A Review of Various Antioxidant Compounds and their Potential Utility as Complementary Therapy in Multiple Sclerosis
Abstract
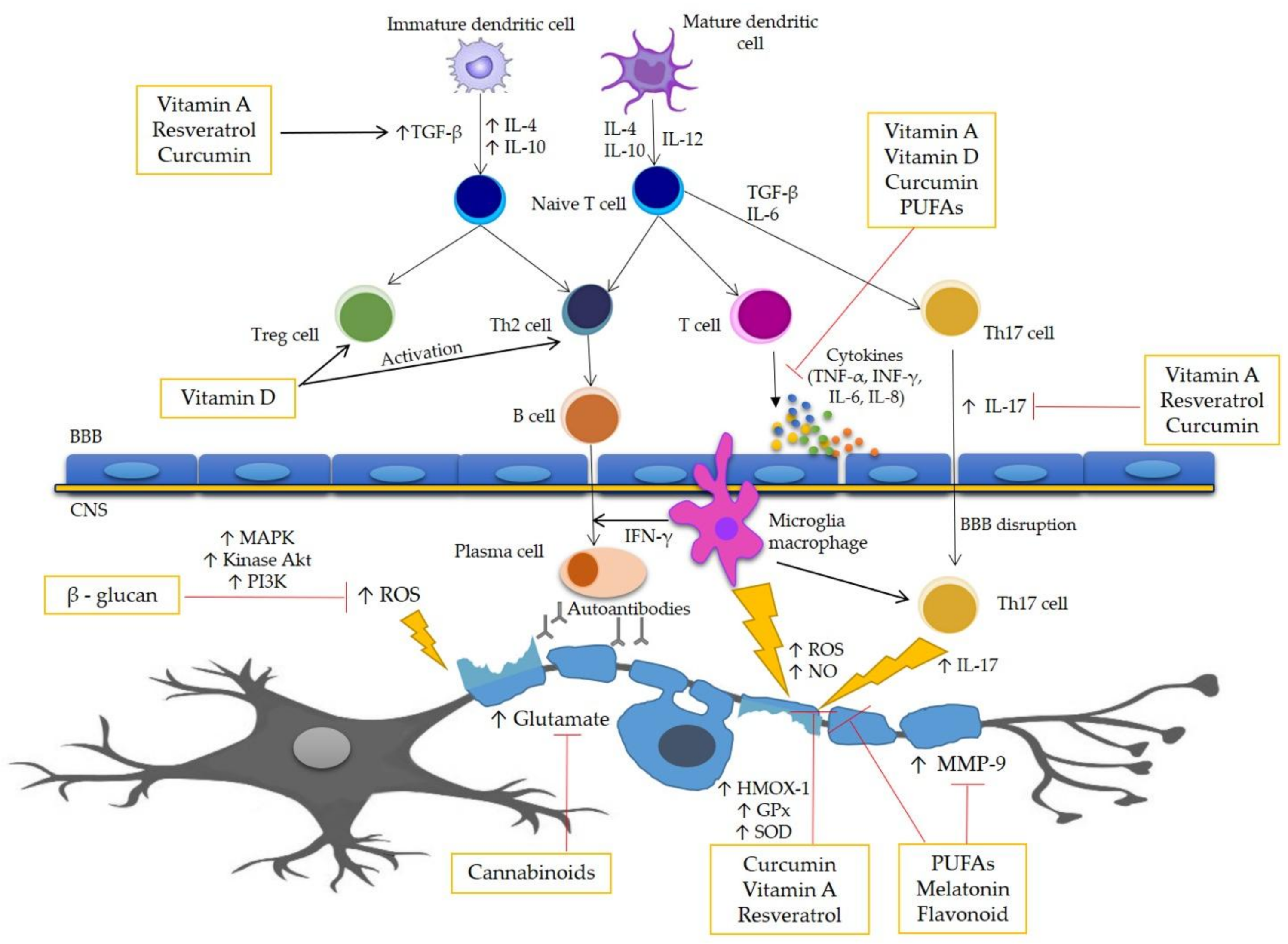
:1. Introduction
2. Pathogenesis of MS
3. Oxidative Stress in MS
4. Antioxidant Compounds as Complementary Therapy in MS
4.1. Curcumin
4.2. Melatonin
4.3. Vitamin D
4.4. Omega-3 Polyunsaturated Fatty Acids (Omega-3 PUFAs)
4.5. Vitamin A
4.6. Flavonoids
4.7. Resveratrol
4.8. Β-glucan
5. Associations between Dietary Patterns and MS
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, E. Multiple sclerosis. Adv. Exp. Med. Biol. 2012, 724, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Bielekova, B.; Martin, R. Development of biomarkers in multiple sclerosis. Brain 2004, 127, 1463–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohl, K.; Tenbrock, K.; Kipp, M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp. Neurol. 2016, 277, 58–67. [Google Scholar] [CrossRef]
- Bendszus, M.; Storch-Hagenlocher, B. Multiple sclerosis and other demyelinating diseases. In Inflammatory Diseases of the Brain; Hähnel, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–18. [Google Scholar]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation induces neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar] [PubMed]
- Lublin, F.D.; Reingold, S.C. Defining the clinical course of multiple sclerosis: Results of an international survey. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, S.P.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis. The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Trapp, B.D. Relapsing and progressive forms of multiple sclerosis—Insights from pathology. Curr. Opin. Neurol. 2014, 27, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.P.; Simonsen, H.; Frederiksen, J.L.; Rostrup, E.; Larsson, H.B. Abnormal blood-brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by MRI. Neuroimage Clin. 2013, 4, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Ransohoff, R.M. The ins and outs of T-lymphocyte trafficking to the CNS: Anatomical sites and molecular mechanisms. Trends Immunol. 2005, 26, 485–495. [Google Scholar] [CrossRef]
- Ziemssen, T.; Ziemssen, F. The role of the humoral immune system in multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE). Autoimmun. Rev. 2005, 4, 460–467. [Google Scholar] [CrossRef]
- Lee, D.H.; Gold, R.; Linker, R.A. Mechanisms of oxidative damage in multiple sclerosis and neurodegenerative diseases: Therapeutic modulation via fumaric acid esters. Int. J. Mol. Sci. 2012, 13, 11783–11803. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. The role of oxidative stress in the pathogenesis of multiple sclerosis: The need for effective antioxidant therapy. J. Neurol. 2004, 251, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, J.; Witte, M.E.; Schreibelt, G.; deVries, H.E. Radical changes in multiple sclerosis pathogen-esis. Biochim. Biophys. Acta 2011, 1812, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Van der Goes, A.; Wouters, D.; Van Der Pol, S.M.; Huizinga, R.; Ronken, E.; Adamson, P.; Greenwood, J.; Dijkstra, C.D.; De Vries, H.E. Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. FASEB J. 2001, 15, 1852–1854. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Bitzer-Quintero, O.K.; Ramírez-Anguiano, A.C.; Flores-Alvarado, L.J.; Ramírez-Ramírez, V.; Macias-Islas, M.A.; Torres-Sánchez, E.D. Immunology and oxidative stress in multiple sclerosis: Clinical and basic approach. Clin. Dev. Immunol. 2013, 2013, 708659. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, C.; Alvarez, J.I.; Prat, A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011, 585, 3770–3780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vukusic, S.; Confavreux, C. Natural history of multiple sclerosis: Risk factors and prognostic indicators. Curr. Opin. Neurol. 2007, 20, 269–274. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, H.P.; Kappos, L.; Lublin, F.D.; Metz, L.M.; McFarland, H.F.; O’Connor, P.W.; et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 2005, 58, 840–846. [Google Scholar] [CrossRef]
- Witherick, J.; Wilkins, A.; Scolding, N.; Kemp, K. Mechanisms of oxidative damage in multiple sclerosis and a cell therapy approach to treatment. Autoimmune Dis. 2011, 2011, 164608. [Google Scholar] [CrossRef]
- Gonsette, R.E. Oxidative stress and excitotoxicity: A therapeutic issue in multiple sclerosis? Mult. Scler. J. 2008, 14, 22–34. [Google Scholar] [CrossRef]
