Life Style Intervention Improves Retinopathy Status—The Finnish Diabetes Prevention Study
Abstract
1. Introduction
2. Subjects and Methods
2.1. Study Design
2.2. Examination
2.3. Statistical Analysis
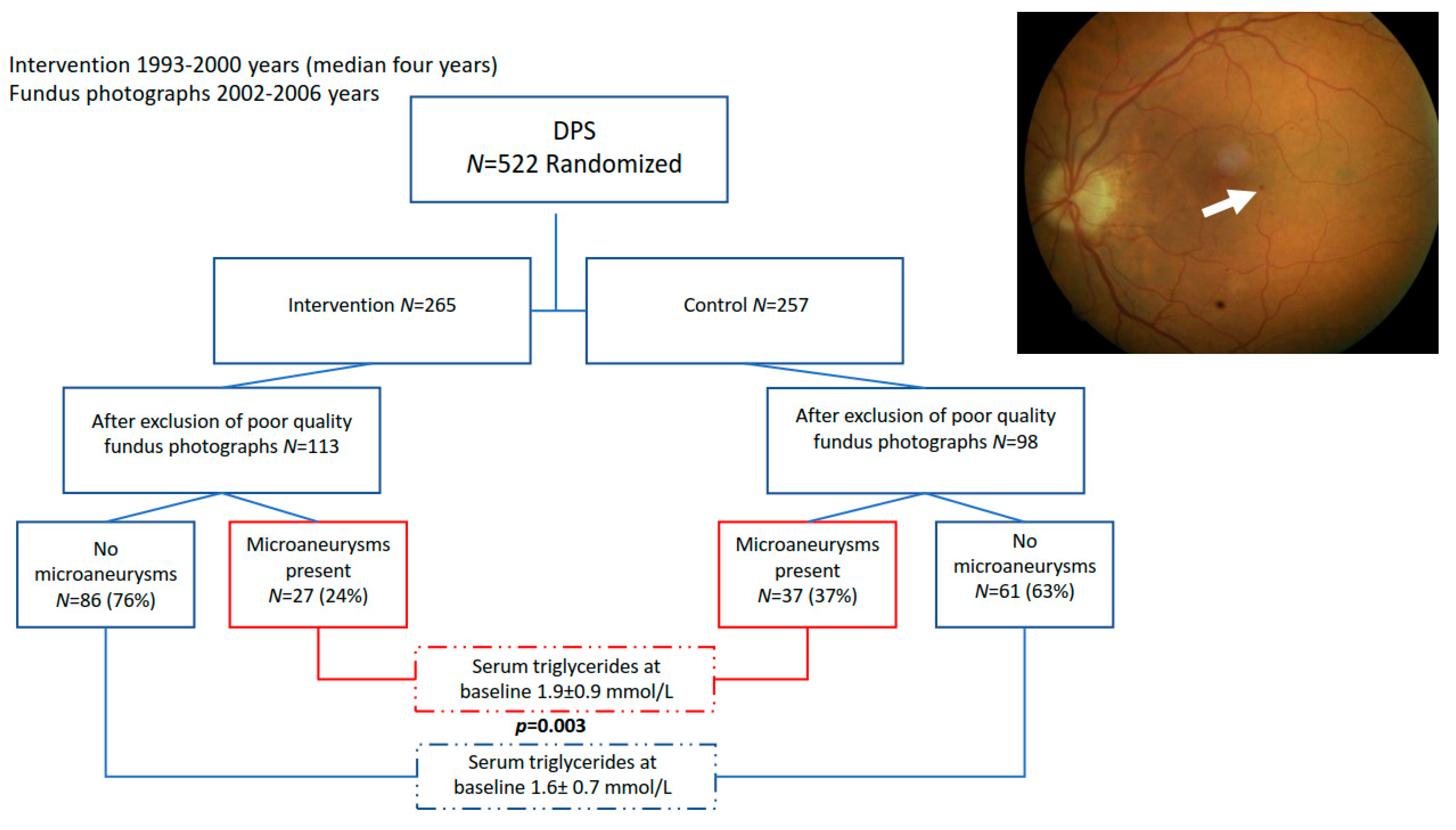
3. Results
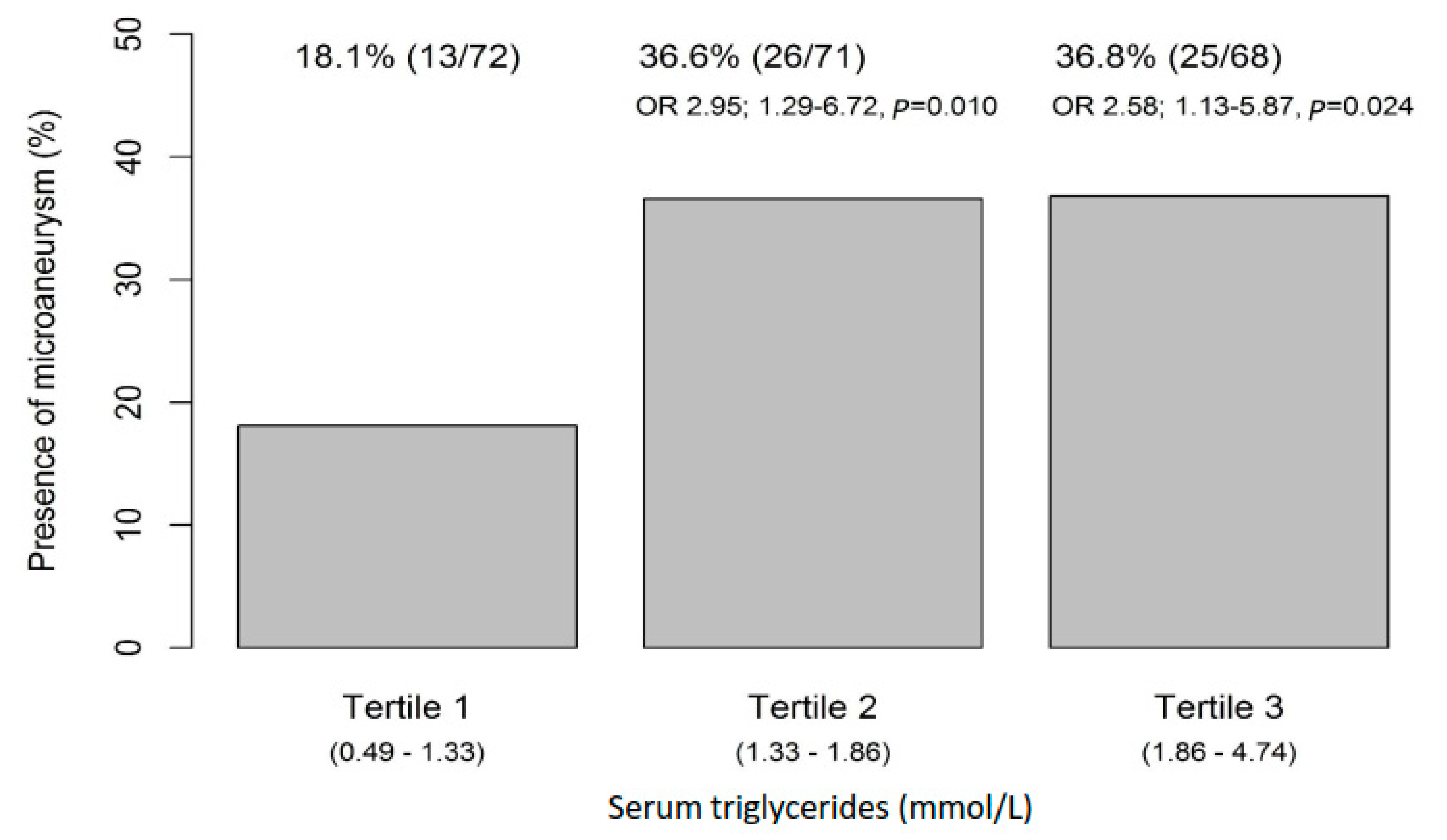
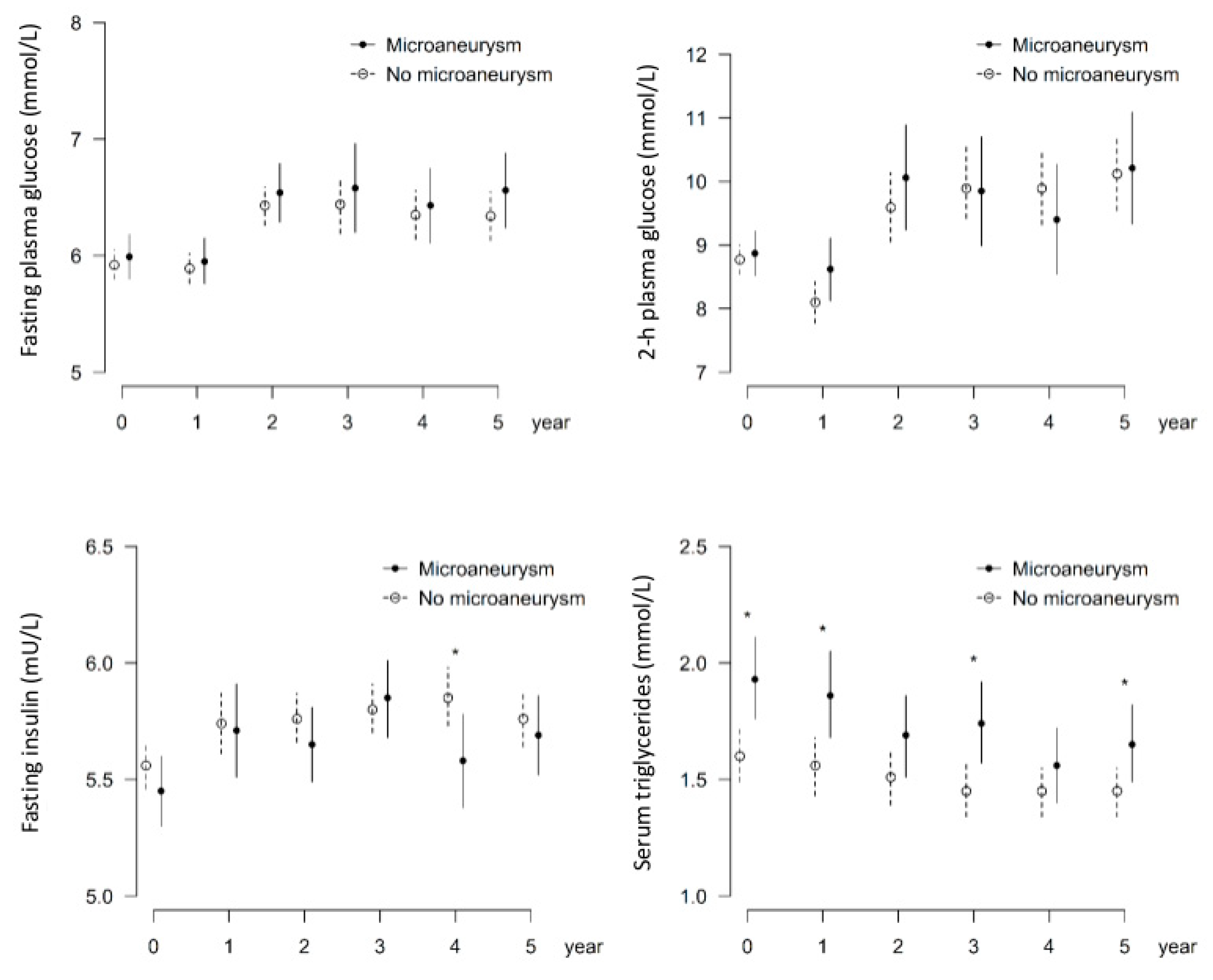
3.1. Higher Serum Triglycerides Are Associated with the Occurrence of Microaneurysms
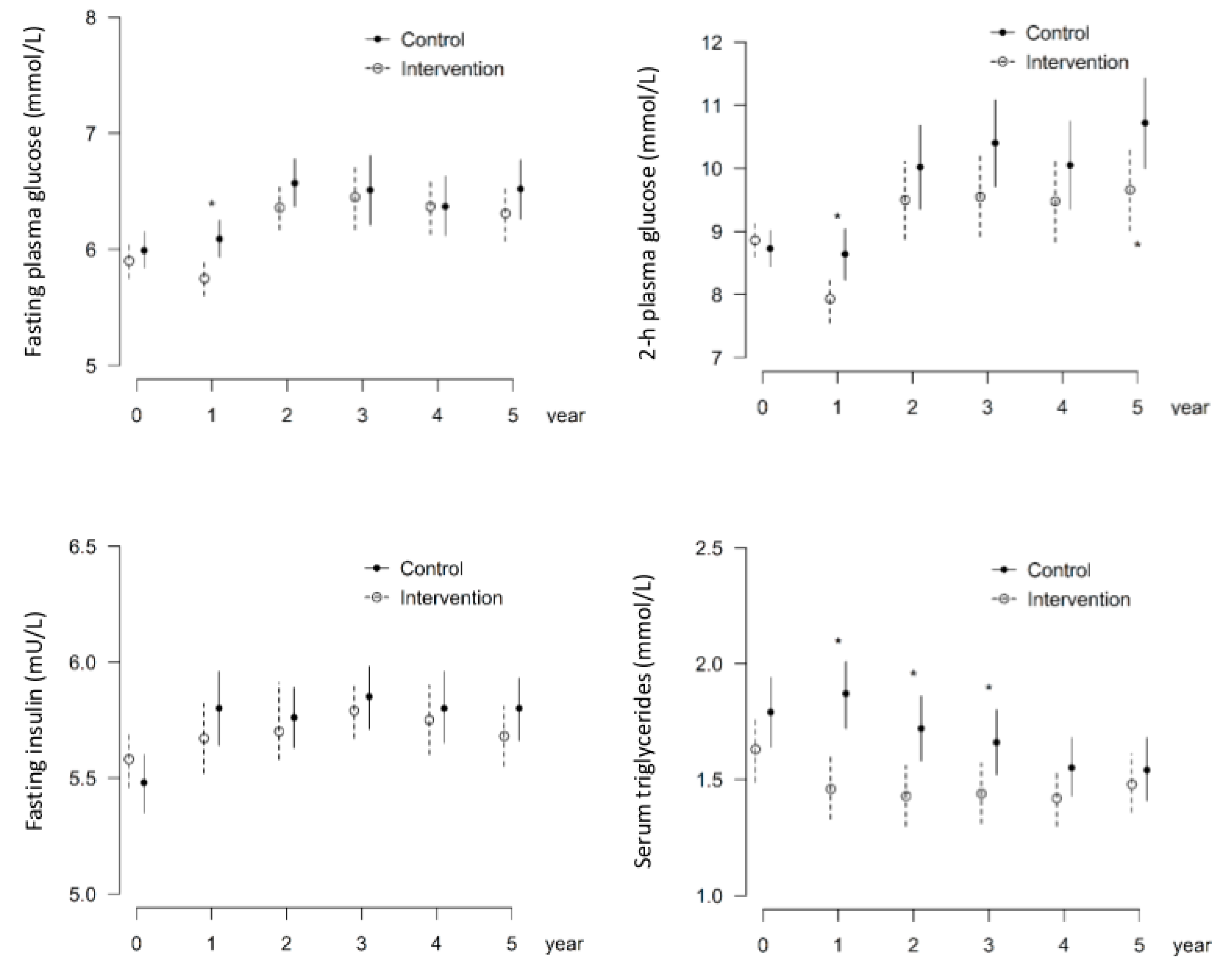
3.2. Intervention Group Shows Decreased Occurrence of Microaneurysms
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Usman Akram, M.; Khalid, S.; Tariq, A.; Khan, S.A.; Azam, F. Detection and classification of retinal lesions for grading of diabetic retinopathy. Comput. Biol. Med. 2014, 45, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Cheung, N.; Tay, W.T.; Wang, J.J.; Aung, T.; Saw, S.M.; Lim, S.C.; Tai, E.S.; Mitchell, P. Prevalence and risk factors for diabetic retinopathy: The Singapore Malay Eye Study. Ophthalmology 2008, 115, 1869–1875. [Google Scholar] [CrossRef]
- Leasher, J.L.; Bourne, R.R.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.; Naidoo, K.; Pesudovs, K.; Price, H.; White, R.A.; Wong, T.Y.; et al. Vision Loss Expert Group of the Global Burden of Disease Study. Global Estimates on the Number of People Blind or Visually Impaired by Diabetic Retinopathy: A Meta-analysis From 1990 to 2010. Diabetes Care 2016, 39, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Lindström, J.; Peltonen, M.; Eriksson, J.G.; Ilanne-Parikka, P.; Aunola, S.; Keinänen-Kiukaanniemi, S.; Uusitupa, M.; Tuomilehto, J. Finnish