The Association between Serum Magnesium Levels and Depression in an Adult Primary Care Population
Abstract
:1. Introduction
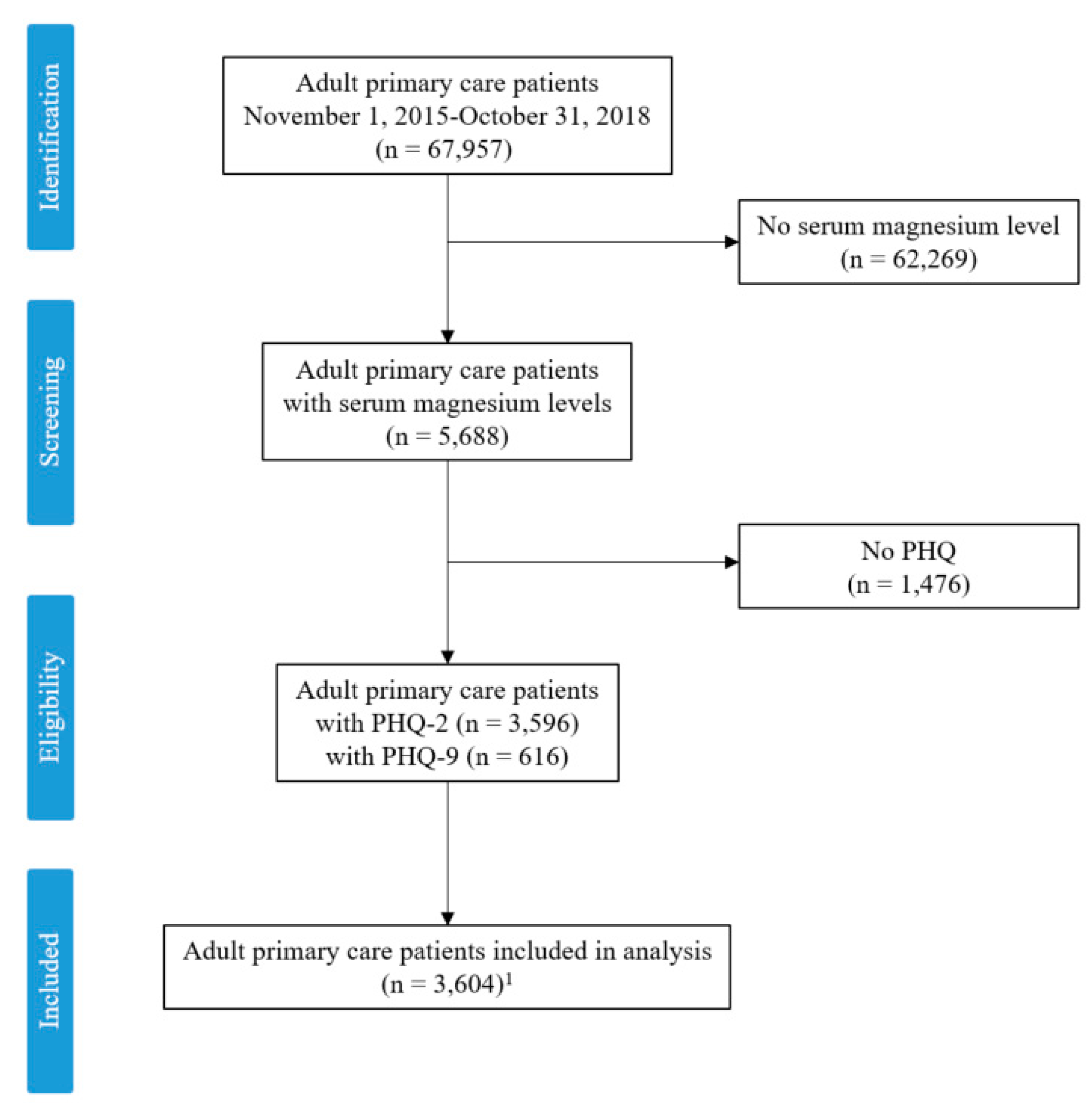
2. Materials and Methods
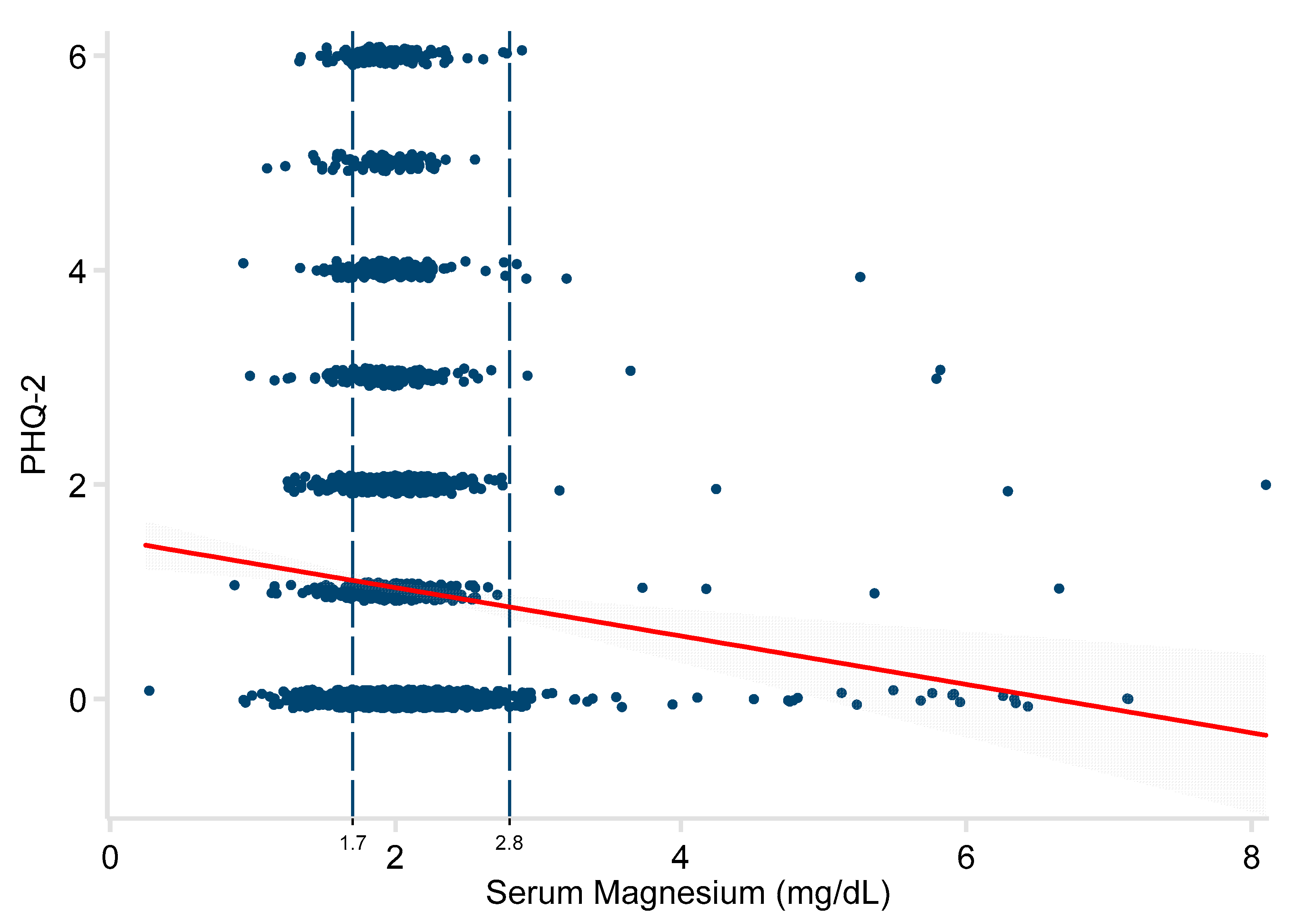
