Remission Effects of Dietary Soybean Isoflavones on DSS-Induced Murine Colitis and an LPS-Activated Macrophage Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and DSS-Induced Colitis Model
2.3. TUNEL Assay
2.4. Histology
2.5. Histological Score
2.6. Quantitative Real-Time PCR
2.7. Cell Culture
2.8. WST-1 Cell Viability Assay
2.9. Measurement of NO
2.10. Measurement of PGE2
2.11. Immunoblotting Assay
2.12. Luciferase activity ASSAY
2.13. Confocal Microscopy
2.14. Statistics
3. Results
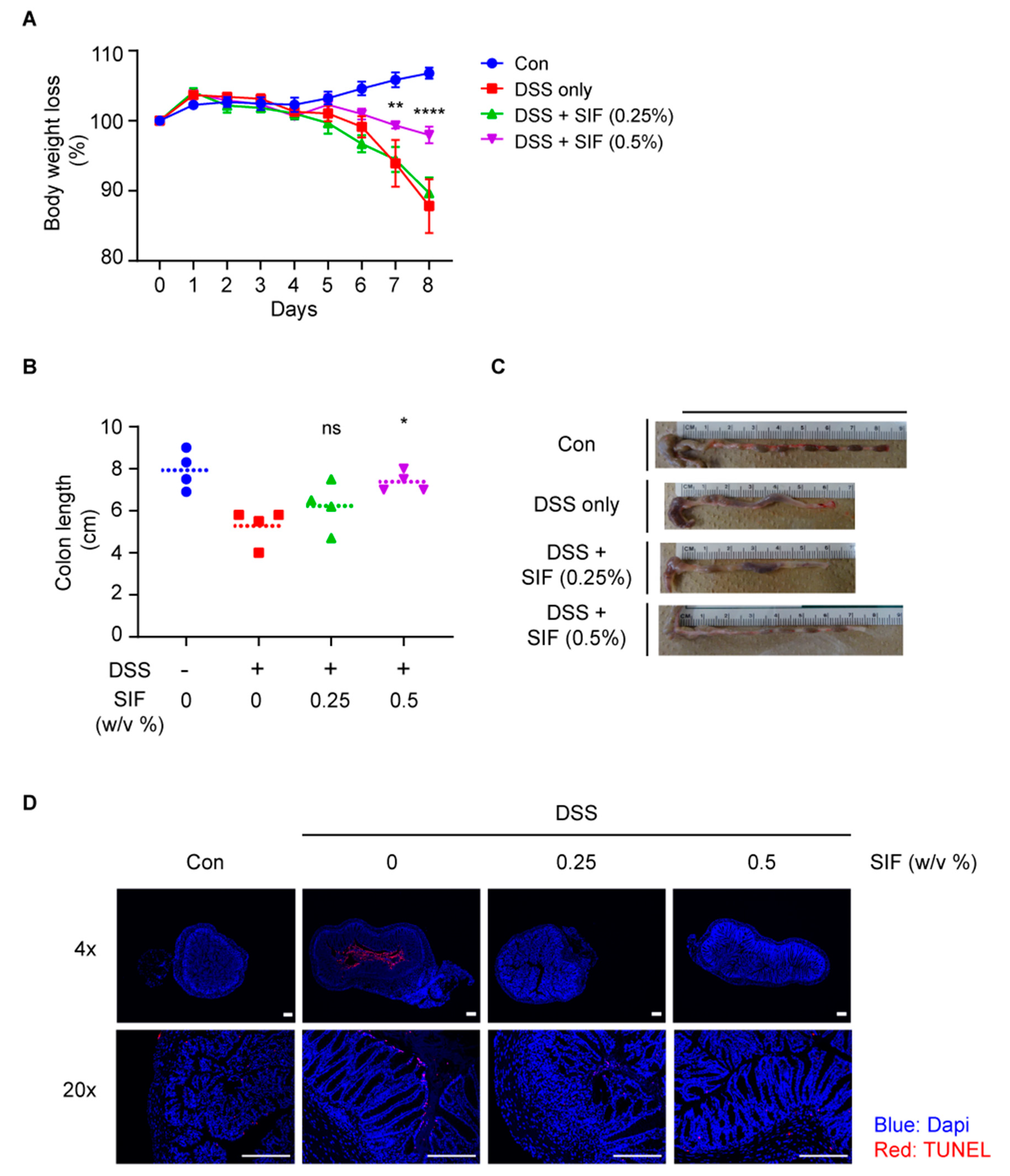
3.1. SIF Alleviates Symptoms of DSS-Induced Colitis in C57BL/6N Mice
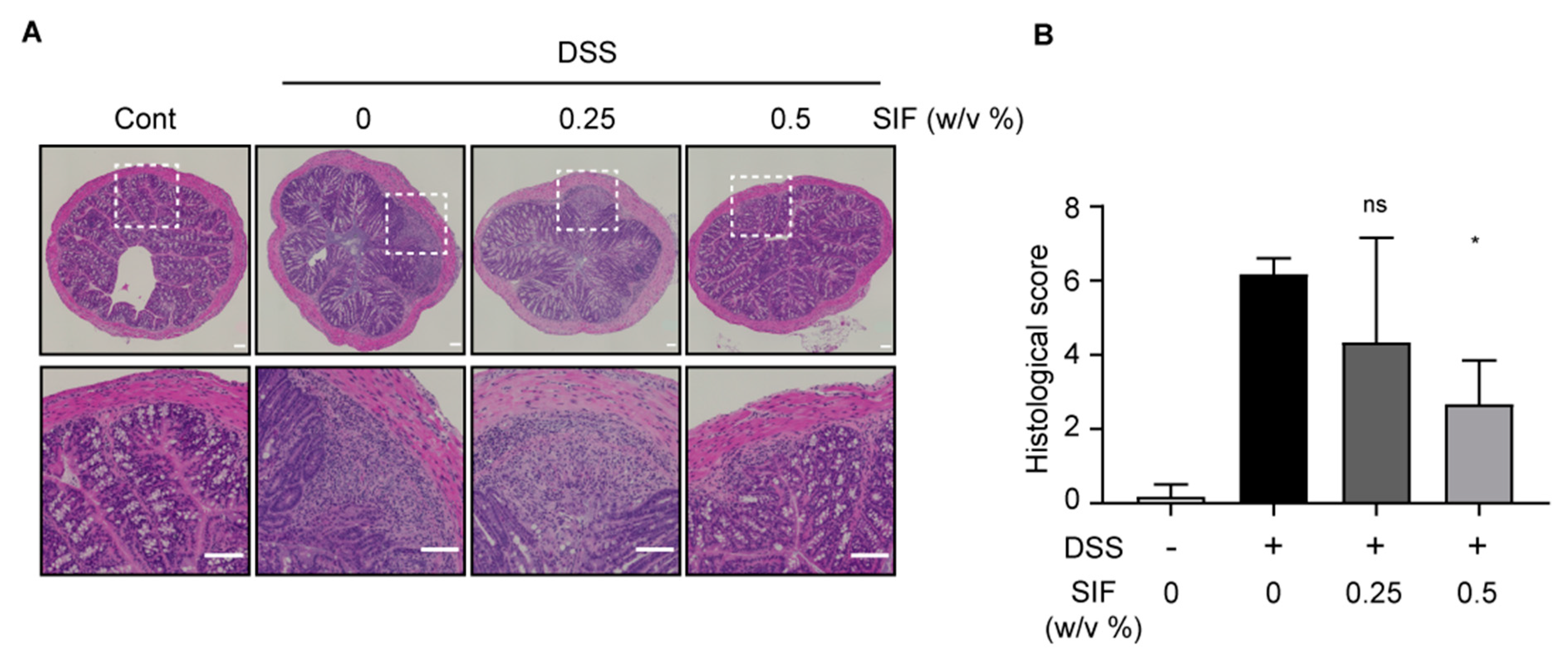
3.2. SIF Mitigates the Histopathology of DSS-Induced Colitis
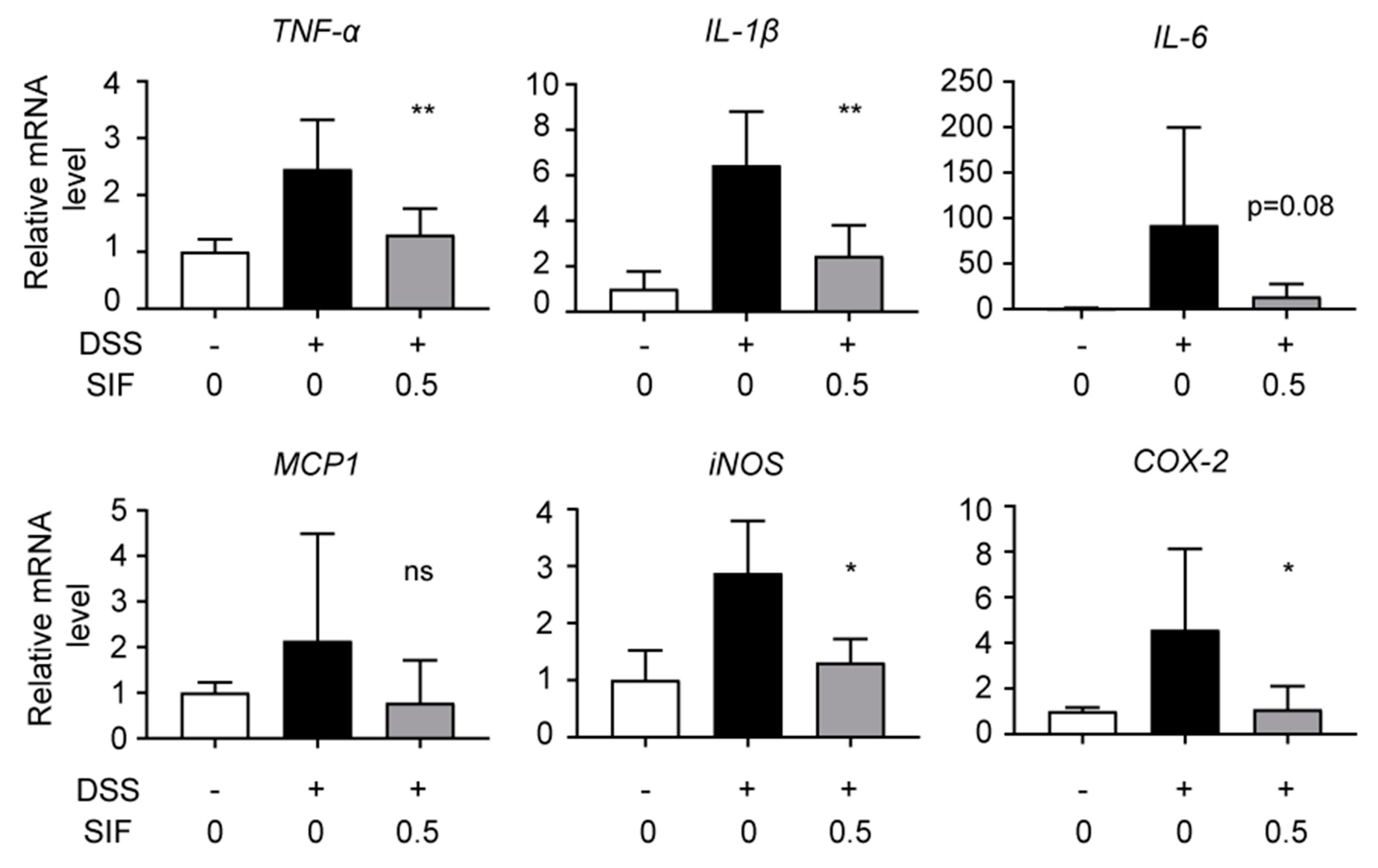
3.3. SIF Suppresses mRNA Expression of Pro-Inflammatory Cytokines and Inflammatory Enzymes in DSS-Induced Colitis Tissue
3.4. SIF Decreases Infiltration of F4/80-Positive Immune Cells in DSS-Induced Colitis Mice
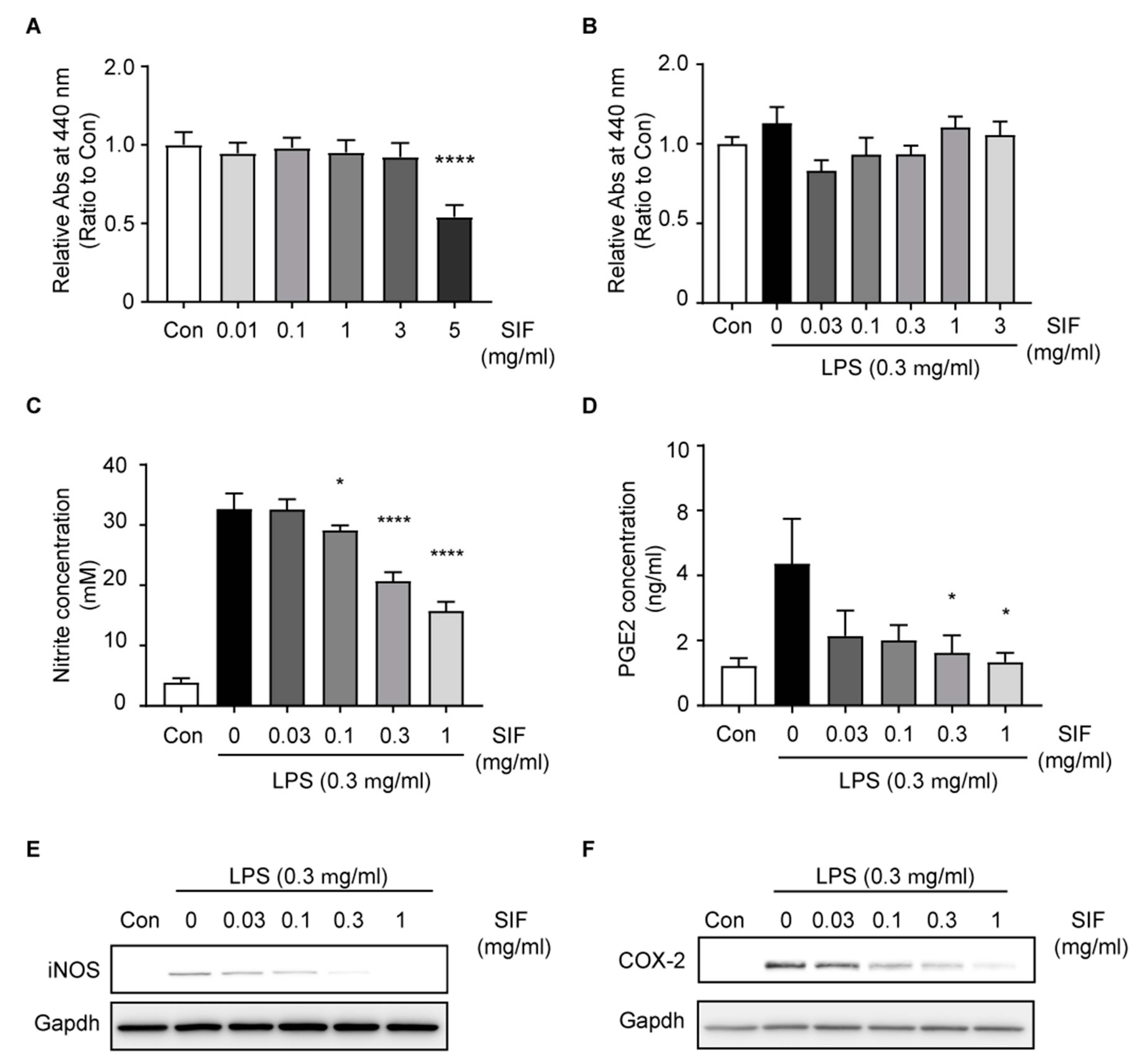
3.5. SIF Inhibits NO and PGE2 Production Via iNOS and COX-2 Expression in LPS-Activated RAW264.7 Macrophages
3.6. Effects of SIF on Pro-Inflammatory Cytokines and Chemokines in LPS-Activated RAW264.7 Macrophages
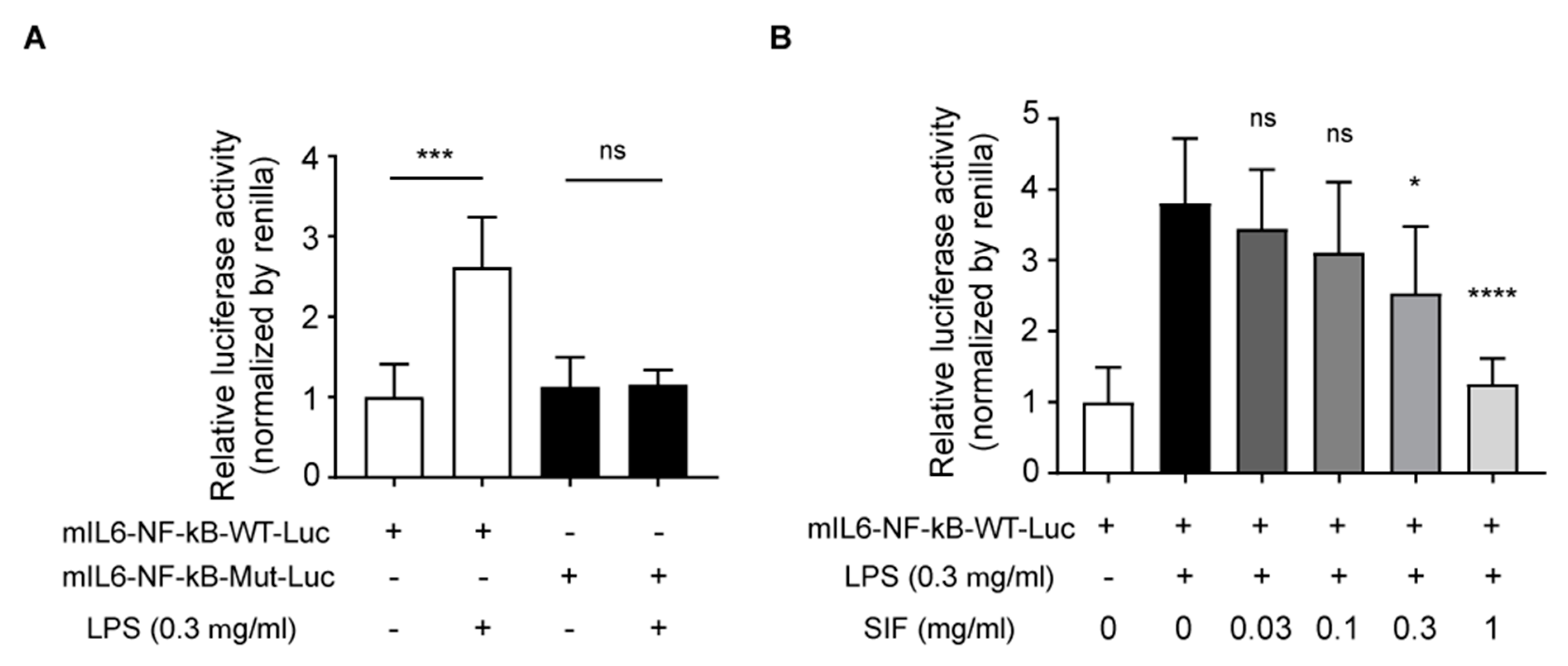
3.7. Effects of SIF on NF-κB Transactivation and LPS-Induced IL-6 Reporter Luciferase Activity
