Effects of Vitamin D3 in Long-Term Ovariectomized Rats Subjected to Chronic Unpredictable Mild Stress: BDNF, NT-3, and NT-4 Implications
Abstract
1. Introduction
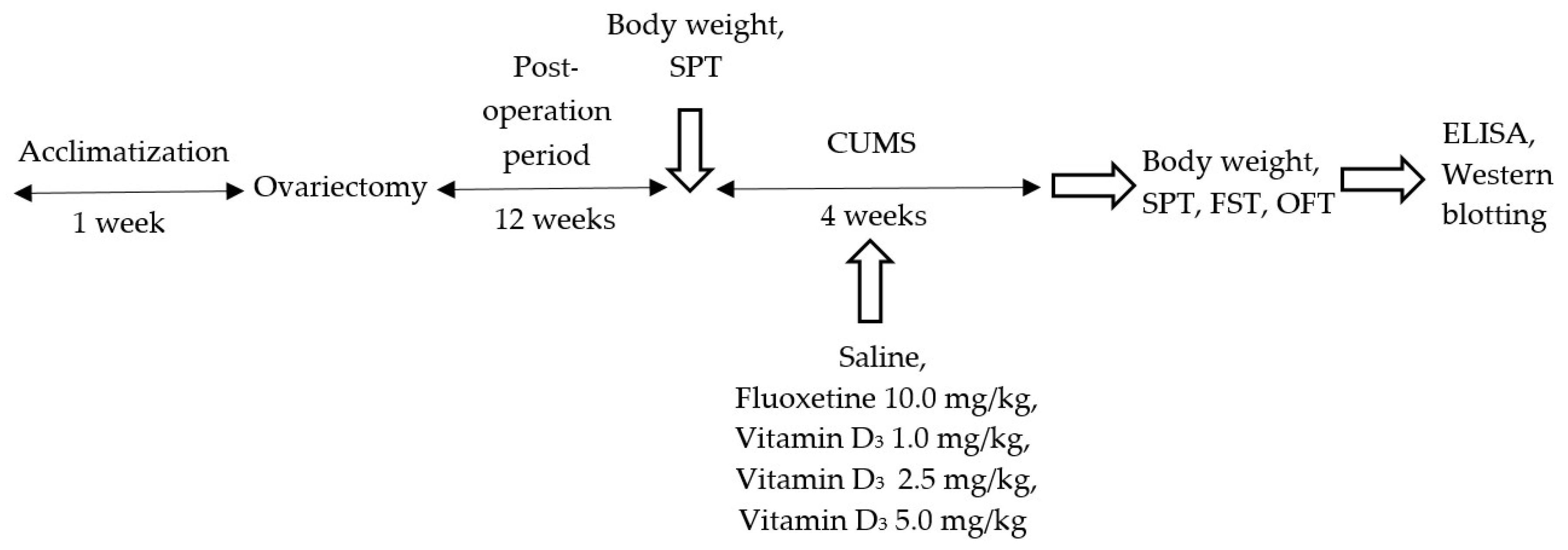
2. Experimental Section
2.1. Animals
2.2. Ovariectomy
2.3. CUMS Model
2.4. Drugs
2.5. Groups of Animals
2.6. Sucrose Preference Test
2.7. Forced Swimming Test
2.8. Open Field Test
2.9. ELISA Measurements
2.10. Western Blotting Analysis
2.11. Statistical Analysis
3. Results
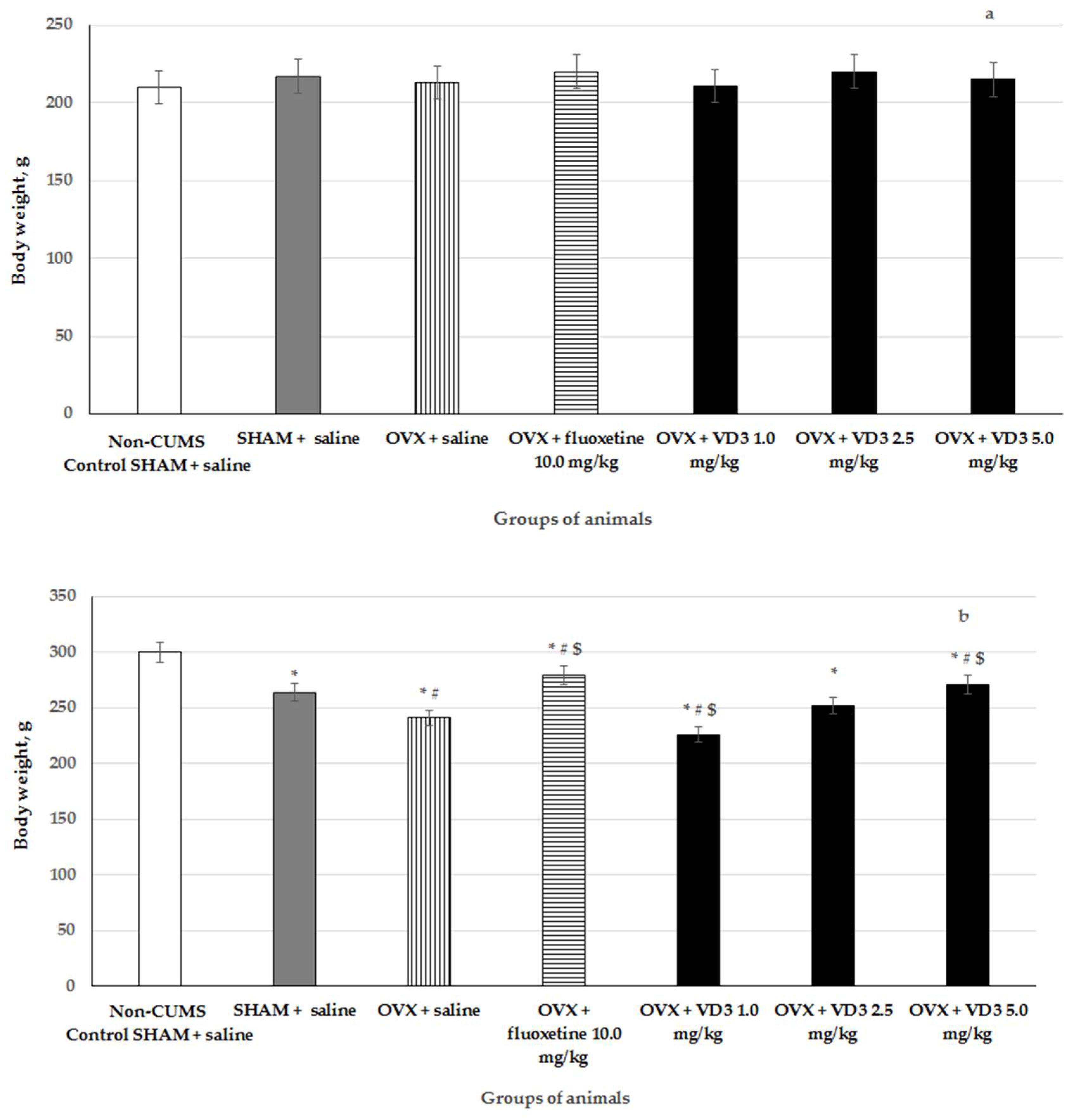
3.1. The Effects of VD3 Administered in Various Doses on the Body Weight in the Long-Term OVX Rats Subjected to CUMS
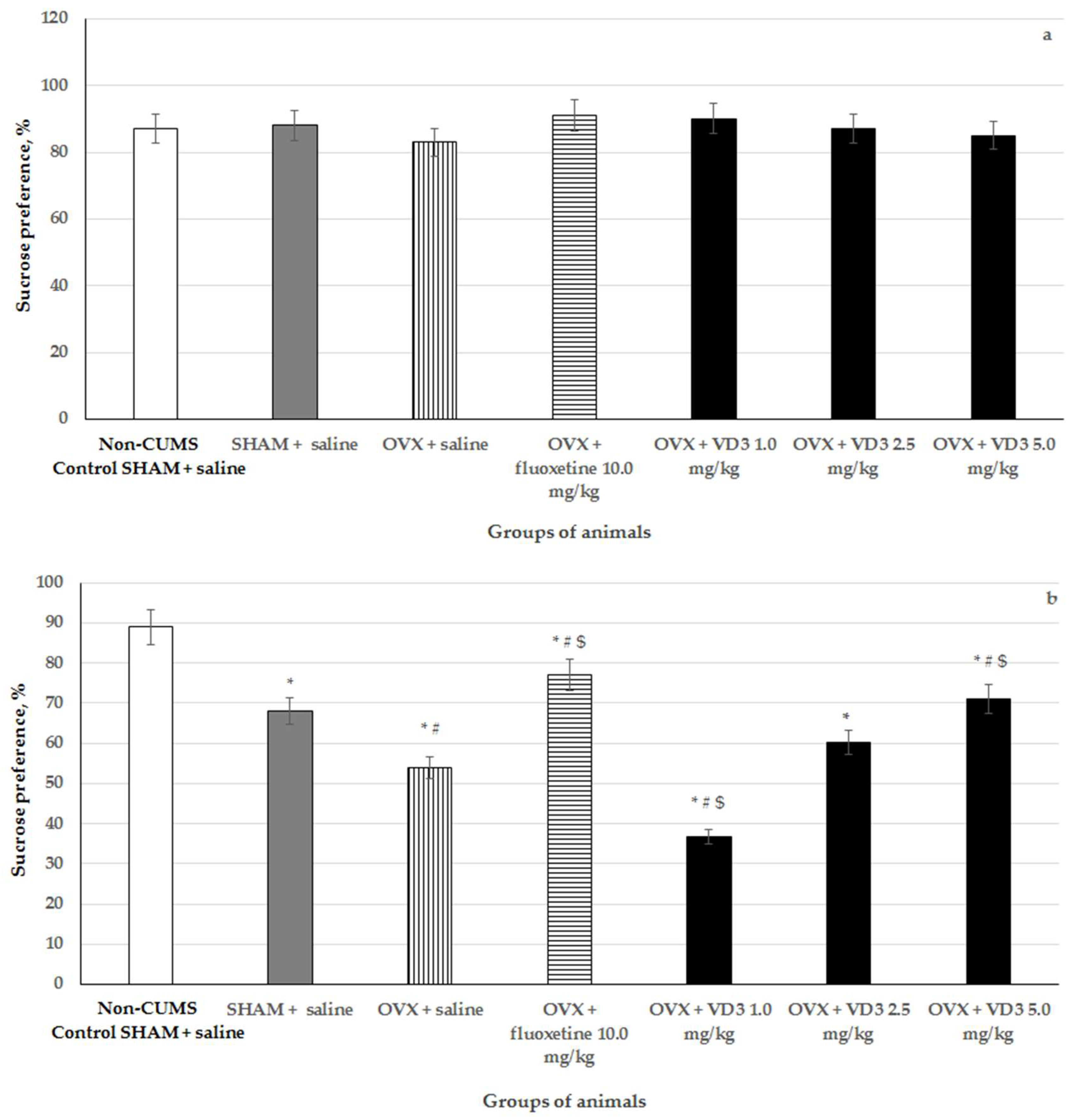
3.2. Effects of VD3 Administered in Various Doses on Sucrose Preference in the Long-Term OVX Rats Subjected to CUMS
3.3. Effects of VD3 Administered in Various Doses on Depression-Like Behavior in the Forced Swimming Test of Long-Term OVX Rats Subjected to CUMS
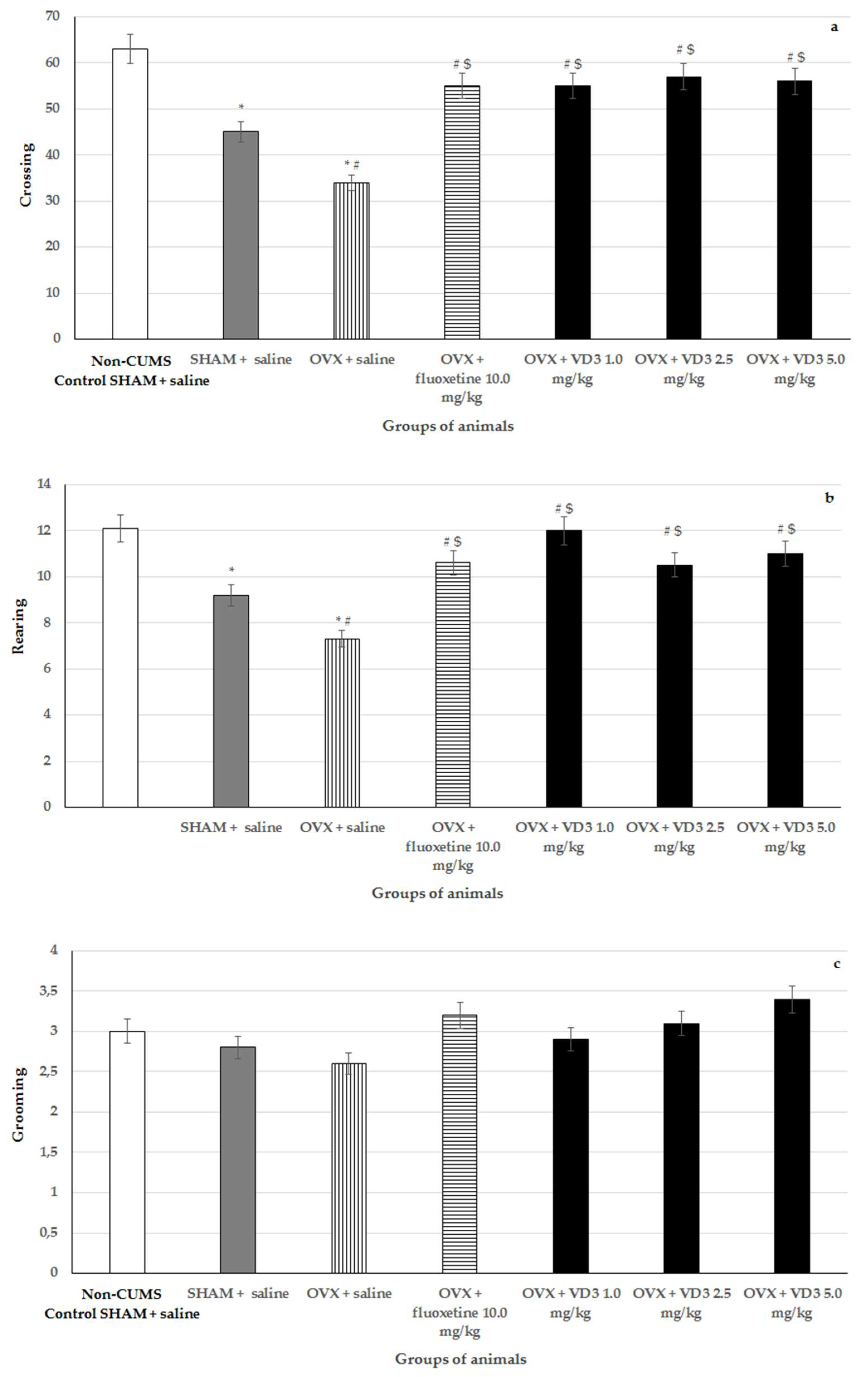
3.4. Effects of VD3 Administered in Various Doses on Behavior in the open Field Test of Long-Term OVX Rats Subjected to CUMS
3.5. Effects of VD3 Administered in Various Doses on Serum Corticosterone, ACTH, VD3 and Estradiol Levels in Long-Term OVX Rats Subjected to CUMS
3.6. Effects of VD3 Administered in Various Doses on Hippocampal BDNF, NT-3, NT-4 Levels in Long-Term OVX Rats Subjected to CUMS
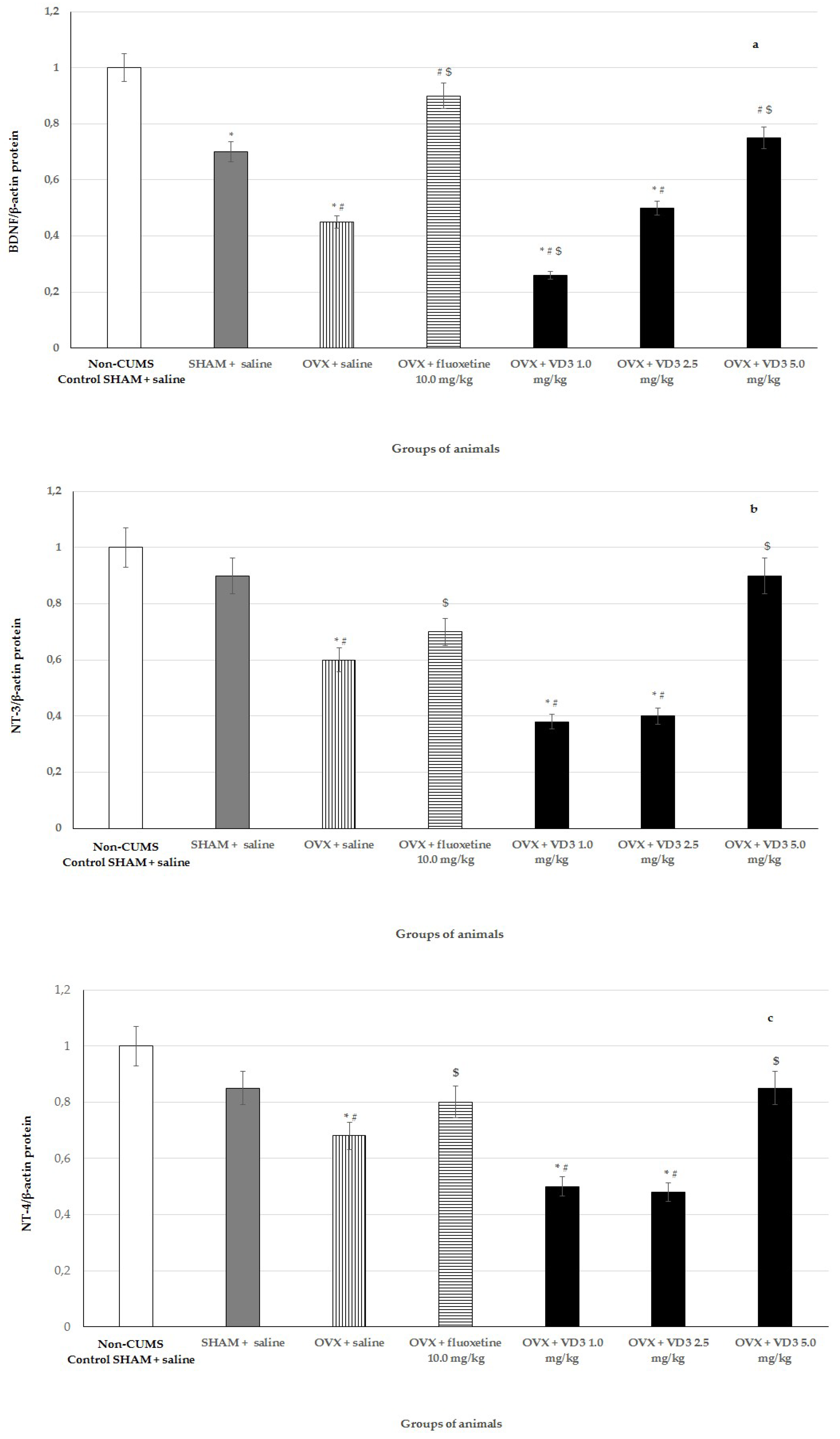
3.7. Effects of VD3 Administered in Various Doses on Protein Expressions of BDNF, NT-3, and NT-4 in Long-Term OVX Rats Subjected to CUMS
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garcia-Portilla, M.P. Depression and perimenopause: A review. Actas. Esp. Psiquiatr. 2009, 37, 213–221. [Google Scholar] [PubMed]
- Frey, B.N.; Lord, C.; Soares, C.N. Depression during menopausal transition: A review of treatment strategies and pathophysiological correlates. Menopause Int. 2008, 14, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Luppa, M.; Sikorski, C.; Luck, T.; Ehreke, L.; Konnopka, A.; Wiese, B.; Weyerer, S.; Konig, H.H.; Riedel-Heller, S.G. Age- and gender-specific prevalence of depression in latest-life—Systematic review and meta-analysis. J. Affect. Disord. 2012, 136, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Burger, H. The menopausal transition—Endocrinology. J. Sex. Med. 2008, 5, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Lagunas, N.; Calmarza-Font, I.; Diz-Chaves, Y.; Garcia-Segura, L.M. Long-term ovariectomy enhances anxiety- and depressive-like behaviors in mice submitted to chronic unpredictable stress. Horm. Behav. 