Gastrointestinal Tolerance, Growth and Safety of a Partly Fermented Formula with Specific Prebiotics in Healthy Infants: A Double-Blind, Randomized, Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Trial Design
2.3. Study Product
2.4. Measurements
2.5. Statistics
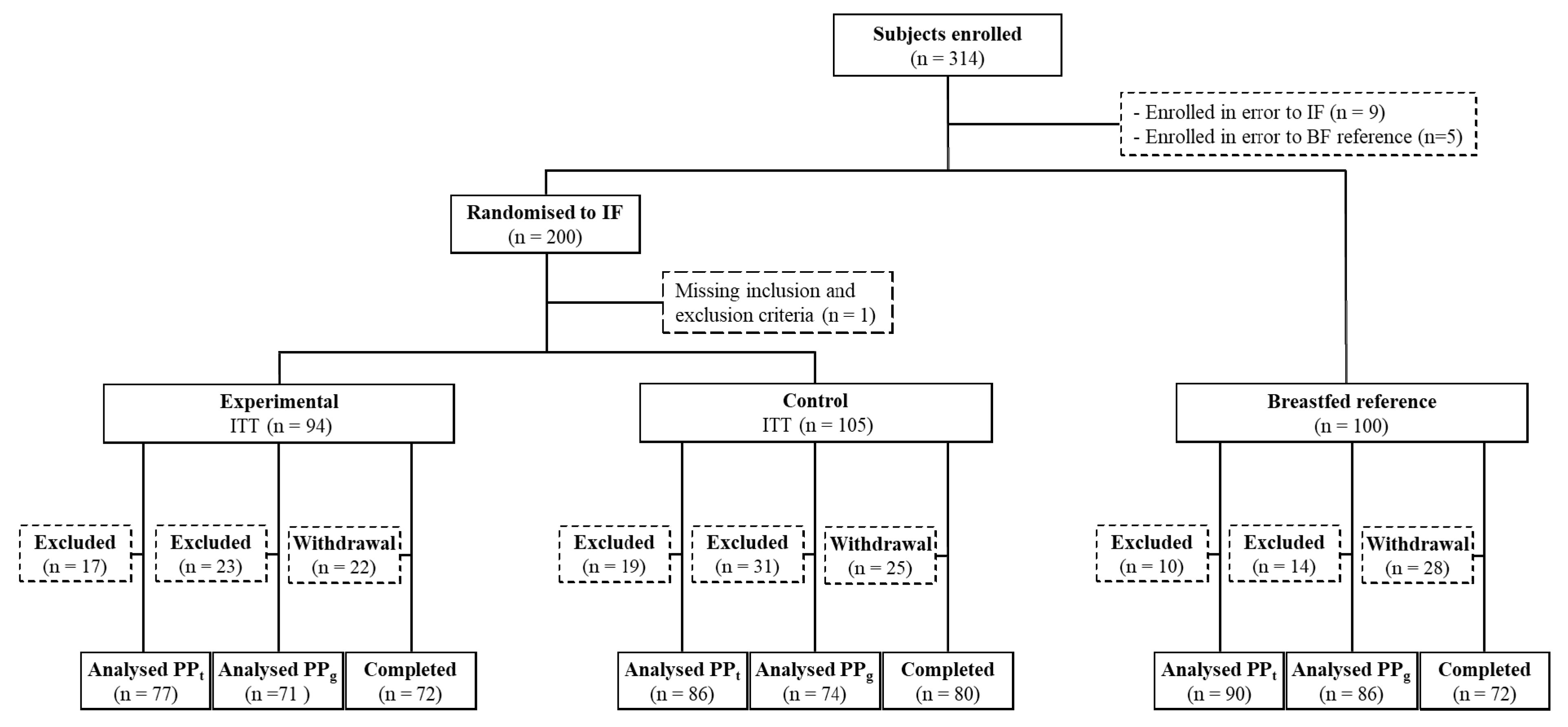
3. Results
3.1. Subject Characteristics
3.2. Study Product Intake
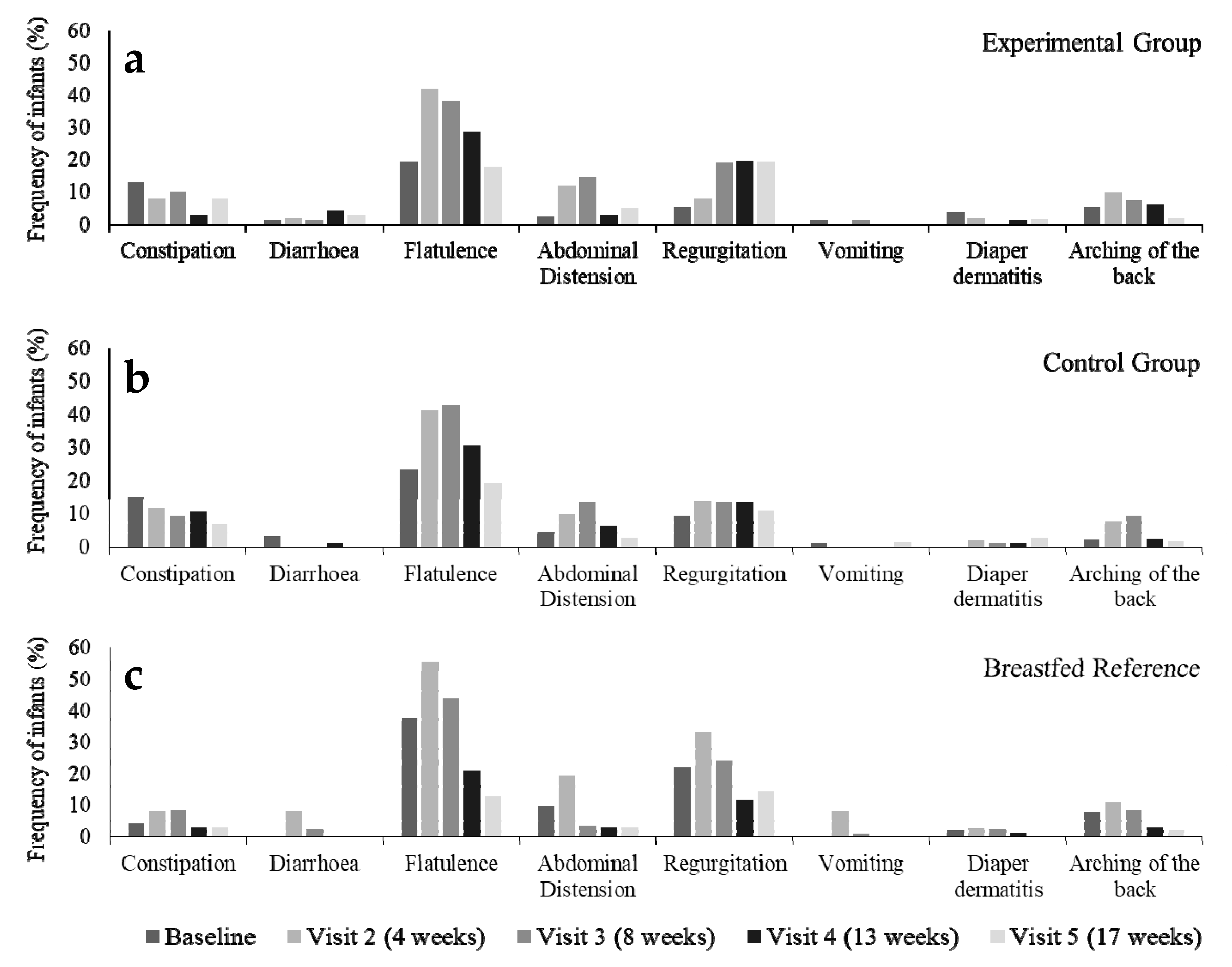
3.3. Parent-Reported Gastrointestinal Symptoms
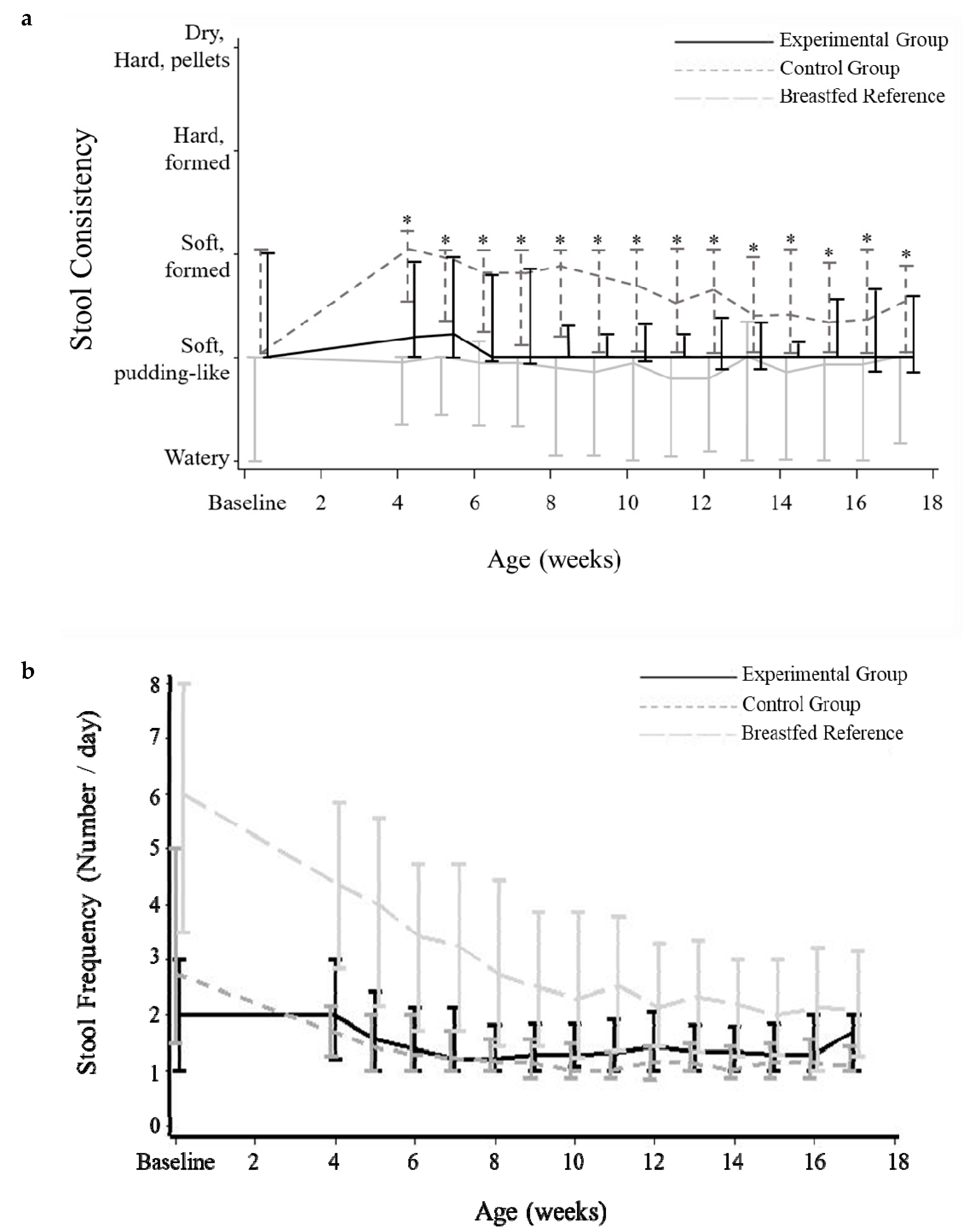
3.4. Stool Characteristics
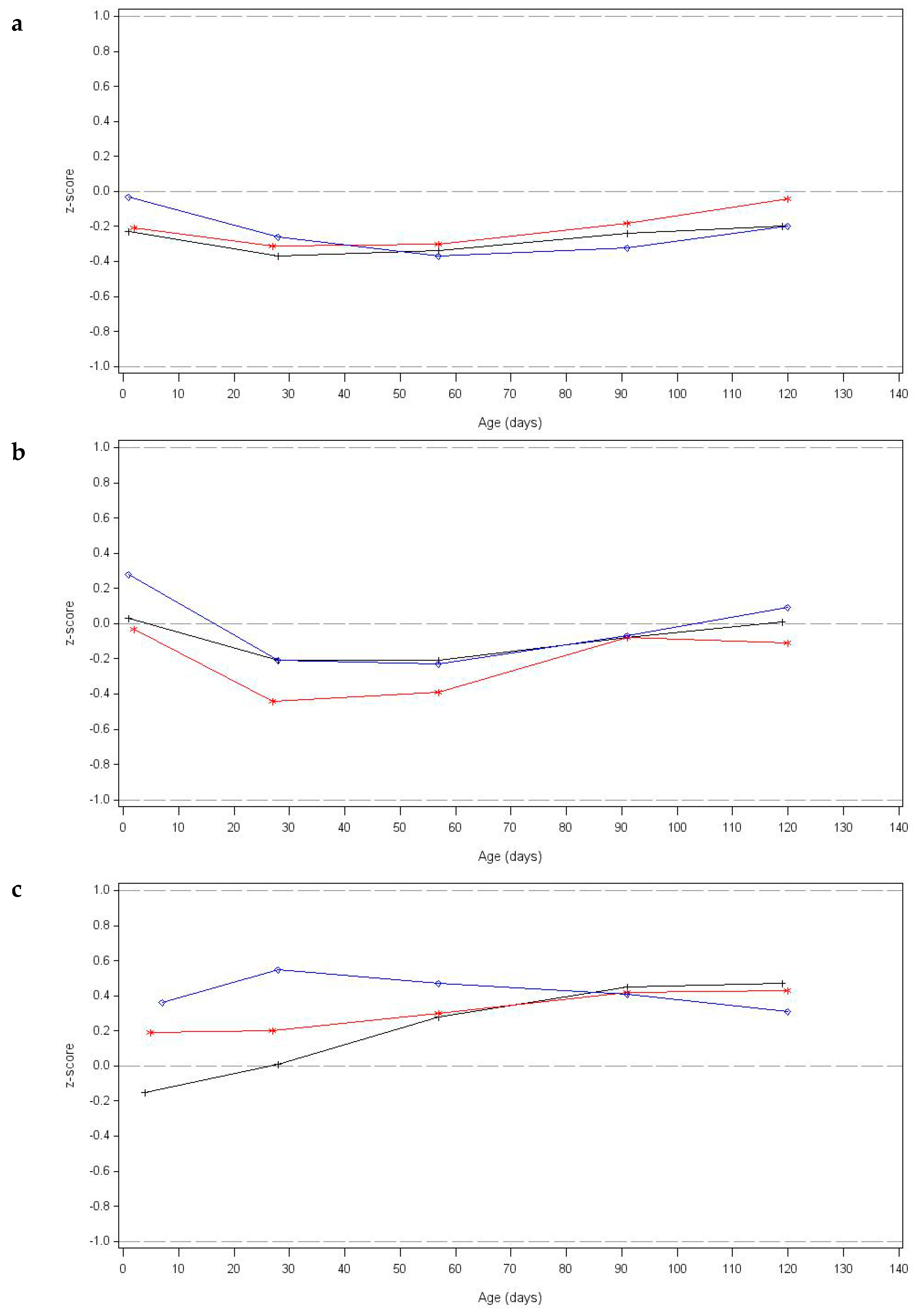
3.5. Growth Outcomes
3.6. Adverse Events
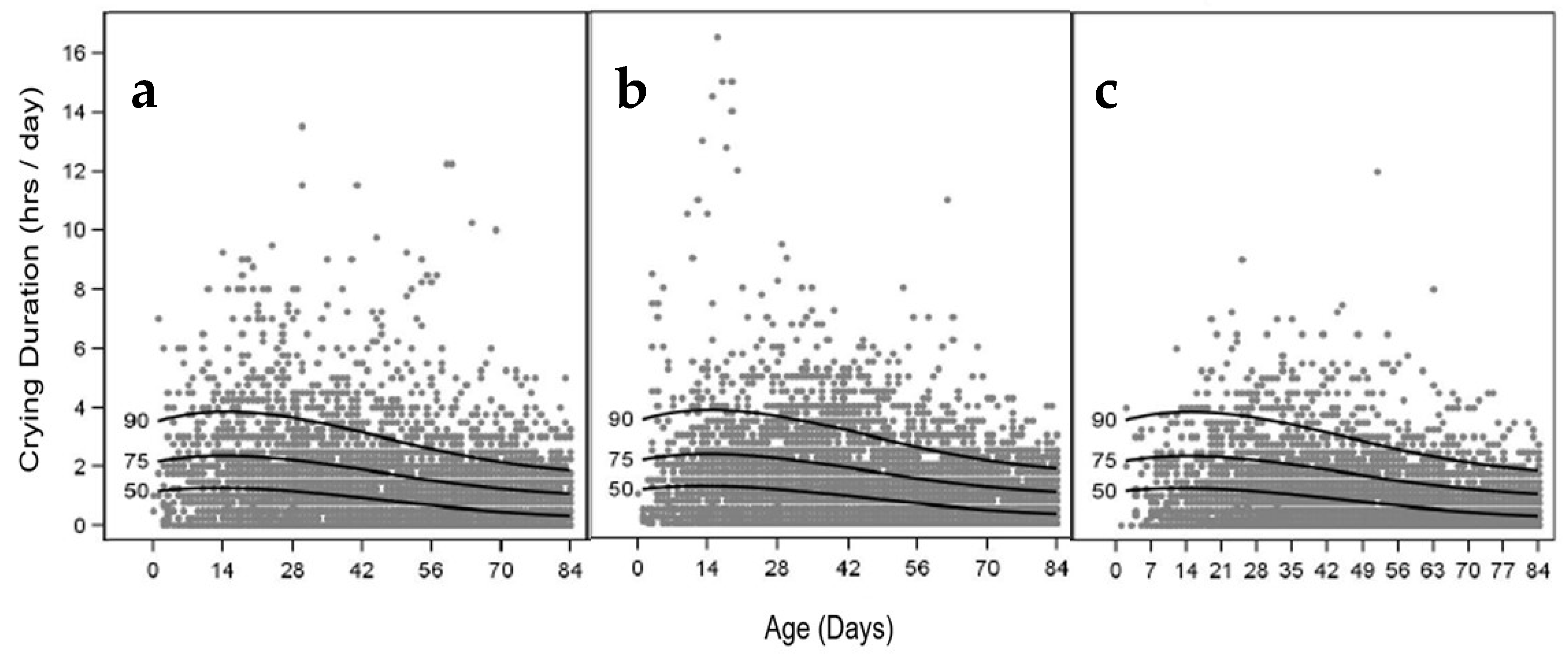
3.7. Infant Crying Frequency and Duration
3.8. Sleep Frequency and Duration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dieterich, C.M.; Felice, J.P.; O’Sullivan, E.; Rasmussen, K.M. Breastfeeding and health outcomes for the mother-infant dyad. Pediatr. Clin. N. Am. 2013, 60, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Lonnerdal, B. Infant formula and infant nutrition: Bioactive proteins of human milk and implications for composition of infant formulae. Am. J. Clin. Nutr. 2014, 99, 712S–717S. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Aubert-Jacquin, C.; Avart, C.; Gontier, C. Benefits of a thickened infant formula with lactase activity in the management of benign digestive disorders in newborns. Archives de Pediatrie Organe Officiel de la