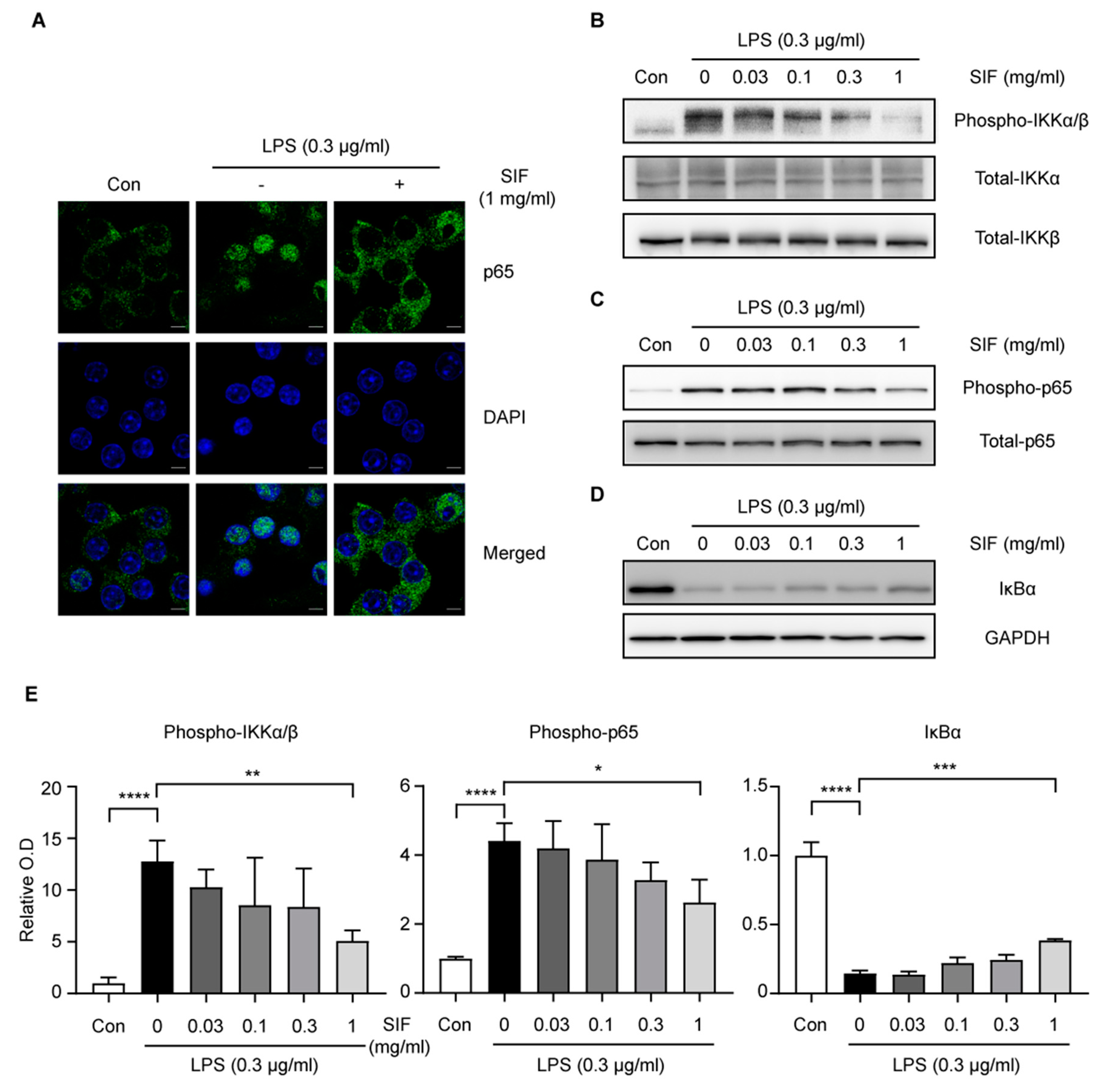
3.8. Effects of SIF Treatment on LPS-Induced NF-κB Nuclear Translocation Via the IKK/IκB Signaling Pathway in RAW264.7 Macrophages
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- References Drewes, A.M.; Frøkjær, J.B.; Larsen, E.; Reddy, H.; Arendt-Nielsen, L.; Gregersen, H. Pain and mechanical properties of the rectum in patients with active ulcerative colitis. Inflamm. Bowel Dis. 2006, 12, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.Q.; Targan, S.R. Immunopathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Söderholm, J.D.; Streutker, C.; Yang, P.C.; Paterson, C.; Singh, P.K.; McKay, D.M.; Sherman, P.M.; Croitoru, K.; Perdue, M.H. Increased epithelial uptake of protein antigens in the ileum of Crohn’s disease mediated by tumour necrosis factor α. Gut 2004, 53, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Auyeung, K.K. Inflammatory bowel disease: Etiology, pathogenesis and current therapy. Curr. Pharm. Des. 2014, 20, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel disease (IBD). Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- M’Koma, A.E. Inflammatory bowel disease: An expanding global health problem. Clin. Med. Insights Gastroenterol. 2013, 6, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef]
