P.M. Release of regulatory gut peptides somatostatin, neurotensin and vasoactive intestinal peptide by acid and hyperosmolal solutions in the intestine in conscious rats. Regul. Pept. 2009, 152, 8–12. [Google Scholar] [CrossRef]
- Sternini, C.; Anselmi, L.; Rozengurt, E. Enteroendocrine cells: A site of “taste” in gastrointestinal chemosensing. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 73–78. [Google Scholar] [CrossRef]
- Sternini, C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G457–G461. [Google Scholar] [CrossRef]
- Buchan, A.M. Nutrient Tasting and Signaling Mechanisms in the Gut III. Endocrine cell recognition of luminal nutrients. Am. J. Physiol. 1999, 277, G1103–G1107. [Google Scholar]
- Montero-Hadjadje, M.; Elias, S.; Chevalier, L.; Benard, M.; Tanguy, Y.; Turquier, V.; Galas, L.; Yon, L.; Malagon, M.M.; Driouich, A.; et al. Chromogranin A promotes peptide hormone sorting to mobile granules in constitutively and regulated secreting cells: Role of conserved N- and C-terminal peptides. J. Biol. Chem. 2009, 284, 12420–12431. [Google Scholar] [CrossRef]
- Shooshtarizadeh, P.; Zhang, D.; Chich, J.F.; Gasnier, C.; Schneider, F.; Haikel, Y.; Aunis, D.; Metz-Boutigue, M.H. The antimicrobial peptides derived from chromogranin/secretogranin family, new actors of innate immunity. Regul. Pept. 2010, 165, 102–110. [Google Scholar] [CrossRef]
- Rindi, G.; Inzani, F.; Solcia, E. Pathology of gastrointestinal disorders. Endocrinol. Metab. Clin. N. Am. 2010, 39, 713–727. [Google Scholar] [CrossRef]
- Seim, I.; El-Salhy, M.; Hausken, T.; Gundersen, D.; Chopin, L. Ghrelin and the brain-gut axis as a pharmacological target for appetite control. Curr. Pharm. Des. 2012, 18, 768–775. [Google Scholar] [CrossRef]
- Dizdar, V.; Spiller, R.; Singh, G.; Hanevik, K.; Gilja, O.H.; El-Salhy, M.; Hausken, T. Relative importance of abnormalities of CCK and 5-HT (serotonin) in Giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment. Pharmacol. Ther. 2010, 31, 883–891. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gilja, O.H. Abnormalities in ileal stem, neurogenin 3, and enteroendocrine cells in patients with irritable bowel syndrome. BMC Gastroenterol. 2017, 17, 90. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gilja, O.H.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Duodenal chromogranin a cell density as a biomarker for the diagnosis of irritable bowel syndrome. Gastroenterol. Res. Pract. 2014, 2014, 462856. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gilja, O.H.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Endocrine cells in the ileum of patients with irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 2383–2391. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gilja, O.H.; Gundersen, D.; Hausken, T. Endocrine cells in the oxyntic mucosa of the stomach in patients with irritable bowel syndrome. World J. Gastrointest. Endosc. 2014, 6, 176–185. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. Stomach antral endocrine cells in patients with irritable bowel syndrome. Int. J. Mol. Med. 2014, 34, 967–974. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gilja, O.H.; Hausken, T. Chromogranin A cells in the stomachs of patients with sporadic irritable bowel syndrome. Mol. Med. Rep. 2014, 10, 1753–1757. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gundersen, D.; Hatlebakk, J.G.; Gilja, O.H.; Hausken, T. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul. Pept. 2014, 188, 60–65. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Hausken, T. Densities of rectal peptide YY and somatostatin cells as biomarkers for the diagnosis of irritable bowel syndrome. Peptides 2015, 67, 12–19. [Google Scholar] [CrossRef][Green Version]