2010, 58, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Vergoni, A.V.; Sandrini, M.; Tagliavini, S.; Bertolini, A. Influence of ovariectomy, estradiol and progesterone on the behavior of mice in an experimental model of depression. Physiol. Behav. 1989, 45, 1067–1068. [Google Scholar] [CrossRef]
- Su, Q.; Cheng, Y.; Jin, K.; Cheng, J.; Lin, Y.; Lin, Z.; Wang, L.; Shao, B. Estrogen therapy increases BDNF expression and improves post-stroke depression in ovariectomy-treated rats. Exp. Ther. Med. 2016, 12, 1843–1848. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fedotova, J.; Dudnichenko, T. Effects of different doses of Vitamin D3 supplementation in young perimenopausal women on depression and hormonal status. Psychoneuroendocrinol 2017, 83, 35. [Google Scholar] [CrossRef]
- Fedotova, J.O. Vitamin D3 treatment differentially affects anxety-like behavior in the old ovariectomized female rats treated with low dose of 17β-estradiol. BMC Med. Gen. 2019, 20, 49. [Google Scholar]
- Lagana, A.S.; Vitale, S.G.; Ban Frangez, H.; Vrtacnik-Bokal, E.; D’Anna, R. Vitamin D in human reproduction: The more, the better? An evidence-based critical appraisal. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4243–4251. [Google Scholar]
- Milaneschi, Y.; Shardell, M.; Corsi, A.M.; Vazzana, R.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. Serum 25-hydroxyvitamin D and depressive symptoms in older women and men. J. Clin. Endocrinol. Metab. 2010, 95, 3225–3323. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, J.O.; Pivina, S.G.; Volkova, O.V. Vitamin D3 application attenuates anxiety-like profile and increases 25-OH-VD3 levels in the serum blood of the middle-aged female rats at 12 week after overiectomy. Act. Nerv. Super. Rediviva 2018, 60, 55–66. [Google Scholar]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Kesby, J.P.; Eyles, D.W.; Burne, T.H.; McGrath, J.J. The effects of vitamin D on brain development and adult brain function. Mol. Cell. Endocrinol. 2011, 347, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Burne, T.H.; McGrath, J.J. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front. Neuroendocrinol. 2013, 34, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Adams, J.S.; Hewison, M. Update in Vitamin, D. J. Clin. Endocrinol. Metabol. 2010, 95, 471–478. [Google Scholar] [CrossRef]
