Supplementation with Synbiotics and/or Branched Chain Amino Acids in Hepatic Encephalopathy: A Pilot Randomised Placebo-Controlled Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Consent
2.2. Participant Selection
2.3. Psychometric Testing Methods
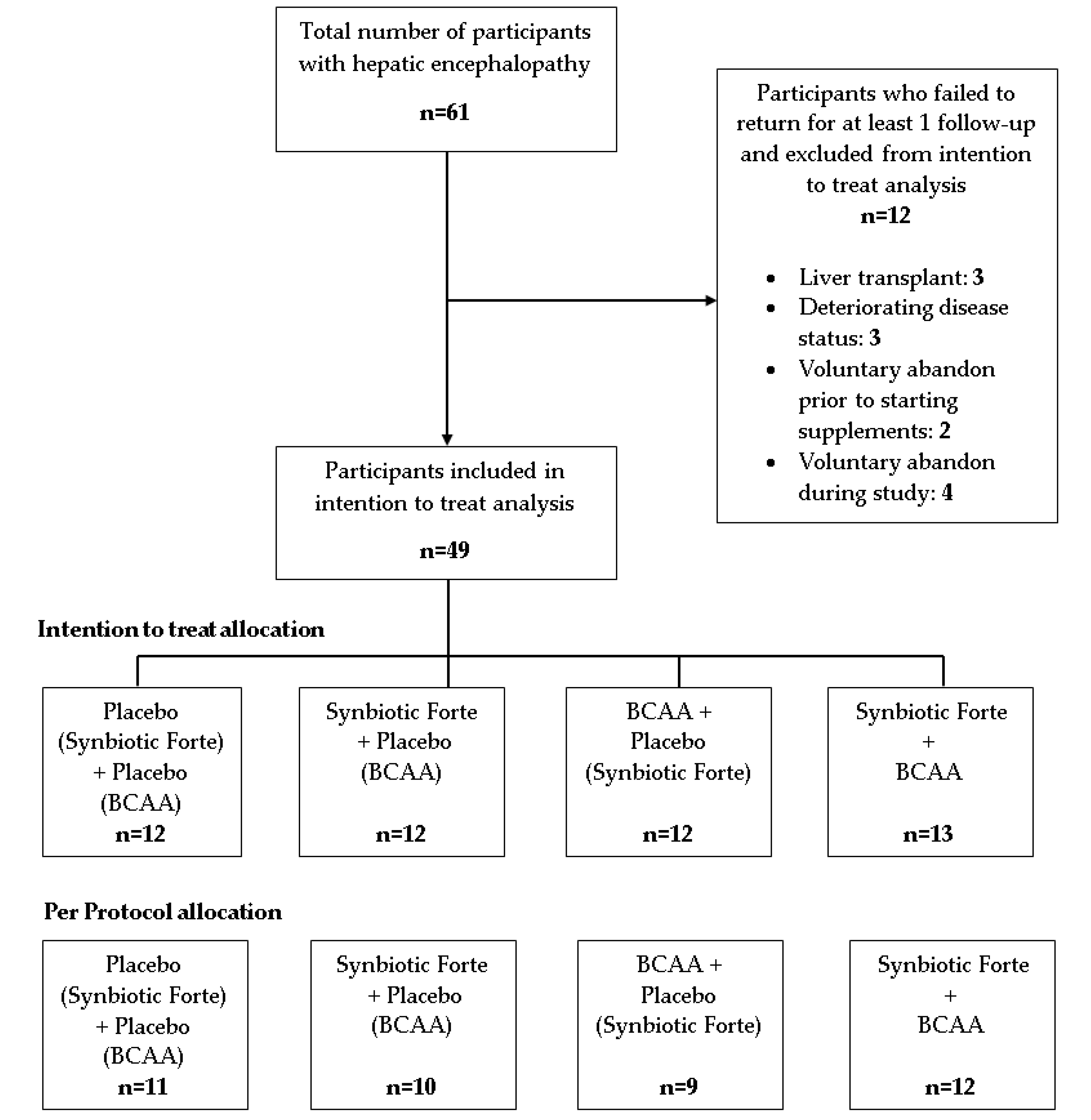
2.4. Participant Allocation
2.5. Interventions Assessed
2.6. Additional Data
2.7. Mood and Cognitive Assessment of Participants
2.8. Statistical Analysis
3. Results
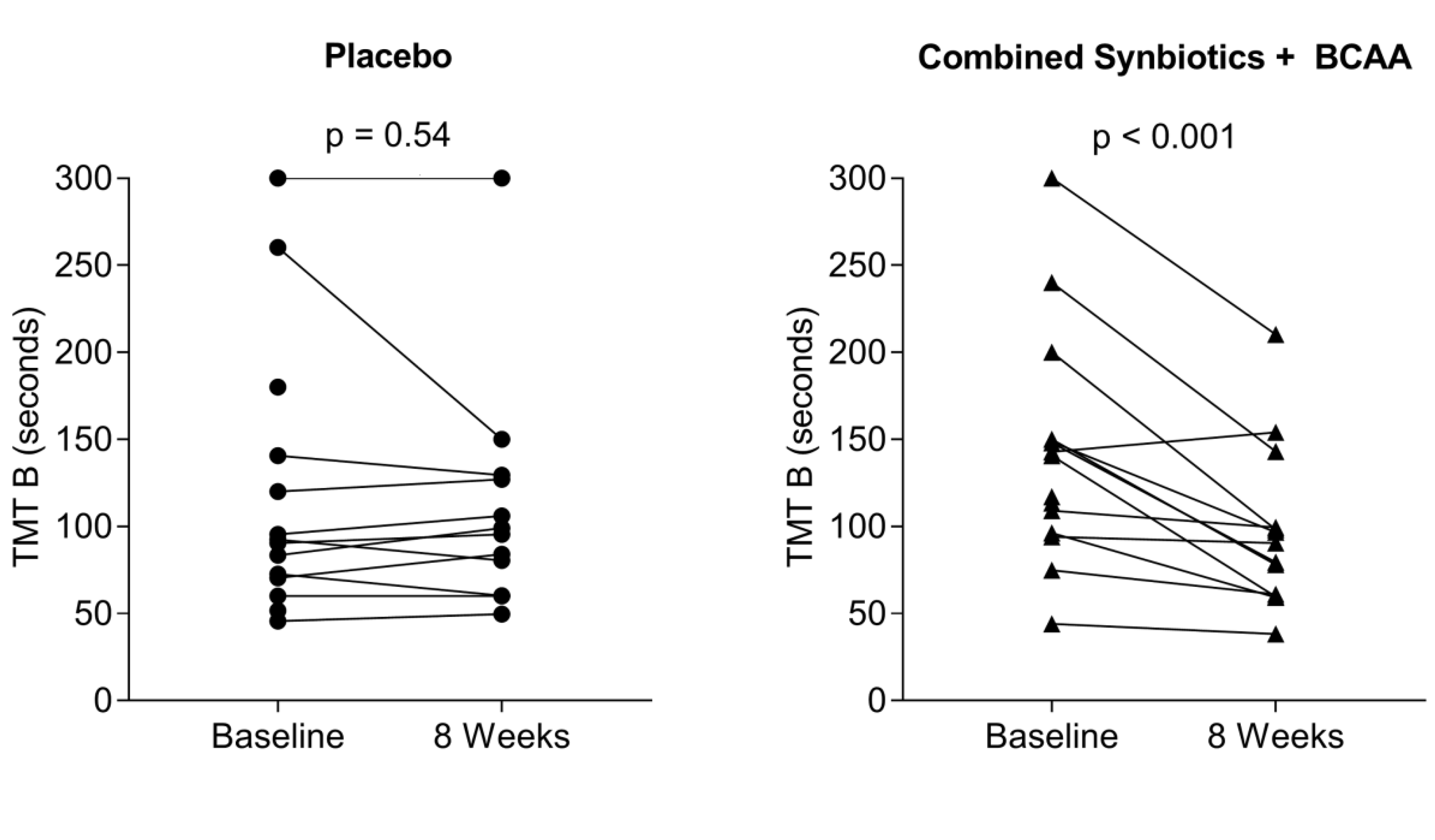
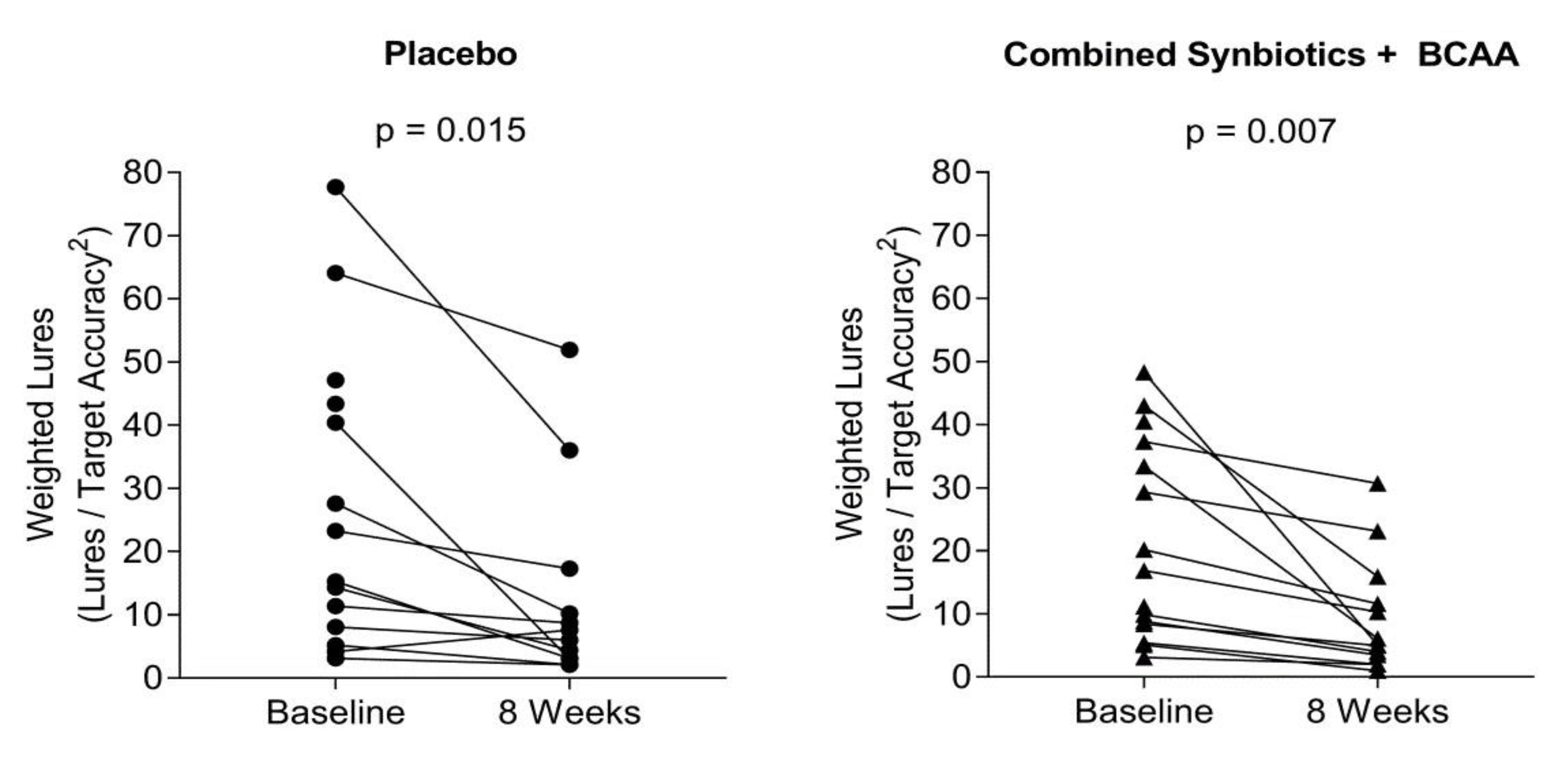
Psychometric Response to Interventions
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amodio, P. Hepatic Encephalopathy: Diagnosis and Management. Liver Int. 2018, 6, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, J. New Assessment of Hepatic Encephalopathy. J. Hepatol. 2011, 54, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Patidar, K.R.; Bajaj, J. Covert and Overt Hepatic Encephalopathy: Diagnosis and Management. Clin. Gastroenterol. Hepatol. 2015, 13, 2048–2061. [Google Scholar] [CrossRef] [PubMed]
- Poordad, F.F. Review article: The burden of hepatic encephalopathy. Aliment. Pharmacol. Ther. 2007, 25, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Schubert, C.; Heuman, D.M.; Wade, J.B.; Gibson, D.P.; Topaz, A.; Saeian, K.; Hafeezullah, M.; Bell, D.E.; Sterling, R.K.; et al. Persistence of Cognitive Impairment after Resolution of Overt Hepatic Encephalopathy. Gastroenterology 2010, 138, 2332–2340. [Google Scholar] [CrossRef] [PubMed]
- Campagna, F.; Montagnese, S.; Schiff, S.; Biancardi, A.; Mapelli, D.; Angeli, P.; Poci, C.; Cillo, U.; Merkel, C.; Gatta, A.; et al. Cognitive Impairment and Electroencephalographic Alterations Before and after Liver Transplantation: What Is Reversible? Liver Transpl. 