- Nair, A.; Frederick, T.J.; Miller, S.D. Astrocytes in multiple sclerosis: A product of their environment. Cell. Mol. Life Sci. 2008, 65, 2702–2720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimsa, U.; Mitchison, N.A.; Brunner-Weinzierl, M.C. Immune privilege as an intrinsic CNS property: Astrocytes protect the CNS against T-cell-mediated neuroinflammation. Mediat. Inflamm. 2013, 2013, 320519. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; van Horssen, J. Oxidativestressanditsimpactonneuronsandgliainmultiplesclerosislesions. Biochim. Biophys. Acta 2016, 1862, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Contestabile, A.; Monti, B.; Polazzi, E. Neuronal-glial Interactions Define the Role of Nitric Oxide in Neural Functional Processes. Curr. Neuropharmacol. 2012, 10, 303–310. [Google Scholar] [CrossRef]
- Mahad, D.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef]
- McFarland, R.; Taylor, R.W.; Turnbull, D.M. A neurological perspective on mitochondrial disease. Lancet Neurol. 2010, 9, 829–840. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Gilgun-Sherk, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Reddy, P.H. Is multiple sclerosis a mitochondrial disease? Biochim. Biophys. Acta 2010, 1802, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Burté, F.; Carelli, V.; Chinnery, P.F.; Yu-Wai-Man, P. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat. Rev. Neurol. 2015, 11, 11–24. [Google Scholar] [CrossRef]
- Pathak, D.; Berthet, A.; Nakamura, K. EnergyFailure: Does It Contribute to Neurodegeneration? Ann. Neurol. 2013, 74, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Barry, J.; Fritz, M.; Brender, J.R.; Smith, P.E.; Lee, D.K.; Ramamoorthy, A. Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: The case of the antioxidant curcumin. J. Am. Chem. Soc. 2009, 131, 4490–4498. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef]
- Miller, E.; Markiewicz, Ł.; Kabziński, J.; Odrobina, D.; Majsterek, I. Potential of redox therapies in neuro-degenerative disorders. Front. Biosci. 2017, 9, 214–234. [Google Scholar] [CrossRef]
- Kimura, K.; Teranishi, S.; Fukuda, K.; Kawamoto, K.; Nishida, T. Delayed disruption of barrier function in cultured human corneal epithelial cells induced by tumor necrosis factor-alpha in a manner dependent on NF-kappaB. Investig. Ophthalmol. Vis. Sci. 2008, 49, 565–571. [Google Scholar] [CrossRef]
- Xie, L.; Li, X.K.; Funeshima-Fuji, N.; Kimura, H.; Matsumoto, Y.; Isaka, Y.; Takahara, S. Amelioration of experimental autoimmune encephalomyelitis by curcumin treatment through inhibition of IL-17 produc-tion. Int. Immunopharmacol. 2009, 9, 575–581. [Google Scholar] [CrossRef]
- Dattilo, S.; Mancuso, C.; Koverech, G.; Di Mauro, P.; Ontario, M.L.; Petralia, C.C.; Petralia, A.; Maiolino, L.; Serra, A.; Calabrese, E.J.; et al. Heat shock proteins and hormesis in the diagnosis and treatment of neurodegenerative diseases. Immun. Ageing 2015, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Mary, C.P.V.; Vijayakumar, S.; Shankar, R. Metal chelating ability and antioxidant properties of Curcu-min-metal complexes—A DFT approach. J. Mol. Graph. Model. 2018, 79, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.; Al-Suhaimi, E.A.; Wahid, F.; Shehzad, O.; Shehzad, A. Therapeutic potential of curcumin for multiple sclerosis. Neurol. Sci. 2018, 39, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21st Century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, C.; Bright, J.J. Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through Janus Kinase-STAT pathway in T lymphocytes. J. Immunol. 2002, 169, 6506–6513. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Sadeghizadeh, M.; Najafi, F.; Javan, M. Polymerized nano-curcumin attenuates neurologi-cal symptoms in EAE model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair. Neuropharmacology 2015, 99, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Grivas, T.B.; Savvidou, O.D. Melatonin the “light of night” in human biology and adolescent idiopathic scoliosis. Scoliosis 2007, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Assesment Report Fir Circadin. Evaluation of Medicines for Human Use. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-Scientific_Discussion/human/000695/ WC500026808.pdf (accessed on 22 June 2019).