- DeLuca, G.C.; Kimball, S.M.; Kolasinski, J.; Ramagopalan, S.V.; Ebers, G.C. Review: The role of vitamin D in nervous system health and disease. Neuropathol. Appl. Neurobiol. 2013, 39, 458–484. [Google Scholar] [CrossRef]
- Eyles, D.W.; Feron, F.; Cui, X.; Kesby, J.P.; Harms, L.H.; Ko, P.; McGrath, J.J.; Burne, T.H.J. Developmental vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinol 2009, 34, S247–S257. [Google Scholar] [CrossRef]
- Obradovic, D.; Gronemeyer, H.; Lutz, B.; Rein, T. Cross-talk of vitamin D and glucocorticoids in hippocampal cells. J. Neurochem. 2006, 96, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.B.; Brotchie, H.; Graham, R.K. Vitamin D and depression. J. Affect. Disord. 2017, 208, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Murphy, J.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M.; Ng, C.H. Adjunctive nutraceuticals for depression: A systematic review and meta-analyses. Am. J. Psych. 2016, 173, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Sepehrmanesh, Z.; Kolahdooz, F.; Abedi, F.; Mazroii, N.; Assarian, A.; Asemi, Z.; Esmaillzadeh, A. Vitamin D supplementation affects the beck depression inventory, insulin resistance, and biomarkers of oxidative stress in patients with major depressive disorder: A Randomized, Controlled Clinical Trial. J. Nutr. 2016, 146, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Spedding, S. Vitamin D and depression: A systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients 2014, 6, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Ohinmaa, A.; Klarenbach, S.; Wong, Z.W.; Veugelers, P. Serum 25Hydroxyvitamin D concentrations and indicators of mental health: An analysis of the Canadian health measures survey. Nutrients 2017, 9, 1116. [Google Scholar] [CrossRef]
- Zhao, G.; Ford, E.S.; Li, C.; Balluz, L.S. No associations between serum concentrations of 25-hydroxyvitamin D and parathyroid hormone and depression among US adults. Br. J. Nutr. 2010, 104, 1696–1702. [Google Scholar] [CrossRef]
- Hanson, N.D.; Owens, M.J.; Nemeroff, C.B. Depression, antidepressants, and neurogenesis: A critical reappraisal. Neuropsychopharmacology 2011, 36, 2589–2602. [Google Scholar] [CrossRef]
- Levada, O.A.; Cherednichenko, N.V. Brain-derived neurotrophic factor (BDNF): Neurobiology and marker value in neuropsychiatry. Likars’ ka Sprava 2015, 3–4, 15–25. [Google Scholar]
- Korte, M.; Kang, H.; Bonhoeffer, T.; Schuman, E. Arolefor BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology 1998, 37, 553–559. [Google Scholar] [CrossRef]