2014, 20, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Saeian, K.; Verber, M.D.; Hischke, D.; Hoffmann, R.G.; Franco, J.; Varma, R.R.; Rao, S.M. Inhibitory Control Test Is a Simple Method to Diagnose Minimal Hepatic Encephalopathy and Predict Development of Overt Hepatic Encephalopathy. Am. J. Gastroenterol. 2007, 102, 754. [Google Scholar] [CrossRef]
- Crowe, S.F. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts A and B of the Trail Making Test. J. Clin. Psychol. 1998, 54, 585–591. [Google Scholar] [CrossRef]
- Tapper, E.B.; Jiang, G.; Patwardhan, V.R. Refining the Ammonia Hypothesis: A Physiology-Driven Approach to the Treatment of Hepatic Encephalopathy. Mayo Clin. Proc. 2015, 90, 646–658. [Google Scholar] [CrossRef]
- Ferenci, P. Hepatic Encephalopathy. Gastroenterol. Rep. 2017, 5, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, C.A.; Patel, V.; Singanayagam, A.; Shawcross, D.L. Review article: The gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment. Pharmacol. Ther. 2017, 47, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Campagna, F.; Amodio, P.; Tuohy, K. Gut Liver Brain axis: The microbial challenge in the hepatic encephalopathy. Food Funct. 2018, 9, 1373–1388. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.H.; La Fata, G.; Steinert, R.E.; Weber, P. Relationship between the gut microbiome and brain function. Nutr. Rev. 2018, 76, 481–496. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Qin, N.; Yang, E.; Prifti, E.; Chen, Y.; Shao, L.; Le Chatelier, E.; Yao, J.; Wu, L.; Zhou, J.; Ni, S.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Iebba, V.; Guerrieri, F.; Di Gregorio, V.; Levrero, M.; Gagliardi, A.; Santangelo, F.; Sobolev, A.P.; Circi, S.; Giannelli, V.; Mannina, L.; et al. Combining amplicon sequencing and metabolomics in cirrhotic patients highlights distinctive microbiota features involved in bacterial translocation, systemic inflammation and hepatic encephalopathy. Sci. Rep. 2018, 8, 8210. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.; McGee, R.G.; Riordan, S.M.; Webster, A.C. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst. Rev. 2017, CD008716. [Google Scholar] [CrossRef]
- Merli, M.; Berzigotti, A.; Zelber-Sagi, S.; Dasarathy, S.; Montagnese, S.; Genton, L.; Plauth, M.; Parés, A. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef]
- Fischer, J.E. Branched-chain-enriched amino acid solutions in patients with liver failure: An early example of nutritional pharmacology. J. Parenter. Enter. Nutr. 1990, 14, 249–256. [Google Scholar] [CrossRef]
- Holecek, M. Branched-chain amino acids and ammonia metabolism in liver disease: Therapeutic implications. Nutrition 2013, 29, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Gluud, L.; Dam, G.; Les, I.; Marchesini, G.; Borre, M.; Aagaard, N.; Vilstrup, H. Branched-Chain Amino Acids Improve Symptoms of Hepatic Encephalopathy (Review); Cochrane Library: London, UK, 2017; pp. 1–86. [Google Scholar]
- Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K.; Blei, A.T. Hepatic encephalopathy—Definition, nomenclature, diagnosis, and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002, 35, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Bass, N.B.; Mullen, K.; Sanyal, A.; Poordad, P.; Neff, G.; Leevy, C.B.; Sigal, A.; Sheikh, M.Y.; Beavers, K.; Frederick, T.; et al. Rifaximin Treatment in Hepatic Encephalopathy. N. Engl. J. Med. 2010, 362, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Varjacic, A.; Mantini, D.; Demeyere, N.; Gillebert, C.R. Neural signatures of Trail Making Test performance: Evidence