- Conklin, C.M.J.; Bechberger, J.F.; MacFabe, D.; Guthrie, N.; Kurowska, E.M.; Naus, C.C. Genistein and quercetin increase connexin43 and suppress growth of breast cancer cells. Carcinogenesis 2007, 28, 93–100. [Google Scholar] [CrossRef]
- Albert Dhayakaran, R.P.; Neethirajan, S.; Xue, J.; Shi, J. Characterization of antimicrobial efficacy of soy isoflavones against pathogenic biofilms. LWT Food Sci. Technol. 2015, 63, 859–865. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Chacko, B.K.; Chandler, R.T.; D’Alessandro, T.L.; Mundhekar, A.; Khoo, N.K.H.; Botting, N.; Barnes, S.; Patel, R.P. Anti-Inflammatory Effects of Isoflavones are Dependent on Flow and Human Endothelial Cell PPARγ. J. Nutr. 2018, 137, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Verdrengh, M.; Jonsson, I.M.; Holmdahl, R.; Tarkowski, A. Genistein as an anti-inflammatory agent. Inflamm. Res. 2003, 52, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Sheu, F.; Lai, H.H.; Yen, G.C. Suppression effect of soy isoflavones on nitric oxide production in RAW 264.7 Macrophages. J. Agric. Food Chem. 2001, 49, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Yerramsetty, V.; Mathias, K.; Bunzel, M.; Ismail, B. Detection and Structural Characterization of Thermally Generated Isoflavone Malonylglucoside