Societe Francaise de Pediatrie 2004, 11, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Thibault, H.; Aubert-Jacquin, C.; Goulet, O. Effects of long-term consumption of a fermented infant formula (with Bifidobacterium breve c50 and Streptococcus thermophilus 065) on acute diarrhea in healthy infants. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Mullié, C.; Yazourh, A.; Thibault, H.; Odou, M.F.; Singer, E.; Kalach, N.; Kremp, O.; Romond, M.B. Increased poliovirus-specific intestinal antibody response coincides with promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in infants: A randomized, double-blind, placebo-controlled trial. Pediatr. Res. 2004, 56, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Ludwig, T.; Bouritius, H.; Alliet, P.; Forde, D.; Peeters, S.; Huet, F.; Hourihane, J. Randomised controlled trial demonstrates that fermented infant formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides reduces the incidence of infantile colic. Acta Paediatr. 2017, 106, 1150–1158. [Google Scholar] [CrossRef]
- Patel, R.M.; Denning, P.W. Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis: What is the current evidence? Clin. Perinatol. 2013, 40, 11–25. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- van de Heijning, B.J.; Berton, A.; Bouritius, H.; Goulet, O. GI symptoms in infants are a potential target for fermented infant milk formulae: A review. Nutrients 2014, 6, 3942–3967. [Google Scholar] [CrossRef]