Diabetes Prevention Study (DPS). Improved lifestyle and decreased diabetes risk over 13 years: Long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia 2013, 56, 284–293. [Google Scholar] [CrossRef]
- de Mello, V.D.; Lindström, J.; Eriksson, J.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Sundvall, J.; Laakso, M.; Tuomilehto, J.; Uusitupa, M. Insulin secretion and its determinants in the progression of impaired glucose tolerance to type 2 diabetes in impaired glucose-tolerant individuals: The Finnish Diabetes Prevention Study. Diabetes Care 2012, 35, 211–217. [Google Scholar] [CrossRef]
- Lindström, J.; Louheranta, A.; Mannelin, M.; Rastas, M.; Salminen, V.; Eriksson, J.; Uusitupa, M.; Tuomilehto, J. Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003, 26, 3230–3236. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [Google Scholar] [CrossRef]
- Gong, Q.; Gregg, E.W.; Wang, J.; An, Y.; Zhang, P.; Yang, W.; Li, H.; Li, H.; Jiang, Y.; Shuai, Y.; et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: The China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011, 54, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.R.; Park, S.W.; Choi, S.Y.; Kim, S.W.; Moon, K.Y.; Kim, J.H.; Lee, K. Association of statin use and hypertriglyceridemia with diabetic macular edema in patients with type 2 diabetes and diabetic retinopathy. Cardiovasc. Diabetol. 2017, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- ACCORD Study Group; Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R., III; Leiter, L.A.; Linz, P.; Friedewald, W.T.; Buse, J.B.; Gerstein, H.C.; Probstfield, J.; et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar] [PubMed]