- McAllister, A.K.; Katz, L.C.; Lo, D.C. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 1999, 22, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Hashimoto, E.; Ukai, W.; Ishii, T.; Yoshinaga, T.; Ono, T.; Tateno, M.; Watanabe, I.; Shirasaka, T.; Saito, S.; et al. Effect of antidepressants on brain-derived neurotrophic factor (BDNF) release from platelets in the rats. Prog. Neuropsychopharmacol. Biol. Psych. 2010, 34, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kim, Y.K. Plasma brain-derived neurotrophic factor as a peripheral marker for the action echanism of antidepressants. Neuropsychobiology 2008, 57, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Ladea, M.; Bran, M. Brain derived neurotrophic factor (BDNF) levels in depressed women treated with open-label escitalopram. Psych. Danubina 2013, 25, 128–132. [Google Scholar]
- Wolkowitz, O.M.; Wolf, J.; Shelly, W.; Rosser, R.; Burke, H.M.; Lerner, G.K.; Reus, V.I.; Nelson, J.C.; Epel, E.S.; Mellon, S.H. Serum BDNF levels before treatment predict SSRI response in depression. Prog. Neuropsychopharmacol. Biol. Psych. 2011, 35, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, C.; Yalcin, E.S.; Aksaray, S.; Kisa, C.; Yildirim, S.G.; Uzbay, T.; Goka, E. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog. Neuropsychopharmacol. Biol. Psych. 2006, 30, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Amadera, J.; Berbel, B.; Volz, M.S.; Rizzerio, B.G.; Fregni, F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int. J. Neuropsychopharmacol. 2011, 14, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Kurita, M.; Nishino, S.; Kato, M.; Numata, Y.; Sato, T. Plasma brain-derived neurotrophic factor levels predict the clinical outcome of depression treatment in a naturalistic study. PLoS ONE 2012, 7, e39212. [Google Scholar] [CrossRef]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Kino, T. Stress, glucocorticoid hormones, and hippocampal neural pro- genitor cells: Implications to mood disorders. Front. Physiol. 2015, 6, 230. [Google Scholar] [CrossRef]
- Bao, A.M.; Meynen, G.; Swaab, D.F. The stress system in depression and neurodegeneration: Focus on the human hypothalamus. Brain Res. Rev. 2008, 57, 531–553. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, J. Post-ovariectomy period influences depression-like behavior in the adult female rats treated with different doses of cholecalciferol. Opera Med. Physiol. 2018, 4, 35–49. [Google Scholar]