- Slominski, A.; Semak, I.; Pisarchik, A.; Sweatman, T.; Szczesniewski, A.; Wortsman, J. Cobversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002, 5111, 102–106. [Google Scholar] [CrossRef]
- Górska-Warsewicz, H.; Laskowski, W.; Kulykovets, O.; Kudlińska-Chylak, A.; Czeczotko, M.; Rejman, K. Food Products as Source of Protein and Amino Acids—The Case of Poland. Nutrients 2018, 10, 1977. [Google Scholar] [CrossRef]
- Lieben, C.K.; Blokland, A.; Deutz, N.E.; Jansen, W.; Han, G.; Hupperts, R.M. Intake of tryptophan-enriched whey protein acutely enhances recall of positive loaded words in patients with multiple sclerosis. Clin. Nutr. 2018, 37, 321–328. [Google Scholar] [CrossRef]
- Hickman, A.B.; Klein, D.C.; Dyda, F. Melatonin biosynthesis: The structure of serotonin N-acetyltransferase at 2.5 A resolution suggests a catalytic mechanism. Mol. Cell 1999, 3, 23–32. [Google Scholar] [CrossRef]
- Rathnasamy, G.; Ling, E.-A.; Kaur, C. Therapeutic implications of melatonin in cerebral edema. Histol. Histopathol. 2014, 29, 1525–1538. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiey, A.; Asghari, B.; Ghalamfarsa, G.; Jadidi-Niaragh, F.; Azizi, G. The Significance of matrix metalloproteinases in the immunopathogenesis and treatment of multiple sclerosis. Sultan Qaboos Univ. Med. J. 2014, 14, e13–e25. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Wakatsuki, A.; Kaneda, C. Melatonin increases activities of glutathione peroxidase and superoxide dismutase in fetal rat brain. J. Pineal Res. 2000, 28, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Walczak, A.; Majsterek, I.; Kędziora, J. Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J. Neuroimmunol. 2013, 257, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Melamud, L.; Golan, D.; Luboshitzky, R.; Lavi, I.; Miller, A. Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J. Neurol. Sci. 2012, 314, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Patzek, S.; Wang, Y. Physiologica and pathophysiologic roles of extra renal CYP27b1: Case report and review. Bone Rep. 2018, 8, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Ložnjak, P.; Jakobsen, J. Stability of vitamin D3 and vitamin D2 in oil, fish and mushrooms after household cooking. Food Chem. 2018, 254, 144–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, M.S.; Liu, Y.; Gray, O.M.; Baker, J.E.; Kolbe, S.C.; Ditchfield, M.R.; Egan, G.F.; Mitchell, P.J.; Harrison, L.C.; Butzkueven, H. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology 2011, 77, 1611–1618. [Google Scholar] [CrossRef] [Green Version]
- Dudani, S.J.; Kalhan, S.; Sharma, S.P. Vitamin D and multiple sclerosis: Potential pathophysiological role and clinical implications. Int. J. Appl. Basic Med. Res. 2011, 1, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Jones, A.P.; Trend, S.; Cha, L.; Fabis-Pedrini, M.J.; Cooper, M.N.; d’Este, C.; Geldenhuys, S.; Carroll, W.M.; Byrne, S.N.; et al. A randomized, controlled clonical trial of narrowband UVB phototherapy for clinically isolated syndrome: The PhoCIS study. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318773112. [Google Scholar] [CrossRef] [PubMed]
- Salzer, J.; Hallmans, G.; Nyström, M.; Stenlund, H.; Wadell, G.; Sundström, P. Vitamin D as a protective factor in multiple sclerosis. Neurology 2012, 79, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.R.; Simão, A.N.C.; Alfieri, D.F.; Flauzino, T.; Kallaur, A.P.; Mezzaroba, L.; Lozovoy, M.A.B.; Sabino, B.S.; Ferreira, K.P.Z.; Pereira, W.L.; et al. Vitamin D deficiency is associated with disability and disease progression in multiple sclerosis patients independently of oxidative and nitrosative stress. J. Neurol. Sci. 2017, 381, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Spach, K.M.; Hayes, C.E. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J. Immunol. 2005, 175, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Nashold, F.E.; Hoag, K.A.; Goverman, J.; Hayes, C.E. Rag-1-dependent cells are necessary for 1,25-dihydroxyvitamin D3 prevention of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2001, 119, 16–29. [Google Scholar] [CrossRef]
- Nashold, F.E.; Miller, D.J.; Hayes, C.E. 1,25-dihydroxyvitamin D3 treatment decreases macrophage accumulation in the CNS of mice with experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2000, 103, 171–179. [Google Scholar] [CrossRef]
- Spach, K.M.; Pedersen, L.B.; Nashold, F.E.; Kayo, T.; Yandell, B.S.; Prolla, T.A.; Hayes, C.E. Gene expression analysis suggests that 1,25-dihydroxyvitamin D3 reverse experimental autoimmune encephalomyelitis by stimulating inflammatory cell apoptosis. Physiol. Genom. 2004, 18, 141–151. [Google Scholar] [CrossRef]
- Mayne, C.G.; Spanier, J.A.; Relland, L.M.; Williams, C.B.; Hayes, C.E. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2011, 41, 822–832. [Google Scholar] [CrossRef]
- Soilu-Hänninen, M.; Aivo, J.; Lindström, B.M.; Elovaara, I.; Sumelahti, M.L.; Färkkilä, M.; Tienari, P.; Atula, S.; Sarasoja, T.; Herrala, L.; et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J. Neurol. Neurosurg Psychiatry 2012, 83, 565–571. [Google Scholar] [CrossRef]