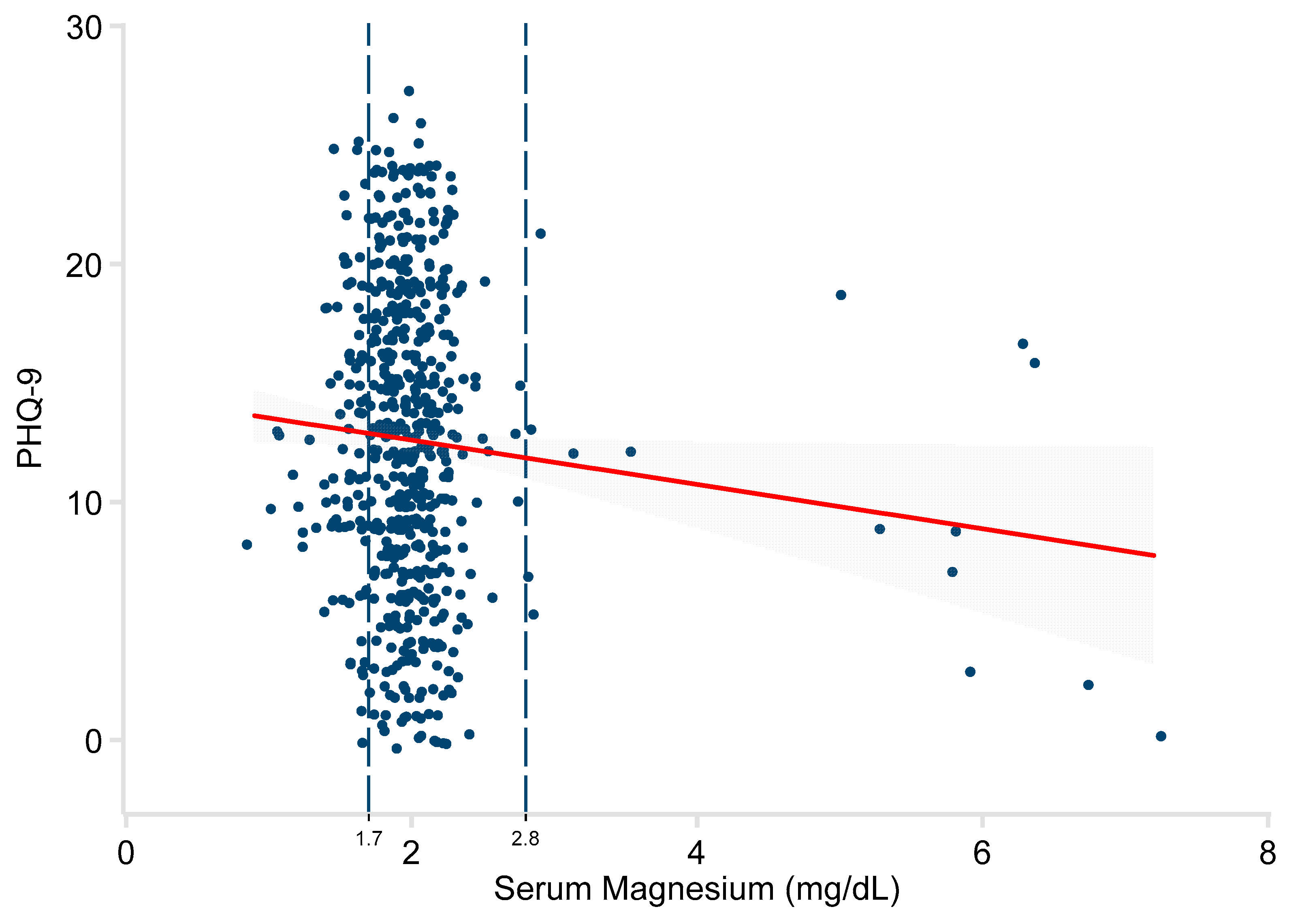
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization. Depression Fact Sheet no. 369. 2012. Available online: http://www.who.int/mediacentre/factsheets/fs369/en/ (accessed on 6 May 2019).
- Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health; Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2017. Available online: https://www.samhsa.gov/data/ (accessed on 6 May 2019).