Derivatives. J. Agric. Food Chem. 2011, 59, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of Pure Isoflavones in Healthy Humans and Analysis of Commercial Soy Isoflavone Supplements. J. Nutr. 2018, 131, 1362S–1375S. [Google Scholar] [CrossRef] [PubMed]
- Zubik, L.; Meydani, M. Bioavailability of soybean isoflavones from aglycone and glucoside. Am. J. Clin. Nutr. 2003, 77, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Yamauchi, A.; Tominaga, K.; Koga, A.; Kai, H.; Kataoka, Y. Soybean Isoflavones Eliminate Nifedipine-Induced Flushing of Tail Skin in Ovariectomized Mice. J. Pharmacol. Sci. 2004, 95, 476–478. [Google Scholar] [CrossRef]
- Nagano, T.; Wu, W.; Tsumura, K.; Yonemoto-Yano, H.; Kamada, T.; Haruma, K. The inhibitory effect of soybean and soybean isoflavone diets on 2,4-dinitrofluorobenzene-induced contact hypersensitivity in mice. Biosci. Biotechnol. Biochem. 2016, 80, 991–997. [Google Scholar] [CrossRef]
- Kurata, M.; Murata, Y.; Momma, K.; Fouad Ali Mursi, I.; Takahashi, M.; Miyamae, Y.; Kambe, T.; Nagao, M.; Narita, H.; Shibuya, Y.; et al. The isoflavone fraction from soybean presents mRNA maturation inhibition activity. Biosci. Biotechnol. Biochem. 2017, 81, 551–554. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104. [Google Scholar] [CrossRef]