- Shaygannejad, V.; Janghorbani, M.; Ashtari, F.; Dehghan, H. Effects of adjunct low-dose vitamin d on relapsing-remitting multiple sclerosis progression: Preliminary findings of a randomized placebo-controlled trial. Mult. Scler. Int. 2012, 2012, 452541. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, D.L.; Healy, B.C.; Muhammad, T.M.; Carruthers, R.L.; Musallam, A.J.; Kivisakk, P.; Weiner, H.L.; Glanz, B.; Chitnis, T. Effect of vitamin D on MS activity by disease-modifying therapy class. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stweart, N.; Simpson, S.; van der Mei, I.; Ponsonby, A.L.; Blizzard, L.; Dwyer, T.; Pittas, F.; Eyles, D.; Ko, P.; Taylor, B.V. Interferon-β and serum 25-hydroxyvitamin D interact to modulate relapse risk in MS. Neurology 2012, 79, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Gallai, V.; Sarchielli, P.; Trequattrini, A.; Franceschini, M.; Floridi, A.; Firenze, C.; Alberti, A.; Di Bene-detto, D.; Stragliotto, E. Cytokine secretion and eicosanoid production in the peripheral blood mononu-clear cells of MS patients undergoing dietary supplementation with n-3 polyunsaturated fatty acids. J. Neuroimmunol. 1995, 56, 143–153. [Google Scholar] [CrossRef]
- Shinto, L.; Marracci, G.; Bumgarner, L.; Yadav, V. The Effects of omega-3 fatty acids on matrix metallo-proteinase-9 production and cell migration in human immune cells: Implications for multiple sclerosis. Autoimmune Dis. 2011, 2011, 134592. [Google Scholar] [CrossRef]
- Sakai, C.; Ishida, M.; Ohba, H.; Yamashita, H.; Uchida, H.; Yoshizumi, M.; Ishida, T. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS ONE 2017, 12, e0187934. [Google Scholar] [CrossRef]
- Pantzaris, M.C.; Loukaides, G.N.; Ntzani, E.E.; Patrikios, I.S. A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: A randomised, double-blind, placebo-controlled proof-of-concept clinical trial. BMJ Open 2013, 3, e002170. [Google Scholar] [CrossRef]
- Shinto, L.; Marracci, G.; Mohr, D.C.; Bumgarner, L.; Murchison, C.; Senders, A.; Bourdette, D. Omega-3 Fatty Acids for Depression in Multiple Sclerosis: A Randomized Pilot Study. PLoS ONE 2016, 11, e0147195. [Google Scholar] [CrossRef]
- Torkildsen, O.; Wergeland, S.; Bakke, S.; Beiske, A.G.; Bjerve, K.S.; Hovdal, H.; Midgard, R.; Lilleås, F.; Pedersen, T.; Bjørnarå, B.; et al. ω-3 fatty acid treatment in multiple sclerosis (OFAMS Study): A randomized, double-blind, placebo-controlled trial. Arch. Neurol. 2012, 69, 1044–1051. [Google Scholar] [CrossRef]
- Shearer, K.D.; Stoney, P.N.; Morgan, P.J.; McCaffery, P.J. A vitamin for the brain. Trends Neurosci. 2012, 35, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Royal, W., 3rd; Gartner, S.; Gajewski, C.D. Retinol measurements and retinoid receptor gene expression in pa-tients with multiple sclerosis. Mult. Scler. 2002, 8, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Raverdeau, M.; Breen, C.J.; Misiak, A.; Mills, K.H. Retinoicacid suppresses IL-17productionandpathogenic activity of γδT-cells in CNS autoimmunity. Immunol. Cell Biol. 2016, 94, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Saboor-Yaraghi, A.A.; Harirchian, M.H.; Mohammadzadeh Honarvar, N.; Bitarafan, S.; Abdolahi, M.; Siassi, F.; Salehi, E.; Sahraian, M.A.; Eshraghian, M.R.; Roostaei, T.; et al. The Effect of vitamin A supplementation on Foxp3 and TGF-β gene expression in Avonex-treated multiple sclerosis patients. J. Mol. Neurosci. 2015, 56, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Grainger, J.R.; Spencer, S.P.; Belkaid, Y. The Role of Retinoic Acid in Tolerance and Immunity. Immunity 2011, 35, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pino-Lagos, K.; Benson, M.J.; Noelle, R.J. Retinoic acid in the immune system. Ann. N. Y. Acad. Sci. 2008, 1143, 170–187. [Google Scholar] [CrossRef] [PubMed]
- Dorosty-Motlagh, A.R.; Honarvar, M.N.; Sedighiyan, M.; Abdolahi, M. The molecular mechanisms of vitamin A deficiency in multiple sclerosis. J. Mol. Neurosci. 2016, 60, 82–90. [Google Scholar] [CrossRef]
- Ahlemeyer, B.; Bauerbach, E.; Plath, M.; Steuber, M.; Heers, C.; Tegtmeier, F.; Krieglstein, J. Retinoic acid reduces apoptosis and oxidative stress by preservation of SOD protein level. Free Radic. Biol. Med. 2001, 30, 1067–1077. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushali, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Karak, P. Biological activities of flavonoids: An overview. IJPSR 2019, 3, 1567–1574. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, Y.; Xu, Y.; Han, X.; Peng, J.; Liu, K.; Sun, C.K. Protective effect of flavonoid-rich extract from Rosa laevigata Michx on cerebral ischemia-reperfusion injury through suppression of apoptosis and inflammation. Neurochem. Int. 2013, 63, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Banjarnahor, S.D.S.; Artant, N. Antioxidant properties of flavonoids. Med. J. Indones. 2014, 23, 239–244. [Google Scholar] [CrossRef]