- Wang, J.; Wu, X.; Lai, W.; Long, E.; Zhang, X.; Li, W. Prevalence of depression and depressive symptoms among outpatients: A systematic review and meta-analysis. BMJ 2017, 7, e017173. [Google Scholar] [CrossRef] [PubMed]
- Ansseau, M.; Dierick, M.; Buntinkx, F.; Cnockaert, P.; De Smedt, J.; Van Den Haute, M. High prevalence of mental disorders in primary care. J. Affect. Disord. 2004, 78, 49–55. [Google Scholar] [CrossRef]
- Siu, A.L.; Bibbins-Domingo, K.; Grossman, D.C.; Baumann, L.C.; Davidson, K.W.; Ebell, M. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA 2016, 315, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Jorm, A.F.; Patten, S.B.; Brugha, T.S.; Mojtabai, R. Has increased provision of treatment reduced the prevalence of common mental disorders? Review of the evidence from four countries. World Psychiatry 2017, 16, 90–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.L.; Lin, C.C. Advances in biomarkers of major depressive disorder. Adv. Clin. Chem. 2015, 68, 177–204. [Google Scholar] [PubMed]
- Tarleton, E.K.; Littenberg, B.; MacLean, C.D.; Kennedy, A.G.; Daley, C. Role of magnesium supplementation in the treatment of depression: A randomized clinical trial. PLoS ONE 2017, 12, e0180067. [Google Scholar] [CrossRef] [PubMed]
- Rajizadeh, A.; Mozaffari-Khosravi, H.; Yassini-Ardakani, M.; Dehghani, A. Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: A randomized, double-blind, placebo-controlled trial. Nutrition 2017, 35, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.L. Magnesium in disease prevention and overall health. Adv. Nutr. 2013, 4, 378s–783s. [Google Scholar] [CrossRef] [PubMed]
- De Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Grober, U.; Schmidt, J.; Kisters, K. Magnesium in prevention and therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H.; Milne, D.B.; Gallagher, S.; Johnson, L.; Hoverson, B. Moderate magnesium deprivation results in calcium retention and altered potassium and phosphorus excretion by postmenopausal women. Magnes. Res. 2007, 20, 19–31. [Google Scholar] [PubMed]
- Martin, K.J.; González, E.A.; Slatopolsky, E. Clinical consequences and management of hypomagnesemia. J. Am. Soc. Nephrol. 2009, 20, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, A.; Sarlo, G.; Holton, K. The role of magnesium in neurological disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Overland, S.; Stewart, R.; Tell, G.S.; Bjelland, I.; Mykletun, A. Association between magnesium intake and depression and anxiety in community-dwelling adults: The Hordaland Health Study. Aust. N. Z. J. Psychiatry 2009, 43, 45–52. [Google Scholar] [CrossRef]
- Yary, T.; Lehto, S.M.; Tolmunen, T.; Tuomainen, T.-P.; Kauhanen, J.; Voutilainen, S. Dietary magnesium intake and the incidence of depression: A 20-year follow-up study. J. Affect. Disord. 2016, 193, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Serefko, A.; Szopa, A.; Poleszak, E. Magnesium and depression. Magnes. Res. 2016, 29, 112–119. [Google Scholar] [CrossRef]
- Szewczyk, B.; Szopa, A.; Serefko, A.; Poleszak, E.; Nowak, G. The role of magnesium and zinc in depression: Similarities and differences. Magnes. Res. 2018, 31, 78–89. [Google Scholar]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef]
- Camardese, G.; Risio, L.D.; Pizi, G.; Mattioli, B.; Buccelletti, F.; Serrani, R.; Leone, B.; Sgambato, A.; Bria, P.; Janiri, L. Plasma magnesium levels and treatment outcome in depressed patients. Nutr. Neurosci. 2012, 15, 78–84. [Google Scholar] [CrossRef]
- Derom, M.L.; Sayon-Orea, C.; Martinez-Ortega, J.M.; Martinez-Gonzalez, M.A. Magnesium and depression: A systematic review. Nutr. Neurosci. 2013, 16, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Chernecky, C.; Berger, B. Magnesium-serum. Laboratory Tests and Diagnostic Procedures, 6th ed.; Elsevier Saunders: St. Louis, MO, USA, 2013; pp. 750–751. [Google Scholar]
- Arroll, B.; Goodyear-Smith, F.; Crengle, S.; Gunn, J.; Kerse, N.; Fishman, T.; Falloon, K.; Hatcher, S. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann. Fam. Med. 2010, 8, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.; Williams, J. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Huber, P.J. Robust estimation of a location parameter. Ann. Math. Statist. 1964, 35, 73–101. [Google Scholar] [CrossRef]
- Wittayanukorn, S.; Qian, J.; Hansen, R.A. Prevalence of depressive symptoms and predictors of treatment among U.S. adults from 2005 to 2010. Gen. Hosp. Psychiatry 2014, 36, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Center for Behavioral Health Statistics and Quality. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables: Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services. 2017. Available online: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#ftn1 (accessed on 8 May 2019).