- Campeotto, F.; Suau, A.; Kapel, N.; Magne, F.; Viallon, V.; Ferraris, L.; Waligora-Dupriet, A.J.; Soulaines, P.; Leroux, B.; Kalach, N.; et al. A fermented formula in pre-term infants: Clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br. J. Nutr. 2011, 105, 1843–1851. [Google Scholar] [CrossRef]
- Indrio, F.; Ladisa, G.; Mautone, A.; Montagna, O. Effect of a fermented formula on thymus size and stool pH in healthy term infants. Pediatr. Res. 2007, 62, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, P.A.; Goossens, D.A.; Staiano, A. Stool characteristics of infants receiving short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides: A review. World J. Gastroenterol. 2014, 20, 13446–13452. [Google Scholar] [CrossRef] [PubMed]
- Knol, J.; Scholtens, P.; Kafka, C.; Steenbakkers, J.; Gro, S.; Helm, K.; Klarczyk, M.; Schöpfer, H.; Böckler, H.M.; Wells, J. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: More like breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J. Nutr. 2008, 138, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; Arslanoglu, S.; Stahl, B.; Jelinek, J.; Wahn, U.; Boehm, G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch. Dis. Child. 2006, 91, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Pawankar, R.; Cuello-Garcia, C.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Beyer, K.; Burks, W.; Canonica, G.W.; Ebisawa, M.; et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Prebiotics. World Allergy Organ. J. 2016, 9, 10. [Google Scholar] [CrossRef]