- Wu, D. Green tea EGCG, T-cell function, and T-cell-mediated autoimmune encephalomyelitis. J. Investig. Med. 2016, 64, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Aktas, O.; Prozorovski, T.; Smorodchenko, A.; Savaskan, N.E.; Lauster, R.; Kloetze, P.M.; Infante-Duarte, C.; Brocke, S.; Zipp, F. Green tea epigallocatechin-3-gallate mediates T-cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J. Immunol. 2004, 173, 5794–5800. [Google Scholar] [CrossRef] [PubMed]
- Inami, S.; Takano, M.; Yamamoto, M.; Murakami, D.; Tajika, K.; Yokoyama, S.; Ohno, N.; Ohba, T.; Sano, J.; Ibuki, C.; et al. Tea catechin consumption reduces circulating oxidized low-density lipoprotein. Int. Heart J. 2007, 48, 725–732. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, L.; Yang, L.; Wang, X. Epigallocatechin Gallate Is the Most Effective Catechin Against Antiox-idant Stress via Hydrogen Peroxide and Radical Scavenging Activity. Med. Sci. Monit. 2018, 24, 8198–8206. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Semnani, M.; Mashayekhi, F.; Azarnia, M.; Salehi, Z. Effects of green tea epigallocatechin-3-gallate on the proteolipid protein and oligodendrocyte transcription factor 1 messenger RNA gene expression in a mouse model of multiple sclerosis. Folia Neuropathol. 2017, 55, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Herges, K.; Milliward, J.M.; Hentschel, N.; Infante-Duarte, C.; Aktas, O.; Zipp, F. Neuroprotective Effect of Combination Therapy of Glatiramer Acetate and Epigallocatechin-3-Gallate in Neuroinflammation. PLoS ONE 2011, 6, e25456. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, Z.; Xu, Y.; Xiao, S.; Meydani, S.N.; Wu, D. Epigallocatechin-3-gallate ameliorates experimental autoimmune encephalomyelitis by altering balance among CD4+ T-cell subsets. Am. J. Pathol. 2012, 180, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Mähler, A.; Steiniger, J.; Bock, M.; Klug, L.; Perreidt, N.; Lorenz, M.; Zimmermann, B.F.; Krannich, A.; Paul, F.; Boschmann, M. Metabolic response to epigallocatechin-3-gallate in relapsing-remitting multiple sclerosis: A randomized clinical trial. Am. J. Clin. Nutr. 2015, 101, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Anti-oxidative action of resveratrol: Implications for human health. Arab. J. Chem. 2011, 4, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Brito, P.; Almeida, L.M.; Dinis, T.C. The interaction of resveratrol with ferrylmyoglobin and peroxyni-trite; protection against LDL oxidation. Free Radic. Res. 2002, 36, 621–631. [Google Scholar] [CrossRef]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef]
- Shindler, K.S.; Ventura, E.; Dutt, M.; Elliott, P.; Fitzgerald, D.C.; Rostami, A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J. Neuroophthalmol. 2010, 30, 328–339. [Google Scholar] [CrossRef]
- Singh, N.P.; Hegde, V.L.; Hofseth, L.J.; Nagarkatti, M.; Nagarkatti, P. Resveratrol (trans-3,5,4′-trihy-droxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T-cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol. Pharmacol. 2007, 72, 1508–1521. [Google Scholar] [CrossRef]
- Sofi, S.A.; Singh, J.; Rafiq, S. β-Glucan and functionality: A review. EC Nutr. 2017, 10, 67–74. [Google Scholar]
- Chen, J.; Seviour, R. Medicinal importance of fungal beta-(1→3), (1→6)-glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Grünebach, F.; Weck, M.M.; Reichert, J.; Brossart, P. Molecular and functional characterization of human Dectin-1. Exp. Hematol. 2002, 30, 1309–1315. [Google Scholar] [CrossRef]
- Lebron, F.; Vassallo, R.; Puri, V.; Limper, A.H. Pneumocystis carinii cell wall beta-glucans initiate macrophage inflammatory responses through NF-kappaB activation. J. Biol. Chem. 2003, 278, 25001–25008. [Google Scholar] [CrossRef] [PubMed]
- Assanasen, C.; Mineo, C.; Seetharam, D.; Yuhanna, I.S.; Marcel, Y.L.; Connelly, M.; Williams, D.L.; de la Llera-Moya, M.; Shaul, P.W.; Silver, D.L. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J. Clin. Investig. 2005, 115, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Opizzi, A.; Monteferrario, F. The biological activity of beta-glucans. Minerva Med. 2009, 100, 237–245. [Google Scholar] [PubMed]
- Salim, H.A.; Abd-Allah, O.A.; Fararh, K.M. Clinicopathological study on the effect of beta-glucan on hematological, immunological and biochemical changes in broiler chicks. Benha Vet. Med. J. 2011, 22, 68–77. [Google Scholar]
- Kogan, G.; Stasko, A.; Bauerova, K.; Polovka, M.; Soltes, L.; Brezova, V.; Navarova, J.; Mihalova, D. Antioxidant properties of yeast (1→3)-β-glucan studied by electron paramagnetic resonance spectroscopy and its activity in the adjuvant arthritis. Carbohydr. Polym. 2005, 61, 18–28. [Google Scholar] [CrossRef]
- Akramiene, D.; Kondrotas, A.; Didziapetriene, J.; Kevelatis, E. Effects of beta-glucans on the immune system. Medicina 2007, 43, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of beta-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef]
- Alternative Healing. Your Guide to Better Health. Available online: http://alternativa-za-vas.com/en/index.php/clanak/article/multiple-sclerosis (accessed on 22 June 2019).