- Nutting, P.A.; Rost, K.; Dickinson, M.; Werner, J.J.; Dickinson, P.; Smith, J.L. Barriers to initiating depression treatment in primary care practice. J. Gen. Int. Med. 2002, 17, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, C.W.M. Managing depression in primary care. Singapore Med. J. 2017, 58, 459–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serefko, A.; Szopa, A.; Wlaz, P.; Nowak, G.; Radziwon-Zaleska, M.; Skalski, M.; Poleszak, E. Magnesium in depression. Pharmacol. Rep. 2013, 65, 547–554. [Google Scholar] [CrossRef]
- Szewczyk, B.; Poleszak, E.; Sowa-Kucma, M.; Siwek, M.; Dudek, D.; Ryszewska-Pokrasniewicz, B. Antidepressant activity of zinc and magnesium in view of the current hypotheses of antidepressant action. Pharmacol. Rep. 2008, 60, 588–589. [Google Scholar]
- Iseri, L.T.; French, J.H. Magnesium: nature’s physiologic calcium blocker. Am. Heart J. 1984, 108, 188–193. [Google Scholar] [CrossRef]
- Derom, M.L.; Martinez-Gonzalez, M.A.; Sayon-Orea Mdel, C.; Bes-Rastrollo, M.; Beunza, J.J.; Sanchez-Villegas, A. Magnesium intake is not related to depression risk in Spanish university graduates. J. Nutr. 2012, 142, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Maes, M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.A.; Sul, J.; Song, Y.; Li, X.; LeBlanc, J.; You, Y.; Butch, A.; Liu, S. Magnesium supplementation, metabolic and inflammatory markers, and global genomic and proteomic profiling: A randomized, double-blind, controlled, crossover trial in overweight individuals. Am. J. Clin. Nutr. 2011, 93, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Bagis, S.; Karabiber, M.; As, I.; Tamer, L.; Erdogan, C.; Atalay, A. Is magnesium citrate treatment effective on pain, clinical parameters and functional status in patients with fibromyalgia? Rheumatol. Int. 2013, 33, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ryszewska-Pokraśniewicz, B.; Mach, A.; Skalski, M.; Januszko, P.; Wawrzyniak, Z.; Poleszak, E.; Nowak, G.; Pilc, A.; Radziwoń-Zaleska, M. Effects of Magnesium Supplementation on Unipolar Depression: A Placebo-Controlled Study and Review of the Importance of Dosing and Magnesium Status in the Therapeutic Response. Nutrients 2018, 10, 1014. [Google Scholar] [CrossRef]
- Wójcik, J.; Dudek, D.; Schlegel-Zawadzka, M.; Grabowska, M.; Marcinek, A.; Florek, E.; Piekoszewski, W.; Nowak, R.J.; Opoka, W.; Nowak, G. Antepartum/postpartum depressive symptoms and serum zinc and magnesium levels. Pharmacol. Rep. 2006, 58, 571. [Google Scholar] [PubMed]
- Rajizadeh, A.; Mozaffari-Khosravi, H.; Yassini-Ardakani, M.; Dehghani, A. Serum Magnesium Status in Patients Subjects with Depression in the City of Yazd in Iran 2013-2014. Biol. Trace Elem. Res. 2016, 171, 275–282. [Google Scholar] [CrossRef]
- Hasey, G.M.; D’Alessandro, E.; Cooke, R.G.; Warsh, J.J. The interface between thyroid activity, magnesium, and depression: A pilot study. Biol. Psychiatry 1993, 33, 133–135. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Mao, M.A.; Srivali, N.; Ungprasert, P.; Varothai, N.; Sanguankeo, A.; Kittanamongkolchai, W.; Erickson, S.B. Hypomagnesaemia linked to depression: A systematic review and meta-analysis. Int. Med. J. 2015, 45, 436–440. [Google Scholar] [CrossRef]
- Murck, H. Ketamine, magnesium and major depression—From pharmacology to pathophysiology and back. J. Psych. Res. 2013, 47, 955–965. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Health and Human Services, United States Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 2015. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 11 June 2019).