- Fedotova, J.; Pivina, S.; Suchko, A. Effects of chronic Vitamin D3 hormone administration on anxiety-like behavior in adult female rats after long-term ovariectomy. Nutrients 2017, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Bekku, N.; Yoshimura, H. Animal model of menopausal depressive-like state in female mice: Prolongation of immobility time in the forced swimming test following ovariectomy. Psychopharmacology 2005, 183, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Bosee, R.; Di Paolo, T. Dopamine and GABAA receptor imbalance after ovariectomy in rats: Model of menopause. J. Psychiatr. Neurosci. 1995, 20, 364–371. [Google Scholar]
- Banasr, M.; Valentine, G.W.; Li, X.Y.; Gourley, S.L.; Taylor, J.R.; Duman, R.S. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol. Psych. 2007, 62, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Katz, R.J. Animal models and human depressive disorders. Neurosci. Biobehav. Rev. 1981, 5, 231–246. [Google Scholar] [CrossRef]
- Willner, P.; Towell, A.; Sampson, D.; Sophokleous, S.; Muscat, R. Reduction of sucrose preference bychronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 1987, 93, 358–364. [Google Scholar] [CrossRef]
- Burstein, O.; Franko, M.; Gale, E.; Handelsman, A.; Barak, S.; Motsan, S.; Shamir, A.; Toledano, R.; Simhon, O.; Hirshler, Y.; et al. Escitalopram and NHT normalized stress-induced anhedonia and molecular neuroadaptations in a mouse model of depression. PLoS ONE 2017, 12, e0188043. [Google Scholar] [CrossRef]
- Estrada-Camarena, E.; Fernandez-Guasti, A.; Lopez-Rubalcava, C. Interaction between estrogens and antidepressants in the forced swimming test in rats. Psychopharmacology 2004, 173, 139–145. [Google Scholar] [CrossRef]
- Olivares-Nazario, M.; Fernandez-Guasti, A.; Martinez-Mota, L. Age-related changes in the antidepressant-like effect of desipramine and fluoxetine in the rat forced-swim test. Behav. Pharmacol. 2016, 27, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.J.; Guo, Y.J.; Zhou, H.; Teng, G.J.; Chen, B.A. Anhedonia and activity deficits in rats: Impact of poststroke depression. J. Psychopharmacol. 2009, 23, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Anisman, H.; Matheson, K. Stress, depression, and anhedonia: Caveats concerning animal models. Neurosci. Biobehav. Rev. 2005, 29, 525–546. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.; Forbes, N.; Reid, I.C. Sucrose consumption as an hedonic measure following chronic unpredictable mild stress. Physiol. Behav. 1995, 57, 241–248. [Google Scholar] [CrossRef]