- Wilding, J.P.; Woo, V.; Rohwedder, K.; Sugg, J.; Parikh, S. Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: Efficacy and safety over 2 years. FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular even 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005, 366, 1849–1861. [Google Scholar]
- Cunha-Vaz, J.; Ribeiro, L.; Lobo, C. Phenotypes and biomarkers of diabetic retinopathy. Prog. Retin. Eye Res. 2014, 41, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, N.; Hietala, K.; Forsblom, C.; Harjutsalo, V.; Mäkinen, V.P.; Kytö, J.; Summanen, P.A.; Thorn, L.M.; Wadén, J.; Gordin, D.; et al. Associations and interactions between lipid profiles, retinopathy and nephropathy in patients with type 1 diabetes: The FinnDiane Study. J. Intern. Med. 2013, 274, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Kohner, E.M.; Stratton, I.M.; Aldington, S.J.; Turner, R.C.; Matthews, D.R. Microaneurysms in the development of diabetic retinopathy (UKPDS 42). UK Prospective Diabetes Study Group. Diabetologia 1999, 42, 1107–1112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klein, R.; Knudtson, M.D.; Lee, K.E.; Gangnon, R.; Klein, B.E. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 2008, 115, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Stratton, I.M.; Aldington, S.J.; Holman, R.R.; Kohner, E.M. UK Prospective Diabetes Study Group Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch. Ophthalmol. 2004, 122, 1631–1640. [Google Scholar] [PubMed]