- Huet, F.; Abrahamse-Berkeveld, M.; Tims, S.; Simeoni, U.; Beley, G.; Savagner, C.; Vandenplas, Y.; Hourihane, J.O. Partly Fermented Infant Formulae With Specific Oligosaccharides Support Adequate Infant Growth and Are Well-Tolerated. J. Pediatr. Gastroenterol. Nutr. 2016, 63, e43–e53. [Google Scholar] [CrossRef]
- Hyman, P.E.; Milla, P.J.; Benninga, M.A.; Davidson, G.P.; Fleisher, D.F.; Taminiau, J. Childhood functional gastrointestinal disorders: Neonate/toddler. Gastroenterology 2006, 130, 1519–1526. [Google Scholar] [CrossRef]
- Loening-Baucke, V. Functional fecal retention with encopresis in childhood. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 79–84. [Google Scholar] [CrossRef]
- IIacono, G.; Merolla, R.; D’Amico, D.; Bonci, E.; Cavataio, F.; Di Prima, L.; Scalici, C.; Indinnimeo, L.; Averna, M.R.; Carroccio, A. Gastrointestinal symptoms in infancy: A population-based prospective study. Dig. Liver Dis. 2005, 37, 432–438. [Google Scholar] [CrossRef]
- Group WMGRS. WHO Child growth standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar]
- Vandenplas, Y.; Abkari, A.; Bellaiche, M.; Benninga, M.; Chouraqui, J.P.; ÇokuÐraþ, F.; Harb, T.; Hegar, B.; Lifschitz, C.; Ludwig, T.; et al. Prevalence and Health Outcomes of Functional Gastrointestinal Symptoms in Infants From Birth to 12 Months of Age. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Van Tilburg, M.A.; Hyman, P.E.; Walker, L.; Rouster, A.; Palsson, O.S.; Kim, S.M.; Whitehead, W.E. Prevalence of functional gastrointestinal disorders in infants and toddlers. J. Pediatr. 2015, 166, 684–689. [Google Scholar] [CrossRef]
- Costalos, C.; Kapiki, A.; Apostolou, M.; Papathoma, E. The effect of a prebiotic supplemented formula on growth and stool microbiology of term infants. Early Hum. Dev. 2008, 84, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.D.; Yan, J.; Bylsma, L.C.; Northington, R.S.; Grathwohl, D.; Steenhout, P.; Erdmann, P.; Spivey-Krobath, E.; Haschke, F. Growth of infants consuming whey-predominant term infant formulae with a protein content of 1.8 g/100 kcal: A multicenter pooled analysis of individual participant data. Am. J. Clin. Nutr. 2016, 104, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products NaAN. Scientific Opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014, 12, 3760. [Google Scholar] [CrossRef]
- Barr, R.G. The normal crying curve: What do we really know? Dev. Med. Child Neurol. 1990, 32, 356–362. [Google Scholar] [CrossRef]
- Michelsson, K.; Rinne, A.; Paajanen, S. Crying, feeding and sleeping patterns in 1 to 12-month-old infants. Child Care Health Dev. 1990, 16, 99–111. [Google Scholar] [CrossRef]