- Ismail, Y.; Ismail, A.A. The underestimated problem of using serum magnesium measurements to exclude magnesium deficiency in adults; a health warning is needed for "normal" results. Clin. Chem. Lab. Med. 2010, 48, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Hubert, J.; Mazur, A. Methods of assessment of magnesium status in humans: A systematic review. Magnes. Res. 2011, 24, 163–180. [Google Scholar] [PubMed]
- Barragan-Rodriguez, L.; Rodriguez-Moran, M.; Guerrero-Romero, F. Efficacy and safety of oral magnesium supplementation in the treatment of depression in the elderly with type 2 diabetes: A randomized, equivalent trial. Magnes. Res. 2008, 21, 218–223. [Google Scholar] [PubMed]
- Leuchter, A.F.; Cook, I.A.; Marangell, L.B.; Gilmer, W.S.; Burgoyne, K.S.; Howland, R.H.; Trivedi, M.H.; Zisook, S.; Jain, R.; McCracken, J.T.; et al. Comparative effectiveness of biomarkers and clinical indicators for predicting outcomes of SSRI treatment in Major Depressive Disorder: Results of the BRITE-MD study. Psych. Res. 2009, 169, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Eby, G.A.; Eby, K.L. Magnesium for treatment-resistant depression: A review and hypothesis. Med. Hypoth. 2010, 74, 649–660. [Google Scholar] [CrossRef] [PubMed]
| PHQ-2 | PHQ-9 | |||
|---|---|---|---|---|
| (n = 3596) | (n = 616) 1 | |||
| Characteristic | Mean | Range | Mean | Range |
| Age (years) | 62.0 | 18–99 | 56.1 | 18–99 |
| Male | 41.9% | 38.8% | ||
| White Race | 94.6% | 93.8% | ||
| Diabetes | 22.2% | 24.0% | ||
| Chronic Kidney Disease | 8.2% | 8.6% | ||
| Months Between PHQ and Mg Test | 7.4 | 0–35.1 | 8.1 | 0–34.4 |
| Serum Mg (mg/dL) 2 | 2.0 | <0.5–8.1 | 2.0 | 0.9–7.2 |
| PHQ Score | 1.0 | 0–6 | 12.6 | 0–27 |
| 0–2 (“Negative screen”) | 84.8% | -- | ||
| 3–6 (“Positive screen”) | 15.2% | -- | ||
| 0–4 (“None”) | -- | 11.2% | ||
| 5–9 (“Mild”) | -- | 22.2% | ||
| 10–14 (“Moderate”) | -- | 27.4% | ||
| 15–19 (“Severe”) | -- | 24.2% | ||
| 20–27 (“Very severe”) | -- | 14.9% | ||
| ≥10 (“Positive”) | 66.5% | |||
| PHQ-2 | PHQ-9 | |||||
|---|---|---|---|---|---|---|
| (n = 3590) | (n = 616) 1 | |||||
| β | 95% CI | P | β | 95% CI | P | |
| Serum Mg (mg/dL) | −0.23 | −0.35, −0.11 | <0.001 | −1.09 | −1.96, −0.21 | 0.015 |
| Age (year) | −0.02 | −0.02, −0.01 | <0.001 | −0.05 | −0.08, −0.02 | 0.001 |
| Male | −0.19 | −0.30, −0.09 | <0.001 | −0.78 | −1.79, 0.23 | 0.13 |
| Months Between PHQ and Mg | −0.01 | −0.02, −0.00 | 0.011 | −0.08 | −0.14, −0.01 | 0.016 |
| White | 0.01 | −0.22, 0.25 | 0.90 | 1.21 | −0.82, 3.24 | 0.24 |
| Diabetes | 0.21 | 0.08, 0.34 | 0.001 | 0.74 | −0.46, 1.93 | 0.23 |
| Chronic Kidney Disease | 0.35 | 0.15, 0.54 | 0.001 | −1.10 | −2.92, 0.72 | 0.24 |
| Constant (intercept) | 2.63 | 2.22, 3.03 | <0.001 | 17.19 | 13.96, 20.41 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarleton, E.K.; Kennedy, A.G.; Rose, G.L.; Crocker, A.; Littenberg, B. The Association between Serum Magnesium Levels and Depression in an Adult Primary Care Population. Nutrients 2019, 11, 1475. https://doi.org/10.3390/nu11071475
Tarleton EK, Kennedy AG, Rose GL, Crocker A, Littenberg B. The Association between Serum Magnesium Levels and Depression in an Adult Primary Care Population. Nutrients. 2019; 11(7):1475. https://doi.org/10.3390/nu11071475
Chicago/Turabian StyleTarleton, Emily K., Amanda G. Kennedy, Gail L. Rose, Abigail Crocker, and Benjamin Littenberg. 2019. "The Association between Serum Magnesium Levels and Depression in an Adult Primary Care Population" Nutrients 11, no. 7: 1475. https://doi.org/10.3390/nu11071475
APA StyleTarleton, E. K., Kennedy, A. G., Rose, G. L., Crocker, A., & Littenberg, B. (2019). The Association between Serum Magnesium Levels and Depression in an Adult Primary Care Population. Nutrients, 11(7), 1475. https://doi.org/10.3390/nu11071475