- Higgins, G.T.; Khan, J.; Pearce, I.A. Glycaemic control and control of risk factors in diabetes patients in an ophthalmology clinic: What lessons have we learned from the UKPDS and DCCT studies? Acta Ophthalmol. Scand. 2007, 85, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Diabetes Prevention Program Research Group.Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef]
- Thomas, D.E.; Elliott, E.J.; Naughton, G.A. Exercise for type 2 diabetes mellitus. Hypertension 2013, 62, 1021–1026. [Google Scholar]
- Huai, P.; Xun, H.; Reilly, K.H.; Wang, Y.; Ma, W.; Xi, B. Physical activity and risk of hypertension: A meta-analysis of prospective cohort studies. Diabetologia 2013, 56, 284–293. [Google Scholar] [CrossRef]
- Mansi, I.A.; English, J.L.; Morris, M.J.; Zhang, S.; Mortensen, E.M.; Halm, E.A. Statins for primary prevention in physically active individuals: Do the risks outweigh the benefits? J. Sci. Med. Sport 2017, 20, 627–632. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Yang, X.; Hemler, E.C.; Fang, Y.; Zhao, L.; Zhang, J.; Yang, Z.; Wang, Z.; He, L.; et al. The dietary transition and its association with cardiometabolic mortality among Chinese adults, 1982–2012: A cross-sectional population-based study. Lancet Diabetes Endocrinol. 2019, 7, 540–548. [Google Scholar] [CrossRef]
- Eckel, R.H.; McLean, E.; Albers, J.J.; Cheung, M.C.; Bierman, E.L. Plasma lipids and microangiopathy in insulin-dependent diabetes mellitus. Diabetes Care 1981, 4, 447–453. [Google Scholar]