- Perše, M.; Cerar, A. Dextran sodium sulphate colitis mouse model: Traps and tricks. J. Biomed. Biotechnol. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Rose, W.A.; Sakamoto, K.; Leifer, C.A. Multifunctional role of dextran sulfate sodium for in vivo modeling of intestinal diseases. BMC Immunol. 2012, 13, 41. [Google Scholar] [CrossRef]
- Schölmerich, J. Inflammatory bowel disease. Endoscopy 1996, 28, 77–82. [Google Scholar] [CrossRef]
- Goyal, N.; Rana, A.; Ahlawat, A.; Bijjem, K.R.V.; Kumar, P. Animal models of inflammatory bowel disease: A review. Inflammopharmacology 2014, 22, 219–233. [Google Scholar] [CrossRef]
- Barton, G.M.; Barton, G.M. A calculated response: Control of inflammation by the innate immune system Find the latest version: Review series A calculated response: Control of inflammation by the innate immune system. Am. Soc. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef]
- Blay, M.; Espinel, A.E.; Delgado, M.A.; Baiges, I.; Bladé, C.; Arola, L.; Salvadó, J. Isoflavone effect on gene expression profile and biomarkers of inflammation. J. Pharm. Biomed. Anal. 2010, 51, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.W. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J. Exp. Med. 2004, 177, 1779–1784. [Google Scholar] [CrossRef]