- Dixon, S. Nutrition in complementary and alternative medicine. Semin. Oncol. Nurs. 2012, 28, 75–84. [Google Scholar] [CrossRef]
- Pucci, E.; Cartechini, E.; Taus, C.; Giuliani, G. Why physicians need to look more closely at the use of complementary and alternative medicine by multiple sclerosis patients. Eur. J. Neurol. 2004, 11, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Leong, E.M.; Semple, S.J.; Angley, M.; Siebert, W.; Petkov, J.; McKinnon, R.A. Complementary and alternative medicines and dietary interventions in multiple sclerosis: What is being used in South Australia and why? Complement. Ther. Med. 2009, 17, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Dayapoglu, N.; Tan, M. Use of complementary and alternative medicine among people with multiple sclerosis in eastern turkey. Neurol. Asia 2016, 21, 63–71. [Google Scholar]
- Nayak, S.; Matheis, R.J.; Schoenberger, N.E.; Shiflett, S.C. Use of unconventional therapies by individuals with multiple sclerosis. Clin. Rehabil. 2003, 17, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Namjooyan, F.; Ghanavati, R.; Majdinasab, N.; Jokari, S.; Janbozorgi, M. Uses of Com-plementary and Alternative Medicine in Multiple Sclerosis. J. Tradit. Complement. Med. 2014, 4, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Bowling, A.C.; Ibrahim, R.; Stewart, T.M. Alternative medicine and multiple sclerosis: An objective review from an American perspective. Int. J. MS Care 2000, 2, 15–28. [Google Scholar] [CrossRef]
- Marrie, R.A.; Hadjimichael, O.; Vollmer, T. Predictors of alternative medicine use by multiple sclerosis patients. Mult. Scler. J. 2003, 9, 461–466. [Google Scholar] [CrossRef]
- Sand, I.K. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Habek, M.; Hojsak, I.; Brinar, V.V. Nutrition in multiple sclerosis. Clin. Neurol. Neurosurg. 2010, 112, 616–620. [Google Scholar] [CrossRef] [Green Version]
- Beckett, J.M.; Bird, M.-L.; Pittaway, J.K.; Ahuja, K.D.K. Diet and Multiple Sclerosis: Scoping Review of Web-Based Recommendations. Interact. J. Med. Res. 2019, 8, e10050. [Google Scholar] [CrossRef]
- Wahls, T.L.; Chenard, C.A.; Snetselaar, L.G. Review of Two Popular Eating Plans within the Multiple Sclerosis Community: Low Saturated Fat and Modified Paleolithic. Nutrients 2019, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Rountree, R. The Wahls Diet for multiple sclerosis: A clinical conversation with Terry Wahls, and Robert Rountree. Altern. Complement. Ther. 2017, 23, 79–86. [Google Scholar]
- Chenard, C.A.; Rubenstein, L.M.; Snetselaar, L.G.; Wahls, T.L. Nutrient Composition Comparison between a Modified Paleolithic Diet for Multiple Sclerosis and the Recommended Healthy U.S.-Style Eating Pattern. Nutrients 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Bisht, B.; Darling, W.G.; Grossmann, R.E.; Shivapour, E.T.; Lutgendorf, S.K.; Snetselaar, L.G.; Hall, M.J.; Zimmerman, M.B.; Wahls, T.L. A multimodal intervention for patients with secondary progressive multiple sclerosis: Feasibility and effect on fatigue. J. Altern. Complement. Med. 2014, 20, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Bisht, B.; Darling, W.G.; Grossmann, R.E.; Shivapour, E.T.; Lutgendorf, S.K.; Snetselaar, L.G.; Chendar, C.A.; Wahls, L. Miltimodal intervention improves fatigue and quality of life of subjects with progressive multiple sclerosis: A pilot study. Degener. Neurol. Neuromuscul. Dis. 2015, 5, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Reese, D.; Shivapour, E.T.; Wahls, T.L.; Dudley-Javoroski, D.; Shields, R. Neuromuscular electrical stimulation and dietary interventions to reduce oxidative stress in a secondary progressive multiple sclerosis patient leads to marked gains in function: A case report. Cases J. 2009, 2, 7601. [Google Scholar] [CrossRef] [PubMed]
- Swank MS Foundation the Swank Low-Fat Diet for the Treatment of MS. Available online: http://www.swankmsdiet.org/the-diet/ (accessed on 23 June 2019).