- Heffner, T.G.; Hartmann, J.A.; Seiden, L.S. A rapid method for the regional dissection of the rat brain. Pharmacol. Biochem. Behav. 1980, 13, 453–456. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microcram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, J.; Jiang, L.; Xie, Y.; Xia, Y.; Lv, R.; Dong, L. Paeoniflorin improves menopause depression in ovariectomized rats under chronic unpredictable mild stress. Int. J. Clin. Exp. Med. 2015, 8, 5103–5111. [Google Scholar] [PubMed]
- Magni, L.R.; Purgato, M.; Gastaldon, C.; Papola, D.; Furukawa, T.A.; Cipriani, A.; Barbui, C. Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database Syst. Rev. 2013, 7, CD004185. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, K.; Yousefian, Z.; Vafaei, A.A.; Rashidy-Pour, A.; Parsaei, H.; Khaleghian, A.; Choobdar, S. Mesolimbic dopamine system and its modulation by Vitamin D in a chronic mild stress model of depression in the rat. Behav. Brain Res. 2019, 356, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Dalmagro, A.P.; Rikel, L.; da Silva, E.B.; Simão da Silva, K.A.B.; Zeni, A.L.B. Cholecalciferol counteracts depressive-like behavior and oxidative stress induced by repeated corticosterone treatment in mice. Eur. J. Pharmacol. 2018, 833, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Groves, N.J.; McGrath, J.J.; Burne, T.H. Vitamin D as a neurosteroid affecting the developing and adult brain. Annu. Rev. Nutr. 2014, 34, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F.; Bao, A.M.; Lucassen, P.J. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005, 4, 141–194. [Google Scholar] [CrossRef] [PubMed]
- Workman, J.L.; Gobinath, A.R.; Kitay, N.K.; Chow, C.; Brummelte, S.; Galea, L.A. Parity modifies the effects of fluoxetine and corticosterone on behavior, stress reactivity, and hippocampal neurogenesis. Neuropharmacol. 2016, 105, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.; Caldeira, M.V.; Santos, S.D.; Duarte, C.B. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br. J. Pharmacol. 2008, 183, S310–S324. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Li, N. A neurotrophic hypothesis of depression: Role of synaptogenesis in the actions of NMDA receptor antagonists. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Lessmann, V.; Brigadski, T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: An update. Neurosci. Res. 2009, 65, 11–22. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Holick, K.A.; Gundersen, B.; Hen, R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 2004, 29, 1321–1330. [Google Scholar] [CrossRef]