- Valledor, A.F.; Comalada, M.; Santamaría-Babi, L.F.; Lloberas, J.; Celada, A. Macrophage Proinflammatory Activation and Deactivation. Adv. Immunol. 2010, 108, 1–20. [Google Scholar]
- Lenon, G.B.; Li, C.G.; Xue, C.C.; Thien, F.C.K.; Story, D.F. Inhibition of inducible nitric oxide production and iNOS protein expression in lipopolysaccharide-stimulated rat aorta and Raw 264.7 macrophages by ethanol extract of a Chinese herbal medicine formula (RCM-101) for allergic rhinitis. J. Ethnopharmacol. 2008, 116, 547–553. [Google Scholar] [CrossRef]
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-B.; Han, A.-R.; Park, E.-Y.; Kim, J.-Y.; Cho, W.; Lee, J.; Seo, E.-K.; Lee, K.-T. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264.7 macrophage cells. Biol. Pharm. Bull. 2007, 30, 2345–2351. [Google Scholar] [CrossRef]
- DiDonato, J.A.; Mercurio, F.; Karin, M. Phosphorylation of I kappa B alpha precedes but is not sufficient for its dissociation from NF-kappa B. Mol. Cell. Biol. 1995, 15, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Tsugawa, T.; Kawagishi, H.; Asai, A.; Sugimoto, M. Loss of HuR leads to senescence-like cytokine induction in rodent fibroblasts by activating NF-κB. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 3079–3087. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Xie, Q.W.; Kashiwabara, Y.; Nathan, C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994, 269, 4705–4708. [Google Scholar]