- Partty, A.; Kalliomaki, M.; Salminen, S.; Isolauri, E. Infantile Colic Is Associated With Low-grade Systemic Inflammation. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 691–695. [Google Scholar] [CrossRef]
- de Weerth, C.; Fuentes, S.; de Vos, W.M. Crying in infants: On the possible role of intestinal microbiota in the development of colic. Gut Microbes 2013, 4, 416–421. [Google Scholar] [CrossRef]
- Rhoads, J.M.; Collins, J.; Fatheree, N.Y.; Hashmi, S.S.; Taylor, C.M.; Luo, M.; Hoang, T.K.; Gleason, W.A.; Van Arsdall, M.R.; Navarro, F.; et al. Infant Colic Represents Gut Inflammation and Dysbiosis. J. Pediatr. 2018, 203, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Renes, I.B.; Beaufrand, C.; Van Limpt, K.; Tondereau, V.; Theodorou, V.; Knol, J.; Eutamene, H. A partly fermented infant milk formula with scGOS/lcFOS reduces stress-induced gut permeability and gut hypersensitivity in rats. In Proceedings of the ESPGHAN, Geneva, Switzerland, 9–12 May 2018. [Google Scholar]
| Experimental Group | Control Group | Breastfed Reference | |
|---|---|---|---|
| n = 94 | n = 105 | n = 100 | |
| Infant sex, % male/female | 50.0/50.0 | 49.5/50.5 | 50.0/50.0 |
| Gestational Age (wks), Median (Q1–Q3) | 40.0 (38.0–40.0) | 39.0 (38.0–40.0) | 40.0 (39.0–41.0) |
| Country, % Italy/Spain | 5.3/94.7 | 7.6/92.4 | 5.0/95.0 |
| Birth weight (g), Median (Q1–Q3) | 3265 (2960–3550) | 3170 (2932–3520) | 3350 (3015–3650) |
| Birth length (g), Median (Q1–Q3) | 50.0 (48.5–51.0) | 50.0 (48.0–51.0) | 50.0 (49.5–52.0) |