- Nibuya, M.; Morinobu, S.; Duman, R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [CrossRef]
- Sen, S.; Duman, R.; Sanacora, G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Meta-analyses and implications. Biol. Psych. 2008, 64, 527–532. [Google Scholar] [CrossRef]
- Satomura, E.; Baba, H.; Nakano, Y.; Maeshima, H.; Suzuki, T.; Arai, H. Correlations between brain-derived neurotrophic factor and clinical symptoms in medicated patients with major depression. J. Affect. Disord. 2011, 135, 332–335. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, F.; Chen, J.; Dong, W. Chronic antidepressant administration alleviates frontal and hippocampal BDNF deficits in CUMS rat. Brain Res. 2010, 1366, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Luellen, B.A.; Bianco, L.E.; Schneider, L.M.; Andrews, A.M. Reduced brain-derived neurotrophic factor is associated with a loss of serotonergic innervation in the hippocampus of aging mice. Genes Brain Behav. 2007, 6, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, S.J.; Kiraly, M.A.; Hawe, R.D.; Makhani, V. Vitamin D as neuroactive substance: Review. Sci. World J. 2006, 6, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, C.F. Neurotrophin-4: The odd one out in the neurotrophin family. Neurochem. Res. 1996, 21, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Friedman, W.J.; Greene, L.A. Neurotrophin signaling via Trks and p75. Exp. Cell Res. 1999, 253, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Mbuagbaw, L.; Samaan, Z.; Falavigna, M.; Zhang, S.; Adachi, J.D.; Cheng, J.; Papaioannou, A.; Thabane, L. Efficacy of vitamin D supplementation in depression in adults: A systematic review. J. Clin. Endocrinol. Metab. 2014, 99, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D and depression: Cellular and regulatory mechanisms. Pharmacol. Rev. 2017, 69, 80–92. [Google Scholar] [CrossRef]
- Gaugris, S.; Heaney, R.P.; Boonen, S.; Kurth, H.; Bentkover, J.D.; Sen, S.S. Vitamin D inadequacy among post-menopausal women: A systemic review. QJM 2005, 98, 667–676. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; Powers, S.I.; Spangler, L.; Brunner, R.L.; Michael, Y.L.; Larson, J.C.; Millen, A.E.; Bueche, M.N.; Salmoirago-Blotcher, E.; Liu, S.; et al. Vitamin D intake from foods and supplements and depressive symptoms in a diverse population of older women. Am. J. Clin. Nutr. 2011, 94, 1104–1112. [Google Scholar] [CrossRef]