- Israël, A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a000158. [Google Scholar] [CrossRef]
- Targan, S.R. Current Limitations of IBD Treatment: Where Do We Go from Here? Ann. N. Y. Acad. Sci. 2006, 1072, 1–8. [Google Scholar] [CrossRef]
- Mi, J.C.; Kang, A.Y.; Kyung, M.L.; Oh, E.; Jun, H.J.; Kim, S.Y.; Joong, H.A.; Moon, T.W.; Lee, S.J.; Park, K.H. Water-soluble genistin glycoside isoflavones up-regulate antioxidant metallothionein expression and scavenge free radicals. J. Agric. Food Chem. 2006, 54, 3819–3826. [Google Scholar]
- Roland, W.S.U.; Vincken, J.P.; Gouka, R.J.; Van Buren, L.; Gruppen, H.; Smit, G. Soy isoflavones and other isoflavonoids activate the human bitter taste receptors hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 2011, 59, 11764–11771. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.T.; Huang, Y.L.; Huang, Y.J.; Chiou, W.F. Miyabenol A inhibits LPS-induced NO production via IKK/IκB inactivation in RAW 264.7 macrophages: Possible involvement of the p38 and PI3K pathways. J. Agric. Food Chem. 2008, 56, 8911–8918. [Google Scholar] [CrossRef] [PubMed]


| Isoflavone Form | Composition 2 (w/w%) |
|---|---|
| Daidzin | 22.58 |
| Glycitin | 8.08 |
| Genistin | 1.19 |
| Malonyl daidzin | 37.96 |