- van Leiden, H.A.; Dekker, J.M.; Moll, A.C.; Nijpels, G.; Heine, R.J.; Bouter, L.M.; Stehouwer, C.D.; Polak, B.C. Blood pressure, lipids, and obesity are associated with retinopathy: The hoorn study. Diabetes Care 2002, 25, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Chou, P.; Lee, A.F.; Lee, F.L.; Hsu, W.M.; Liu, J.H.; Tung, T.H. Microaneurysm number and distribution in the macula of Chinese type 2 diabetics with early diabetic retinopathy: A population-based study in Kinmen, Taiwan. Acta Diabetol. 2010, 47, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Zhang, P.; Wang, J.; Ma, J.; An, Y.; Chen, Y.; Zhang, B.; Feng, X.; Li, H.; Chen, X.; et al. Morbidity and mortality after lifestyle intervention for people withimpaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019, 7, 452–461. [Google Scholar] [CrossRef]
| Variable | No Microaneurysms, N = 147 | Microaneurysms Present, N = 64 | p-Value |
|---|---|---|---|
| Age, years | 54.3 ± 7.3 | 52.6 ± 6.2 | 0.083 baseline |
| Body weight, kg | 87.4 ± 14.9 −1.1 ± 6.1 | 85.1 ± 13.8 −1.0 ± 6.1 | 0.327 baseline 0.916 change |
| Fasting plasma glucose, mmol/L | 5.9 ± 0.8 0.0 ± 0.7 | 6.0 ± 0.7 0.0 ± 0.6 | 0.585 baseline 0.960 change |
| 2 h plasma glucose, mmol/L | 8.8 ± 1.4 −0.7 ± 1.9 | 8.9 ± 1.5 −0.3 ± 2.0 | 0.676 baseline 0.152 change |
| Fasting insulin, mU/L | 14.1 ± 6.6 −2.0 ± 6.1 | 14.9 ± 8.9 −1.0 ± 6.2 | 0.526 baseline 0.282 change |
| Systolic blood pressure, mmHg | 138.6 ± 18.7 −4.9 ± 14.0 | 136.9 ± 19.0 −2.4 ± 15.2 | 0.530 baseline 0.238 change |