- Weinstock-Guttman, B.; Zivadinov, R.; Mahfooz, N.; Carl, E.; Drake, A.; Schneider, J.; Teter, B.; Hussein, S.; Mehta, B.; Weiskopf, M.; et al. Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J. Neuroinflamm. 2011, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhornitsky, S.; McKay, K.A.; Metz, L.M.; Teunissen, C.E.; Rangachari, M. Cholesterol and markers of cholesterol turnover in multiple sclerosis: Relationship with disease outcomes. Mult. Scler. Relat. Disord. 2016, 5, 53–65. [Google Scholar] [CrossRef]
- Marrie, R.A.; Rudick, R.; Horwitz, R.; Cutter, G.; Tyry, T.; Campagnolo, D.; Vollmer, T. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 2010, 74, 1041–1047. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, K. Diet and disease modification in multiple sclerosis: A nutritional epidemiology perspective. J. Neurol. Neurosurg. Psychiatry 2018, 89, 3. [Google Scholar] [CrossRef]
- Azary, S.; Schreiner, T.; Graves, J.; Waldman, A.; Belman, A.; Guttman, B.W.; Aaen, G.; Tillema, J.M.; Mar, S.; Hart, J.; et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Moravejolahkami, A.R.; Paknahad, Z.; Chitsaz, A. Association of dietary patterns with systematic inflammation, quality of life, disease severity, relapse rate, severity of fatigue and anthropometic measurements in MS patients. Nutr. Neurosci. 2019, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Willett, W.C.; Hernán, M.A.; Olek, M.J.; Ascherio, A. Dietary Fat in Relation to Risk of Multiple Sclerosis among Two Large Cohorts of Women. Am. J. Epidemiol. 2000, 152, 1056–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotstein, D.L.; Cortese, M.; Fung, T.T.; Chitnis, T.; Ascherio, A.; Munger, K.L. Diet quality and risk of multiple sclerosis in two cohorts of US women. Mult. Scler. J. 2018, 1352458518807061. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munger, K.L.; White, R.; Köchert, K.; Simon, K.C.; Polman, C.H.; Freedman, M.S.; Hartung, H.P.; Miller, D.H.; Montalbán, X.; et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014, 71, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, V.; Dalla Costa, G.; Colombo, B.; Dalla Libera, D.; Rubinacci, A.; Filippi, M.; Furlan, R.; Comi, G. Vitamin D levels and risk of multiple sclerosis in patients with clinically isolated syndromes. Mult. Scler. 2014, 20, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Niino, M.; Sato, S.; Fukazawa, T.; Masaki, K.; Miyazaki, Y.; Matsuse, D.; Yamasaki, R.; Takahashi, E.; Kikuchi, S.; Kira, J. Decreased serum vitamin D levels in Japanese patients with multiple sclerosis. J. Neuroimmunol. 2015, 279, 40–45. [Google Scholar] [CrossRef]
- Dehghan, M.; Ghaedi-Heidari, F. Environmental Risk Factors for Multiple Sclerosis: A Case-control Study in Kerman, Iran. Iran. J. Nurs. Midwifery Res. 2018, 23, 431–436. [Google Scholar] [CrossRef]
| Compounds | Research Model | Dosage/Days/Criteria | Potential Role in MS | Ref. |
|---|---|---|---|---|
| Curcumin | EAE (adult female Lewis rats) | 100 and 200 mg/kg/day per 14 days | - reduces clinical severity - decreases CNS inflammatory cells infiltration - inhibits neural Ag-specific T cell responses and IL-17 mRNA expression - suppresses gene expression of TGF-β, IL-6, IL-21 and STAT3 in spinal cord - inhibits phosphorylation of STAT3 in Jurkat T cells | [41] |
| Curcumin | EAE (SJL/mice) | 50 and 100 μg /kg/day per 25 days | - inhibits of clinical and pathological symptoms of EAE - decreases CNS inflammation and demyelination in spinal cord - inhibits neural Ag-specific T cell responses - inhibits of MBP-specific T cell responses - inhibits IL-12 production in spleen cells, macrophages and microglia - inhibits IL-12-induced T cell responses - inhibits IL-12-induced tyrosine phosphorylation of STAT3, STAT4, JAK2 and TYK2 in T cells | [46] |
| Polymerized form of nanocurcumin (PNC) | EAE (adult female Lewis rats) | 12.5 mg/kg/day per 29 days | - diminishes pro-inflammatory genes expression: MCP-1, IL-1, IL-17, NFκB in the lumbar spinal cord - augments expression of anti-inflammatory genes expression: IL-4, Foxp3, TGF-β in spinal cord - increases expression of oxidative stress marker genes: iNOS, HMOX-1 and Nrf2 in spinal cord | [47] |
| Melatonin | Fetal rats | 10 mg/kg/day per 20 days | - increases gene expression of anti-oxidant enzymes: SOD and GPx in rat fetal brain | [57] |
| Melatonin | SPMS (n = 16) | 10 mg per 30 days | - increases concentration of SOD and GPx in erythrocytes - decreases concentration of MDA in erythrocytes | [54] |
| 25(OH)D | MS(n = 256) | MS patients >63.3 nmol/L | - diminishes the risk of MS (high circulating levels of vitamin D ae associated with a lower risk of MS) | [63] |
| 25(OH)D | MS (n = 196) | MS patients ≥75 nmol/L | - decreases (approximately in 61%) risk of MS | [65] |