from lesion-mapping and neuroimaging studies. Neuropsychologia 2018, 115, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Nabi, E.; Bajaj, J. Useful Tests for Hepatic Encephalopathy in Clinical Practice. Curr. Gastroenterol. Rep. 2014, 16, 362. [Google Scholar] [CrossRef] [PubMed]
- Amodio, P.; Ridola, L.; Schiff, S.; Montagnese, S.; Pasquale, C.; Nardelli, S.; Tressa, M.; Marzano, C.; Flaiban, C.; Angeli, P.; et al. Improving the Inhibitory Control Task to Detect Minimal Hepatic Encephalopathy. Gastroenterology 2010, 139, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Senior, G.; Piovesana, A.; Beaumont, P. Discrepancy analysis and Australian norms for the Trail Making Test. Clin. Neuropsychol. 2018, 32, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Müller, U.; Blackwell, A.D.; Clark, L.; Robbins, T.W.; Sahakian, B.J. Neurochemical Modulation of Response Inhibition and ProbabilisticLearning in Humans. Science 2006, 311, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Doig, D.S.; Simpson, F. Randomization and allocation concealment: A practical guide for researchers. J. Crit. Care 2005, 20, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Hasse, J.M.; Strong, S.; Gorman, M.A.; Liepa, U. Subjective global assessment: Alternative nutrition-assessment technique for liver-transplant candidates. Nutrition 1993, 9, 339–343. [Google Scholar] [PubMed]
- Henry, J.D.; Crawford, J. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2005, 44, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Valla, D. Assessment of the prognosis of cirrhosis: Child–Pugh versus MELD. J. Hepatol. 2005, 42, S100–S107. [Google Scholar] [CrossRef] [PubMed]
- Foodworks. FoodWorks Professional, Computer Program; Xyris Software Inc.: Brisbane, Australia, 2003; Volume 2003.