| Diastolic blood pressure, mmHg | 85.4 ± 9.3 −3.2 ± 8.7 | 86.5 ± 10.3 −3.2 ± 8.7 | 0.463 baseline 0.926 change |
| Serum total cholesterol, mmol/L | 5.6 ± 1.0 −0.1 ± 0.6 | 5.6 ± 0.9 −0.1 ± 0.6 | 0.532 baseline 0.798 change |
| High-density lipoprotein cholesterol, mmol/L | 1.2 ± 0.3 0.0 ± 0.2 | 1.2 ± 0.3 0.0 ± 0.2 | 0.162 baseline 0.691 change |
| Serum triglycerides, mmol/L | 1.6 ± 0.7 0.0 ± 0.6 | 1.9 ± 0.9 −0.1 ± 0.7 | 0.003 baseline 0.795 change |
| Variable | Participants, N = 211 | No Participants, N = 311 | p-Value |
|---|---|---|---|
| Age, years | 53.8 ± 7.0 | 56.1 ± 7.1 | <0.001 |
| Body weight, kg | 86.7 ± 14.6 | 85.8 ± 13.9 | 0.470 |
| Fasting plasma glucose, mmol/L | 5.9 ± 0.8 | 6.3 ± 0.7 | <0.001 |
| 2 h plasma glucose, mmol/L | 8.8 ± 1.4 | 9.0 ± 1.5 | 0.254 |
| Fasting insulin, mU/L | 14.4 ± 7.4 | 15.1 ± 7.5 | 0.332 |
| Systolic blood pressure, mmHg | 138.1 ± 18.8 | 138.0 ± 16.9 | 0.968 |
| Diastolic blood pressure, mmHg | 85.7 ± 9.6 | 85.7 ± 9.8 | 0.985 |
| Serum total cholesterol, mmol/L | 5.6 ± 1.0 | 5.6 ± 0.9 | 0.642 |
| High-density lipoprotein cholesterol, mmol/L | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.553 |
| Serum triglycerides, mmol/L | 1.7 ± 0.7 | 1.7 ± 0.8 | 0.716 |
| Variable | OR (95% CI) | p-Value |
|---|---|---|
| Age, years | 0.97 (0.93–1.01) | 0.171 |
| Sex, woman | 1.60 (0.77–3.33) | 0.208 |
| Diabetes, yes | 0.80 (0.42–1.54) | 0.508 |
| BMI | 1.02 (0.96–1.09) | 0.523 |
| Group, intervention | 0.52 (0.28–0.97) | 0.039 |
| Variable | OR (95% CI) | p-Value |