| Malonyl glycitin | 20.46 |
| Malonyl genistin | 8.08 |
| Acetyl daidzin | 0.46 |
| Acetyl glycitin | 1.12 |
| Acetyl genistin | 0.08 |
| Daidzein | ND 3 |
| Glycitein | ND |
| Genistein | ND |
| Total | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-E.; Kawaguchi, K.; Hayashi, H.; Furusho, K.; Maruyama, M. Remission Effects of Dietary Soybean Isoflavones on DSS-Induced Murine Colitis and an LPS-Activated Macrophage Cell Line. Nutrients 2019, 11, 1746. https://doi.org/10.3390/nu11081746
Kim S-E, Kawaguchi K, Hayashi H, Furusho K, Maruyama M. Remission Effects of Dietary Soybean Isoflavones on DSS-Induced Murine Colitis and an LPS-Activated Macrophage Cell Line. Nutrients. 2019; 11(8):1746. https://doi.org/10.3390/nu11081746
Chicago/Turabian StyleKim, Sang-Eun, Koichiro Kawaguchi, Hiroko Hayashi, Katsuhiro Furusho, and Mitsuo Maruyama. 2019. "Remission Effects of Dietary Soybean Isoflavones on DSS-Induced Murine Colitis and an LPS-Activated Macrophage Cell Line" Nutrients 11, no. 8: 1746. https://doi.org/10.3390/nu11081746
APA StyleKim, S.-E., Kawaguchi, K., Hayashi, H., Furusho, K., & Maruyama, M. (2019). Remission Effects of Dietary Soybean Isoflavones on DSS-Induced Murine Colitis and an LPS-Activated Macrophage Cell Line. Nutrients, 11(8), 1746. https://doi.org/10.3390/nu11081746