- Bottesi, G.; Ghisi, M.; Altoè, G.; Conforti, E.; Melli, G.; Sica, C. The Italian version of the Depression Anxiety Stress Scales-21: Factor structure and psychometric properties on community and clinical samples. Compr. Psychiatry 2015, 60, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.S.; Trezza, B.; Busse, A.L.; Jacob-Filho, W. Learning effect of computerized cognitive tests in older adults. Einstein 2014, 12, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.F.; Altman, D.; Moher, D. For the CONSORT Group: CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010, 7, e1000251. [Google Scholar]
- Krag, A.; Schuchmann, M.; Sodatonou, H.; Pilot, J.; Whitehouse, J.; Strasser, S.; Hudson, M. Design of the Prospective Real-world Outcomes Study of hepatic encephalopathy Patients’ Experience on Rifaximin-α (PROSPER): An observational study among 550 patients. Hepatol. Med. Policy 2018, 3, 4. [Google Scholar] [CrossRef]
- Biyik, M.; Ucar, R.; Gungor, G.; Polat, I.; Gaipov, A.; Cakir, O.O.; Ataseven, H.; Demir, A.; Turk, S.; Polat, H. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 435–441. [Google Scholar] [CrossRef]
- Peng, J.K.; Hepgul, N.; Higginson, I.J.; Gao, W. Symptom prevalence and quality of life of patients of patients with end-stage liver disease: A systematic review and meta-analysis. Palliat. Med. 2019, 33, 24–36. [Google Scholar] [CrossRef]
- Dang, T.; Mitchell, N.; Farhat, K.; Abraldes, J.; Ma, M.; Bailey, R.; Tandon, P. A183 Anxiety impacts health-related quality of life and hospitalizations in patients with cirrhosis. J. Can. Assoc. Gastroenterol. 2018, 1, 271–272. [Google Scholar] [CrossRef][Green Version]
- Wood, B.M.; Nicholas, M.; Blyth, F.; Ashgari, A.; Gibson, S. The Utility of the Short Version of the Depression Anxiety Stress Scales (DASS-21) in Elderly Patients with Persistent Pain: Does Age Make a Difference? Pain Med. 2010, 11, 1780–1790. [Google Scholar] [CrossRef]
- Dhiman, R.K.; Rana, B.; Agrawal, S.; Garg, A.; Chopra, M.; Thumburu, K.K.; Khattri, A.; Malhotra, S.; Duseja, A.; Chawla, Y.K. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: A randomized, controlled trial. Gastroenterology 2014, 147, 1327–1337. [Google Scholar] [PubMed]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; Puri, P.; Sterling, R.K.; Luketic, V.; Stravitz, R.T.; Siddiqui, M.S.; Fuchs, M.; et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment. Pharmacol. Ther. 2014, 38, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
| Placebo (n = 12) | Synbiotics (n = 12) | BCAA (n = 12) | Synbiotics + BCAA (n = 13) | Total (n = 49) | |
|---|---|---|---|---|---|
| Gender (n) | |||||
| Male | 11 | 11 | 9 | 11 | 42 |
| Female | 1 | 1 | 3 | 2 | 7 |
| Age | 54.1 ± 6.7 | 56.7 ± 7.5 | 5.7 ± 0.9 | 55.3 ± 4.4 | 55.8 ± 6.1 |
| Primary Diagnosis (n) | |||||
| Viral | 6 | 5 | 9 | 6 | 26 |
| Alcohol | 3 | 2 | 0 | 3 | 8 |
| NASH | 1 | 3 | 2 | 2 | 8 |
| Cholestatic | 2 | 0 | 0 | 1 | 3 |
| Other | 0 | 2 | 1 | 1 | 4 |