| Mode of delivery, % vaginal/c-section 1 | 68.1/31.9 | 61.0/38.1 | 82.0/17.0 |
| Order in household, % 1, 2, ≥3 | 86.2/12.8/1.1 | 81.9/15.2/2.9 | 84.0/11.1/5.0 |
| Maternal age (years; mean ± SD) | 32.9 ± 5.0 | 33.2 ± 5.0 | 32.3 ± 3.8 |
| Maternal BMI (kg/m2; mean ± SD) | 26.0 ± 5.8 | 25.2 ± 4.8 | 24.6 ± 5.0 |
| Maternal education, % | |||
| Primary School | 15 | 18 | 11 |
| High School | 47 | 51 | 43 |
| University | 36 | 29 | 41 |
| Age at inclusion (days), Median (Q1–Q3) | 14.0 (5.0–24.0) | 15.0 (5.0–25.0) | 23.0 (9.5–27.0) |
| Adverse Events | Experimental Group n = 94 | Control Group n = 104 | Breastfed Reference n = 105 | |
|---|---|---|---|---|
| n (%) | n (%) | p-Value | n (%) | |
| Any Gastrointestinal Event | 13 (13.8) | 19 (18.3) | 0.443 | 6 (6.0) |
| Abdominal Pain | 1 (1.1) | 0 (0) | 0.475 | 0 (0) |
| Constipation | 6 (6.4) | 9 (8.7) | 0.600 | 3 (3.0) |
| Diarrhoea | 1 (1.1) | 0 (0) | 0.475 | 0 (0) |
| Flatulence | 2 (2.1) | 1 (1.0) | 0.605 | 0 (0) |
| Gastroesophageal Reflux Disease | 2 (2.1) | 3 (2.9) | 1.000 | 2 (2.0) |
| Infantile Colic | 1 (1.1) * | 9 (8.7) | 0.020 | 1 (1.0) |
| Regurgitation | 1 (1.1) | 0 (0) | 0.475 | 1 (1.0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Herrera, A.; Mulder, K.; Bouritius, H.; Rubio, R.; Muñoz, A.; Agosti, M.; Lista, G.; Corvaglia, L.; Ludwig, T.; Abrahamse-Berkeveld, M.; et al. Gastrointestinal Tolerance, Growth and Safety of a Partly Fermented Formula with Specific Prebiotics in Healthy Infants: A Double-Blind, Randomized, Controlled Trial. Nutrients 2019, 11, 1530. https://doi.org/10.3390/nu11071530