|---|---|---|
| Age, years | 0.97 (0.93–1.02) | 0.261 |
| Sex, woman | 1.86 (0.90–4.05) | 0.105 |
| Fasting glucose 0 h | 1.04 (0.71–1.49) | 0.845 |
| Serum triglycerides, mmol/L | 1.92 (1.12–3.35) | 0.018 |
| Group, intervention | 0.62 (0.33–1.15) | 0.129 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aro, A.; Kauppinen, A.; Kivinen, N.; Selander, T.; Kinnunen, K.; Tuomilehto, J.; Keinänen-Kiukaanniemi, S.; Lindström, J.; Uusitupa, M.; Kaarniranta, K. Life Style Intervention Improves Retinopathy Status—The Finnish Diabetes Prevention Study. Nutrients 2019, 11, 1691. https://doi.org/10.3390/nu11071691
Aro A, Kauppinen A, Kivinen N, Selander T, Kinnunen K, Tuomilehto J, Keinänen-Kiukaanniemi S, Lindström J, Uusitupa M, Kaarniranta K. Life Style Intervention Improves Retinopathy Status—The Finnish Diabetes Prevention Study. Nutrients. 2019; 11(7):1691. https://doi.org/10.3390/nu11071691
Chicago/Turabian StyleAro, A., A. Kauppinen, N. Kivinen, T. Selander, K. Kinnunen, J. Tuomilehto, S. Keinänen-Kiukaanniemi, J. Lindström, M. Uusitupa, and K. Kaarniranta. 2019. "Life Style Intervention Improves Retinopathy Status—The Finnish Diabetes Prevention Study" Nutrients 11, no. 7: 1691. https://doi.org/10.3390/nu11071691
APA StyleAro, A., Kauppinen, A., Kivinen, N., Selander, T., Kinnunen, K., Tuomilehto, J., Keinänen-Kiukaanniemi, S., Lindström, J., Uusitupa, M., & Kaarniranta, K. (2019). Life Style Intervention Improves Retinopathy Status—The Finnish Diabetes Prevention Study. Nutrients, 11(7), 1691. https://doi.org/10.3390/nu11071691