| MELD | 13.3 ± 2.9 | 13.2 ± 3.2 | 13.8 ± 2.9 | 14.4 ± 5.6 | 13.7 ± 3.8 |
| CP score | 9.2 ± 1.9 | 8.5 ± 0.9 | 9.2 ± 1.6 | 9.4 ± 2.4 | 9.1 ± 1.8 |
| Albumin (g/L) | 30.5.1 ± 5.3 | 34.6 ± 1.0 | 33.1 ± 7.2 | 33.3 ± 5.0 | 32.9 ± 5.6 |
| Ammonia (µmol/L) | 72.2 ± 29.5 | 84.0 ± 59.9 | 87.0.1 ± 37.9 | 80.8 ± 59.3 | 80.9 ± 47.7 |
| Neutrophils (×109 g/L) | 3.0 ± 1.4 | 2.6 ± 1.3 | 3.2 ± 1.5 | 3.1 ± 1.4 | 3.0 ± 1.4 |
| Lymphocyte (×109 g/L) | 1.1 ± 0.6 | 1.0 ± 0.4 | 1.0 ± 0.4 | 1.0 ± 0.5 | 1.0 ± 0.5 |
| N:L ratio | 3.0 ± 1.5 | 3.2 ± 1.5 | 4.1 ± 2.2 | 4.0 ± 02.4 | 3.6 ± 2.0 |
| INR | 1.5 ± 0.2 | 1.4 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.4 | 1.5 ± 0.3 |
| Ascites (n) | |||||
| None | 4 | 2 | 0 | 4 | 10 |
| Mild | 3 | 2 | 6 | 4 | 15 |
| Moderate | 4 | 7 | 3 | 3 | 17 |
| Severe | 1 | 1 | 3 | 1 | 5 |
| SGA (n) | |||||
| A | 3 | 3 | 4 | 4 | 14 |
| B | 4 | 5 | 1 | 5 | 15 |
| C | 5 | 4 | 7 | 4 | 20 |
| Daily Energy Intake (kcals/kg) | 22.3 ± 6.4 | 23.4 ± 5.4 | 28.9 ± 6.5 | 26.7 ± 10.6 | 25.4 ± 7.7 |
| Daily Protein (g/kg) | 1.3 ± 0.6 | 1.2 ± 0.3 | 1.4 ± 0.4 | 1.3 ± 0.5 | 1.3 ± 0.4 |
| Lactulose dose (mL/day) | 72 ± 36 | 51 ± 40 | 67 ± 50 | 64 ± 56 | 64 ± 46 |
| DASS-21 | |||||
| Depression | 12.8 ± 9.0 | 15.0 ± 10.3 | 12.2 ± 8.5 | 16.6 ± 9.6 | 14.2 ± 9.3 |
| Anxiety | 9.3 ± 7.7 | 10.2 ± 6.0 | 8.8 ± 7.9 | 12.0 ± 7.3 | 10.1 ± 7.2 |
| Stress | 15.2 ± 11.5 | 15.2 ± 10.3 | 15.2 ± 10.0 | 16.9 ± 11.9 | 15.6 ± 10.7 |
| TMT (seconds) | |||||
| TMT A | 49.9 ± 22.7 | 47.6 ± 15.6 | 47.4 ± 15.8 | 51.6 ± 17.7 | 49.2 ± 17.7 |
| TMT B | 119.3 ± 79.7 | 100.2 ± 66.8 | 123.4 ± 53.5 | 145.3 ± 69.3 | 122.5 ± 67.8 |
| Inhibitory Control Test (ICT) | |||||
| Correct target response (n) | 186.1 ±31.3 | 191.3 ± 21.1 | 196.2 ±17.4 | 184.4 ± 33.6 | 189.4 ± 26.4 |
| Target accuracy * | 0.88 ± 0.15 | 0.90 ± 0.10 | 0.93 ± 0.08 | 0.87 ± 0.16 | 0.89 ± 0.12 |
| Incorrect lure response (n) | 14.9 ± 10.9 | 13.9 ± 11.8 | 14.8 ± 12.1 | 14.0 ± 10.6 | 14.4 ± 11.0 |
| Weighted lure response # | 24.6 ± 26.4 | 18.8 ± 18.2 | 18.3 ± 16.1 | 20.7 ± 15.8 | 20.6 ± 18.4 |
| Intention to Treat Analysis (ITT) | TMT-A (Seconds) | TMT-B (Seconds) |
|---|---|---|
| Placebo—baseline | 49.93 ± 5.03 | 119.34 ± 17.97 |
| Placebo—4 weeks | 41.45 ± 5.03 | 107.82 ± 17.97 |
| Placebo—8 weeks | 41.29 ± 5.03 | 111.80 ± 17.97 |
| Synbiotics—baseline | 47.60 ±5.03 | 100.20 ± 17.97 |
| Synbiotics—4 weeks | 56.02 ± 5.17 | 123.44 ± 18.24 |
| Synbiotics—8 weeks | 49.53 ± 5.17 | 121.45 ± 18.24 |
| BCAA—baseline | 47.42 ± 5.03 | 123.44 ± 17.97 |
| BCAA—4 weeks | 43.30 ± 5.17 | 107.40 ± 18.24 |
| BCCA—8 weeks | 37.41 ± 5.33 | 110.08 ± 18.56 |
| Synbiotics + BCAA—baseline | 51.57 ± 4.83 | 145.3 ± 17.26 |
| Synbiotics + BCAA—4 weeks | 41.09 ± 4.83 | 104.72 ± 17.26 |
| Synbiotics + BCAA—8 weeks | 36.91 ± 4.83 | 94.56 ± 17.26 * |
| Per protocol analysis (PP) | ||
| Placebo—baseline | 45.46 ± 5.21 | 102.92 ± 17.65 |
| Placebo—4 weeks | 41.45 ± 5.03 | 107.82 ± 17.97 |