- El-Salhy, M.; Patcharatrakul, T.; Hatlebakk, J.G.; Hausken, T.; Gilja, O.H.; Gonlachanvit, S. Chromogranin A cell density in the large intestine of Asian and European patients with irritable bowel syndrome. Scand. J. Gastroenterol. 2017, 52, 691–697. [Google Scholar] [CrossRef]
- El-Salhy, M.; Patcharatrakul, T.; Hatlebakk, J.G.; Hausken, T.; Gilja, O.H.; Gonlachanvit, S. Enteroendocrine, Musashi 1 and neurogenin 3 cells in the large intestine of Thai and Norwegian patients with irritable bowel syndrome. Scand. J. Gastroenterol. 2017, 52, 1331–1339. [Google Scholar] [CrossRef]
- El-Salhy, M.; Vaali, K.; Dizdar, V.; Hausken, T. Abnormal small-intestinal endocrine cells in patients with irritable bowel syndrome. Dig. Dis. Sci. 2010, 55, 3508–3513. [Google Scholar] [CrossRef]
- Wang, J.; Cortina, G.; Wu, S.V.; Tran, R.; Cho, J.H.; Tsai, M.J.; Bailey, T.J.; Jamrich, M.; Ament, M.E.; Treem, W.R.; et al. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N. Engl. J. Med. 2006, 355, 270–280. [Google Scholar] [CrossRef]
- Fishbein, T.M.; Novitskiy, G.; Lough, D.M.; Matsumoto, C.; Kaufman, S.S.; Shetty, K.; Zasloff, M. Rejection reversibly alters enteroendocrine cell renewal in the transplanted small intestine. Am. J. Transplant. 2009, 9, 1620–1628. [Google Scholar] [CrossRef]
- Jenny, M.; Uhl, C.; Roche, C.; Duluc, I.; Guillermin, V.; Guillemot, F.; Jensen, J.; Kedinger, M.; Gradwohl, G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002, 21, 6338–6347. [Google Scholar] [CrossRef]
- Montgomery, R.K.; Breault, D.T. Small intestinal stem cell markers. J. Anat. 2008, 213, 52–58. [Google Scholar] [CrossRef]
- Potten, C.S.; Booth, C.; Tudor, G.L.; Booth, D.; Brady, G.; Hurley, P.; Ashton, G.; Clarke, R.; Sakakibara, S.; Okano, H. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 2003, 71, 28–41. [Google Scholar] [CrossRef]
- Kayahara, T.; Sawada, M.; Takaishi, S.; Fukui, H.; Seno, H.; Fukuzawa, H.; Suzuki, K.; Hiai, H.; Kageyama, R.; Okano, H.; et al. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003, 535, 131–135. [Google Scholar] [CrossRef]
- He, X.C.; Yin, T.; Grindley, J.C.; Tian, Q.; Sato, T.; Tao, W.A.; Dirisina, R.; Porter-Westpfahl, K.S.; Hembree, M.; Johnson, T.; et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat. Genet. 2007, 39, 189–198. [Google Scholar] [CrossRef]
- Schonhoff, S.E.; Giel-Moloney, M.; Leiter, A.B. Minireview: Development and differentiation of gut endocrine cells. Endocrinology 2004, 145, 2639–2644. [Google Scholar] [CrossRef]
- Schonhoff, S.E.; Giel-Moloney, M.; Leiter, A.B. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev. Biol. 2004, 270, 443–454. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Hausken, T. The reduction in duodenal endocrine cells in IBSis associated with stem cell abnormalities. World. J. Gastroenterol. 2015, 21, 9577–9587. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Hausken, T. Irritable bowel syndrome: Recent developments in diagnosis, pathophysiology, and treatment. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 435–443. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gundersen, D.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. Is irritable bowel syndrome an organic disorder? World J. Gastroenterol. 2014, 20, 384–400. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hausken, T.; Gilja, O.H.; Hatlebakk, J.G. The possible role of gastrointestinal endocrine cells in the pathophysiology of irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 139–148. [Google Scholar] [CrossRef]
- Hill, P.; Muir, J.G.; Gibson, P.R. Controversies and Recent Developments of the Low-FODMAP Diet. Gastroenterol. Hepatol. 2017, 13, 36–45. [Google Scholar]
- Biesiekierski, J.R.; Jalanka, J.; Staudacher, H.M. Can Gut Microbiota Composition Predict Response to Dietary Treatments? Nutrients 2019, 11, 1134. [Google Scholar] [CrossRef]