- Kjærgaard, M.; Joakimsen, R.; Jorde, R. Low serum 25-hydroxyvitamin D levels are associated with depression in an adult Norwegian population. Psych. Res. 2011, 190, 221–225. [Google Scholar] [CrossRef]
- Accortt, E.E.; Schetter, C.D.; Peters, R.M.; Cassidy-Bushrow, A.E. Lower prenatal vitamin D status and postpartum depressive symptomatology in African American women: Preliminary evidence for moderation by inflammatory cytokines. Arch. Women Ment. Health. 2016, 19, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Harms, L.R.; Burne, T.H.J.; Eyles, D.W.; McGrath, J.J. Vitamin D and the brain. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 657–669. [Google Scholar]
- Mizwicki, M.T.; Norman, A.W. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci. Signal 2009, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinksi, G.G.; Petrie, G.I.; De Luca, H.F. 1,25-Dihydroxyvitamin D3 restores fertility of vitamin D-deficient female rats. Am. J. Physiol. 1989, 256, E483–E487. [Google Scholar] [CrossRef] [PubMed]
- Luk, J.; Torrealday, S.; Neal Perry, G.; Pal, L. Relevance of vitamin D in reproduction. Hum. Reprod. 2012, 27, 3015–3027. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshkina, A.; Dudnichenko, T.; Baranenko, D.; Fedotova, J.; Drago, F. Effects of Vitamin D3 in Long-Term Ovariectomized Rats Subjected to Chronic Unpredictable Mild Stress: BDNF, NT-3, and NT-4 Implications. Nutrients 2019, 11, 1726. https://doi.org/10.3390/nu11081726
Koshkina A, Dudnichenko T, Baranenko D, Fedotova J, Drago F. Effects of Vitamin D3 in Long-Term Ovariectomized Rats Subjected to Chronic Unpredictable Mild Stress: BDNF, NT-3, and NT-4 Implications. Nutrients. 2019; 11(8):1726. https://doi.org/10.3390/nu11081726
Chicago/Turabian StyleKoshkina, Alexandra, Tatyana Dudnichenko, Denis Baranenko, Julia Fedotova, and Filippo Drago. 2019. "Effects of Vitamin D3 in Long-Term Ovariectomized Rats Subjected to Chronic Unpredictable Mild Stress: BDNF, NT-3, and NT-4 Implications" Nutrients 11, no. 8: 1726. https://doi.org/10.3390/nu11081726
APA StyleKoshkina, A., Dudnichenko, T., Baranenko, D., Fedotova, J., & Drago, F. (2019). Effects of Vitamin D3 in Long-Term Ovariectomized Rats Subjected to Chronic Unpredictable Mild Stress: BDNF, NT-3, and NT-4 Implications. Nutrients, 11(8), 1726. https://doi.org/10.3390/nu11081726