Rodriguez-Herrera A, Mulder K, Bouritius H, Rubio R, Muñoz A, Agosti M, Lista G, Corvaglia L, Ludwig T, Abrahamse-Berkeveld M, et al. Gastrointestinal Tolerance, Growth and Safety of a Partly Fermented Formula with Specific Prebiotics in Healthy Infants: A Double-Blind, Randomized, Controlled Trial. Nutrients. 2019; 11(7):1530. https://doi.org/10.3390/nu11071530
Chicago/Turabian StyleRodriguez-Herrera, Alfonso, Kelly Mulder, Hetty Bouritius, Rocio Rubio, Antonio Muñoz, Massimo Agosti, Gianluca Lista, Luigi Corvaglia, Thomas Ludwig, Marieke Abrahamse-Berkeveld, and et al. 2019. "Gastrointestinal Tolerance, Growth and Safety of a Partly Fermented Formula with Specific Prebiotics in Healthy Infants: A Double-Blind, Randomized, Controlled Trial" Nutrients 11, no. 7: 1530. https://doi.org/10.3390/nu11071530
APA StyleRodriguez-Herrera, A., Mulder, K., Bouritius, H., Rubio, R., Muñoz, A., Agosti, M., Lista, G., Corvaglia, L., Ludwig, T., Abrahamse-Berkeveld, M., & Perez-Navero, J. L. (2019). Gastrointestinal Tolerance, Growth and Safety of a Partly Fermented Formula with Specific Prebiotics in Healthy Infants: A Double-Blind, Randomized, Controlled Trial. Nutrients, 11(7), 1530. https://doi.org/10.3390/nu11071530