- Mazzawi, T.; El-Salhy, M. Changes in small intestinal chromogranin A-immunoreactive cell densities in patients with irritable bowel syndrome after receiving dietary guidance. Int. J. Mol. Med. 2016, 37, 1247–1253. [Google Scholar] [CrossRef][Green Version]
- Mazzawi, T.; El-Salhy, M. Dietary guidance and ileal enteroendocrine cells in patients with irritable bowel syndrome. Exp. Ther. Med. 2016, 12, 1398–1404. [Google Scholar] [CrossRef]
- Mazzawi, T.; Gundersen, D.; Hausken, T.; El-Salhy, M. Increased gastric chromogranin A cell density following changes to diets of patients with irritable bowel syndrome. Mol. Med. Rep. 2014, 10, 2322–2326. [Google Scholar] [CrossRef][Green Version]
- Mazzawi, T.; Gundersen, D.; Hausken, T.; El-Salhy, M. Increased chromogranin A cell density in the large intestine of patients with irritable bowel syndrome after receiving dietary guidance. Mol. Med. Rep. 2015, in press. [Google Scholar] [CrossRef]
- Mazzawi, T.; Hausken, T.; Gundersen, D.; El-Salhy, M. Effects of dietary guidance on the symptoms, quality of life and habitual dietary intake of patients with irritable bowel syndrome. Mol. Med. Rep. 2013, 8, 845–852. [Google Scholar] [CrossRef]
- Mazzawi, T.; Hausken, T.; Gundersen, D.; El-Salhy, M. Effect of dietary management on the gastric endocrine cells in patients with irritable bowel syndrome. Eur. J. Clin. Nutr. 2014. [Google Scholar] [CrossRef]
- Mazzawi, T.; Hausken, T.; Gundersen, D.; El-Salhy, M. Dietary guidance normalizes large intestinal endocrine cell densities in patients with irritable bowel syndrome. Eur. J. Clin. Nutr. 2016, 70, 175–181. [Google Scholar] [CrossRef]
- Mazzawi, T.; Lied, G.A.; El-Sahy, M.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. Effect of fecal microbiota transplantation on the symptoms and duodenal enteroendocrine cells in patients with irritable bowel syndrome. United Eur. Gastroenterol. J. 2016, 4 (Suppl. 5), 677. [Google Scholar]
| Vegetables | Fruits | Others |
|---|---|---|
| Onions, garlic, the white portion of leeks and spring onions, cabbage, spring onions, mushrooms, beans, red kidney beans, Brussels sprouts, sugar peas, asparagus, lentils, beets, artichoke, fennel, peas, sugar peas, cauliflower | Apples, pears, peach, mango, watermelon, dried fruit, fruit juice, canned fruit in natural juice, nashiphary, apricot, longan, cherry, lychee, nectarine, plum | Wheat, barley, rye, bread, pasta, couscous, biscuits, cakes |
| Milk and dairy products: cheese, yogurt, soy milk, cream | ||
| Sweeteners containing fructose (for example, corn syrup) | ||
| Sweeteners: sorbitol, mannitol, xylitol, isomalt, maltitol, and other sweeteners with names ending in “ol” |
| Vegetables | Fruits | Others |
|---|---|---|
| Onions, garlic, beans, peas, artichoke, cabbage | Watermelon | Wheat flour and wheat-based products Milk and dairy products Sweeteners containing fructose (for example, corn syrup) Sweeteners: sorbitol, menthol, xylitol, isomalt, maltitol, and other sweeteners with names ending in “ol” Carbonated drinks (soft drinks), coffee, beer |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Salhy, M.; Hatlebakk, J.G.; Hausken, T. Diet in Irritable Bowel Syndrome (IBS): Interaction with Gut Microbiota and Gut Hormones. Nutrients 2019, 11, 1824. https://doi.org/10.3390/nu11081824
El-Salhy M, Hatlebakk JG, Hausken T. Diet in Irritable Bowel Syndrome (IBS): Interaction with Gut Microbiota and Gut Hormones. Nutrients. 2019; 11(8):1824. https://doi.org/10.3390/nu11081824
Chicago/Turabian StyleEl-Salhy, Magdy, Jan Gunnar Hatlebakk, and Trygve Hausken. 2019. "Diet in Irritable Bowel Syndrome (IBS): Interaction with Gut Microbiota and Gut Hormones" Nutrients 11, no. 8: 1824. https://doi.org/10.3390/nu11081824
APA StyleEl-Salhy, M., Hatlebakk, J. G., & Hausken, T. (2019). Diet in Irritable Bowel Syndrome (IBS): Interaction with Gut Microbiota and Gut Hormones. Nutrients, 11(8), 1824. https://doi.org/10.3390/nu11081824