| Placebo—8 weeks | 41.29 ± 5.03 | 111.80 ± 17.97 |
| Synbiotics—baseline | 47.60 ±5.03 | 100.20 ± 17.97 |
| Synbiotics—4 weeks | 56.02 ± 5.17 | 123.44 ± 18.24 |
| Synbiotics—8 weeks | 49.53 ± 5.17 | 121.45 ± 18.24 |
| BCAA—baseline | 47.42 ± 5.03 | 123.44 ± 17.97 |
| BCAA—4 weeks | 43.30 ± 5.17 | 107.40 ± 18.24 |
| BCCA—8 weeks | 37.41 ± 5.33 | 110.08 ± 18.56 |
| Synbiotics + BCAA—baseline | 51.57 ± 4.83 | 145.3 ± 17.26 |
| Synbiotics + BCAA—4 weeks | 41.09 ± 4.83 | 104.72 ± 17.26 |
| Synbiotics + BCAA—8 weeks | 36.91 ± 4.83 | 94.56 ± 17.26 # |
| Intention to Treat Analysis (ITT) | Correct Target Responses | Target Accuracy | Incorrect Lure Responses | Weighted Lures |
|---|---|---|---|---|
| Placebo—Baseline | 186.08 ± 7.33 | 0.88 ± 0.04 | 14.92 ± 3.00 | 24.55 ± 4.33 |
| Placebo—4 weeks | 201.50 ± 7.33 | 0.95 ± 0.04 | 10.67 ± 3.00 | 12.47 ± 4.33 |
| Placebo—8 weeks | 197.08 ± 7.33 | 0.93 ± 0.04 | 9.42 ± 3.00 | 12.71 ± 4.33 |
| Synbiotics—baseline | 191.33 ± 7.33 | 0.90 ± 0.04 | 13.92 ± 3.00 | 18.81 ± 4.33 |
| Synbiotics—4 weeks | 184.27 ± 7.89 | 0.79 ± 0.04 | 11.12 ± 3.05 | 16.53 ± 4.45 * |
| Synbiotics—8 weeks | 197.66 ± 7.59 | 0.93 ± 0.04 | 12.36 ± 3.05 | 15.27 ± 4.39 ** |
| BCAA—baseline | 197.17 ± 7.33 | 0.93 ± 0.04 | 14.75 ± 3.00 | 18.31 ± 4.33 |
| BCAA—4 weeks | 199.34 ± 7.59 | 0.94 ± 0.04 | 13.42 ± 3.05 | 15.05 ±4.39 * |
| BCCA—8 weeks | 198.04 ± 7.89 | 0.93 ± 0.04 | 14.23 ± 3.11 | 19.97 ± 4.45 ** |
| Synbiotics + BCAA—baseline | 184.38 ± 7.05 | 0.87 ± 0.04 | 14.00 ± 2.88 | 20.70 ± 4.16 |
| Synbiotics + BCAA—4 weeks | 183.85 ± 7.05 | 0.87 ± 0.04 | 10.77 ± 2.88 | 15.80 ± 4.16 ** |
| Per protocol analysis (PP) | ||||
| Placebo—baseline | 191.64 ± 7.70 | 0.90 ±0.04 | 13.82 ± 3.10 | 19.72 ± 4.24 |
| Placebo—4 weeks | 203.85 ± 8.03 | 0.96 ± 0.04 | 8.46 ± 3.16 | 8.93 ± 4.30 ## |
| Placebo—8 weeks | 202.20 ± 8.41 | 0.95 ± 0.05 | 7.21 ± 3.23 | 9.50 ± 4.37 ## |
| Synbiotics—baseline | 189.80 ± 8.07 | 0.90 ± 0.04 | 14.80 ± 3.25 | 20.47 ± 4.45 |
| Synbiotics—4 weeks | 179.01 ± 9.48 | 0.74 ± 0.05 | 10.60 ± 3.41 | 16.24 ± 4.73 |
| Synbiotics—8 weeks | 202.14 ± 8.93 | 0.94 ± 0.05 | 10.90 ± 3.41 | 13.38 ± 4.62 # |
| BCAA—baseline | 191.67 ± 8.51 | 0.90 ± 0.05 | 16.67 ± 3.43 | 21.35 ± 4.69 |
| BCAA—4 weeks | 202.03 ± 9.52 | 0.95 ± 0.05 | 15.90 ± 3.61 | 17.82 ± 4.88 |
| BCCA—8 weeks | 192.56 ± 9.52 | 0.91 ± 0.05 | 15.92 ± 3.61 | 19.50 ± 4.88 # |
| Synbiotics + BCAA—baseline | 184.38 ± 7.08 | 0.87 ± 0.04 | 14.00 ± 2.85 | 20.70 ± 3.90 |
| Synbiotics + BCAA—4 weeks | 182.10 ± 7.96 | 0.86 ± 0.04 | 12.39 ± 3.01 | 18.32 ± 4.06 |
| Synbiotics + BCAA—8 weeks | 199.16 ± 7.33 | 0.94 ± 0.04 | 8.21 ± 2.90 * | 9.38 ± 3.95 # |
| Intention to Treat Analysis (ITT) | Depression | Anxiety | Stress |
|---|---|---|---|
| Placebo—Baseline | 12.83 ± 2.57 | 9.33 ± 1.99 | 15.17 ± 3.03 |
| Placebo—4 weeks | 12.30 ± 2.63 | 10.06 ± 2.03 | 11.09 ± 3.07 |
| Placebo—8 weeks | 10.17 ± 2.57 | 10.50 ± 1.99 | 13.67 ± 3.03 |