| 25(OH)D | MS (n = 196) | MS patients ≥50 nmol/L | - enhances disease progression evaluated by MSSS and EDSS - lowers the NOx level in serum | [66] |
| 1,25-(OH)2D3 | EAE (mice) | 0.5 μg/kg/day per 28 days | - down-regulates CYP24A1 gene expression in spinal cord (only in females) | [67] |
| 1,25-(OH)2D3 | EAE (mice) | 50 ng/day (females)/100 ng/day (males) | - limits of occurrence of activated autoreactive T cells in the CNS | [68] |
| 1,25-(OH)2D3 | EAE (mice) | 50 ng/day per 72 h | - reduces accumulation of macrophage in the CNS in spinal cord | [69] |
| 1,25-(OH)2D3 | EAE (B10.PL(73NS)/Sn mice) | 200 ng (in 0.1 mL of soybean oil) | - stimulates inflammatory cell apoptosis, and enhances CNS cell survival in spinal cord | [70] |
| 1,25-(OH)2D3 | EAE (mice) | 50 ng/day (females)/100 ng/day (males) | - acts directly on pathogenic CD4+ T cells and inhibits EAE via VDR in T lymphocytes | [71] |
| 1,25-(OH)2D3 | RRMS (n = 50) | 0.5 μg/day for 12 months | - decreases relapses rate | [73] |
| ESAPENT (with 51% EPA and 31% DHA) | MS (n = 20) | 6 g (in fish oil)/day for 6 months | - suppresses the capacity of monocytes to synthesize IL-1 and TNF-α - decreases in the population of the inflammatory cytokines: IL-1β, IL-2, TNF-α, and IFN-γ | [77] |
| EPA and DHA | Healthy volunteers (PBMCs) | 10 μg/mL,25 μg/mL, and 50 μg/mL for 3 months | - decreases level of MMP-9 in PBMCs - inhibits MMP-9 activity - modulates immune cell production of MMP-9 - inhibits T cell migration | [78] |
| EPA and DHA | MS (n = 80) | EPA 16500 mg and DHA 4650 mg per 30 months | - no beneficial effect on MS patients | [80] |
| TRIOMAR (ω-3 fatty acids) | MS (n = 102) | EPA 270 mg and DHA 170 mg for 24 months | - no beneficial effect on MS patients | [81] |
| RA | EAE (C57BL/6 mice) | 250 μg/kg/day | - inhibits the function of IL-17A-producing γ∆ T cells impairing their proliferation cytokine production and their pathogenic activity - inhibits cytokine production by Th17 cells - suppress IL-1R and IL-23R expression in γ∆ T cells | [85] |
| Vitamin A | RRMS (n = 39) | 400 IU/day for 6 months | - increases expression of TGF-β PBMCs - increases expression of Foxp3 in PBMCs | [86] |
| Flavonoid-rich extract (FRE) | Male Sprague–Dawley rats | 50-200 mg/kg/day per 7 days | - decreases expression of pro-inflammatory cytokines: NFκB, iNOS, COX-2, MMP-9, TNF-α in rats brain - diminishes level of p-ERK, MAPK, and phosphor-p38 in rats brain | [94] |
| EGCG | EAE (Female SJL/J mice) | 300 μg/day per 131 days | - reduces EAE symptoms, brain inflammation, and neuronal damage in mouse brain - inhibits of TNF-α synthesis in T cells - decreases proliferation of CD4+ T cells | [98] |
| Catechins | Healthy volunteers (n = 29) | 500 mg/kg/day for 4 weeks | - reduces plasma oxidized LDL by 18% | [99] |
| EGCG | EAE (C57BL/6 mice) | 50 mg/kg/day for 4 weeks | - increases in PLP and Olig1 expression in cerebral cortex | [100] |
| SRT501 | EAE (SJL/J mice) | 100 mg/kg/day per 30 days | - attenuates neuronal damage and neurological dysfunction in EAE by a mechanism involving SIRT1 activation | [110] |
| Resveratrol | EAE (C57BL/6 mice) | 100 mg/kg/day per 30 days | - decreases the clinical symptoms and inflammatory responses, mainly due to trigger apoptosis in activated T cells in spinal cord and reduces level of pro-inflammatory mediators. | [111] |
| Β-glucan (from baker’s yeast S. cerevisiae) | Broiler chicks | Total volume of carbohydrates from β-glucan 7.5 mg/mL daily per 21 days | - decreases in triglyceride, total cholesterol and glucose concentration (with no significant change in uric acid or creatinine concentration) - increases phagocytic activity and phagocytic index | [118] |
| carboxymethylated (1,3)-β-d-glucan (CMG) | Male Lewis rat | 5 mg/kg/day per 28 days | - shows ability to protects against lipid peroxidation | [119] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, E.D.; Dziedzic, A.; Saluk-Bijak, J.; Bijak, M. A Review of Various Antioxidant Compounds and their Potential Utility as Complementary Therapy in Multiple Sclerosis. Nutrients 2019, 11, 1528. https://doi.org/10.3390/nu11071528
Miller ED, Dziedzic A, Saluk-Bijak J, Bijak M. A Review of Various Antioxidant Compounds and their Potential Utility as Complementary Therapy in Multiple Sclerosis. Nutrients. 2019; 11(7):1528. https://doi.org/10.3390/nu11071528
Chicago/Turabian StyleMiller, Elzbieta Dorota, Angela Dziedzic, Joanna Saluk-Bijak, and Michal Bijak. 2019. "A Review of Various Antioxidant Compounds and their Potential Utility as Complementary Therapy in Multiple Sclerosis" Nutrients 11, no. 7: 1528. https://doi.org/10.3390/nu11071528
APA StyleMiller, E. D., Dziedzic, A., Saluk-Bijak, J., & Bijak, M. (2019). A Review of Various Antioxidant Compounds and their Potential Utility as Complementary Therapy in Multiple Sclerosis. Nutrients, 11(7), 1528. https://doi.org/10.3390/nu11071528