| Synbiotics—baseline | 15.00 ± 2.57 | 10.17 ± 1.99 | 15.00 ± 3.03 |
| Synbiotics—4 weeks | 12.69 ± 2.63 | 10.24 ± 2.03 | 12.22 ± 3.07 |
| Synbiotics—8 weeks | 12.57 ± 2.70 | 11.65 ± 2.06 | 18.17 ± 3.12 |
| BCAA—baseline | 12.17 ± 2.57 | 8.83 ± 1.99 | 15.17 ± 3.03 |
| BCAA—4 weeks | 13.49 ± 2.63 | 9.95 ± 2.03 | 15.96 ± 3.07 |
| BCCA—8 weeks | 12.19 ± 2.70 | 9.19 ± 2.06 | 14.12 ± 3.12 |
| Synbiotics + BCAA—baseline | 16.62 ± 2.47 | 12.00 ± 1.92 | 16.92 ± 2.91 |
| Synbiotics + BCAA—4 weeks | 14.77 ± 2.47 | 10.31 ± 1.92 | 14.15 ± 2.90 |
| Synbiotics + BCAA—8 weeks | 13.38 ± 2.47 | 9.54 ± 1.92 | 14.15 ± 2.90 |
| Per Protocol analysis (PP) | |||
| Placebo—Baseline | 12.91 ± 2.70 | 9.64 ±2.07 | 16.55 ± 3.22 |
| Placebo—4 weeks | 12.99 ± 2.77 | 10.46 ±2.11 | 12.04 ± 3.26 |
| Placebo—8 weeks | 12.15 ± 2.86 | 11.28 ± 2.15 | 16.14 ± 3.32 |
| Synbiotics—baseline | 16.40 ± 2.83 | 11.00 ± 2.17 | 16.40 ± 3.37 |
| Synbiotics—4 weeks | 12.64 ± 3.02 | 10.61 ± 2.26 | 11.72 ± 3.50 |
| Synbiotics—8 weeks | 15.01 ± 3.14 | 11.77 ± 2.32 | 21.31 ± 3.58 |
| BCAA—baseline | 14.44 ± 2.99 | 8.44 ± 2.29 | 17.11 ± 3.56 |
| BCAA—4 weeks | 15.26 ± 3.21 | 8.06 ± 2.40 | 19.89 ± 3.70 |
| BCCA—8 weeks | 12.06 ± 3.21 | 7.81 ± 2.40 | 16.03 ± 3.70 |
| Synbiotics + BCAA—baseline | 16.62 ± 2.49 | 12.00 ± 1.91 | 16.92 ± 2.96 |
| Synbiotics + BCAA—4 weeks | 13.97 ± 2.67 | 8.95 ± 2.00 | 14.23 ± 3.08 |
| Synbiotics + BCAA—8 weeks | 12.79 ± 2.54 | 9.44 ± 1.93 * | 14.20 ± 2.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidot, H.; Cvejic, E.; Finegan, L.J.; Shores, E.A.; Bowen, D.G.; Strasser, S.I.; McCaughan, G.W.; Carey, S.; Allman-Farinelli, M.; Shackel, N.A. Supplementation with Synbiotics and/or Branched Chain Amino Acids in Hepatic Encephalopathy: A Pilot Randomised Placebo-Controlled Clinical Study. Nutrients 2019, 11, 1810. https://doi.org/10.3390/nu11081810
Vidot H, Cvejic E, Finegan LJ, Shores EA, Bowen DG, Strasser SI, McCaughan GW, Carey S, Allman-Farinelli M, Shackel NA. Supplementation with Synbiotics and/or Branched Chain Amino Acids in Hepatic Encephalopathy: A Pilot Randomised Placebo-Controlled Clinical Study. Nutrients. 2019; 11(8):1810. https://doi.org/10.3390/nu11081810
Chicago/Turabian StyleVidot, Helen, Erin Cvejic, Liam J. Finegan, E. Arthur Shores, David G. Bowen, Simone I. Strasser, Geoffrey W. McCaughan, Sharon Carey, Margaret Allman-Farinelli, and Nicholas A. Shackel. 2019. "Supplementation with Synbiotics and/or Branched Chain Amino Acids in Hepatic Encephalopathy: A Pilot Randomised Placebo-Controlled Clinical Study" Nutrients 11, no. 8: 1810. https://doi.org/10.3390/nu11081810
APA StyleVidot, H., Cvejic, E., Finegan, L. J., Shores, E. A., Bowen, D. G., Strasser, S. I., McCaughan, G. W., Carey, S., Allman-Farinelli, M., & Shackel, N. A. (2019). Supplementation with Synbiotics and/or Branched Chain Amino Acids in Hepatic Encephalopathy: A Pilot Randomised Placebo-Controlled Clinical Study. Nutrients, 11(8), 1810. https://doi.org/10.3390/nu11081810






