Fermented Oyster Extract Prevents Ovariectomy-Induced Bone Loss and Suppresses Osteoclastogenesis
Abstract
1. Introduction
2. Results
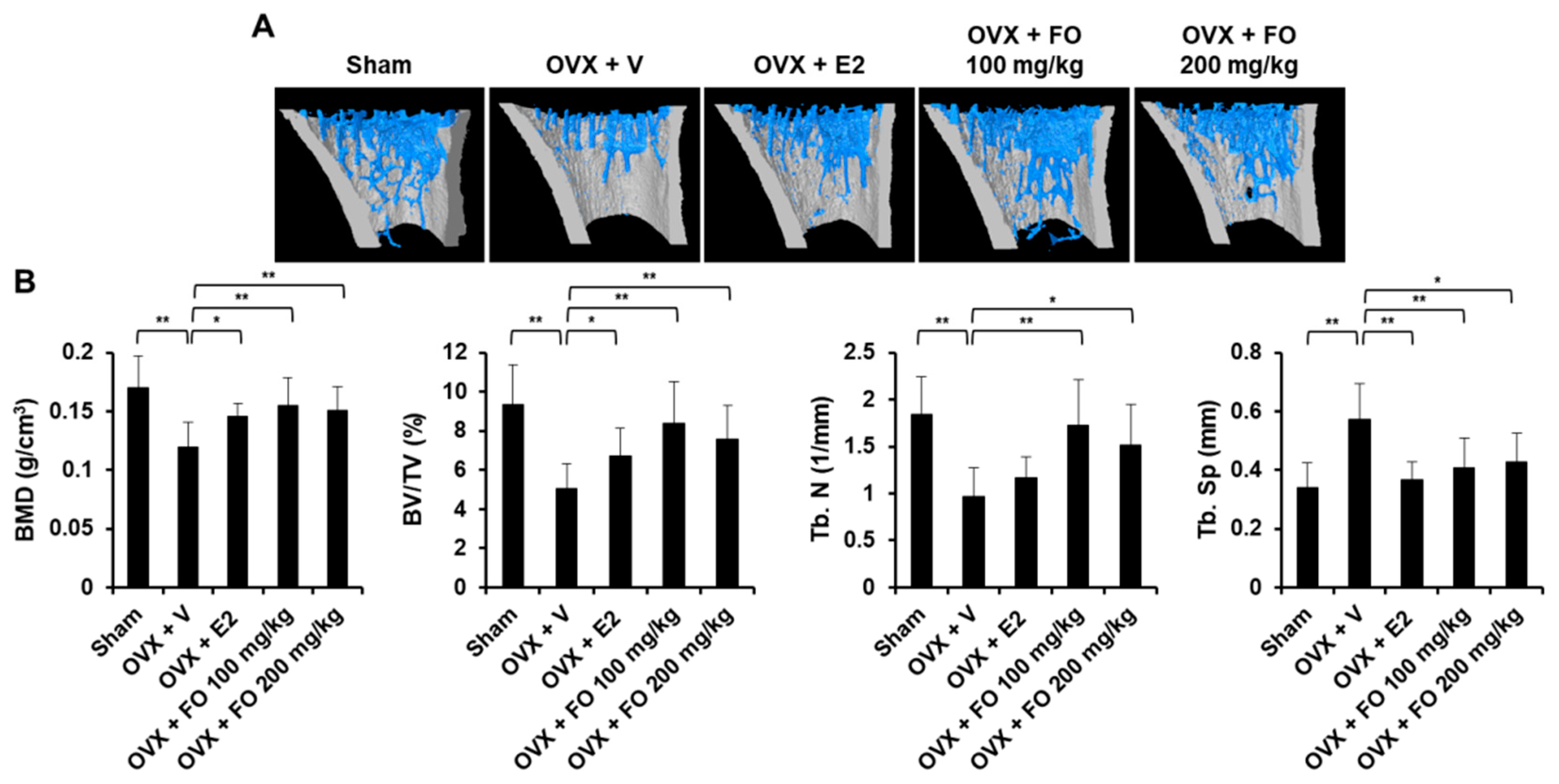
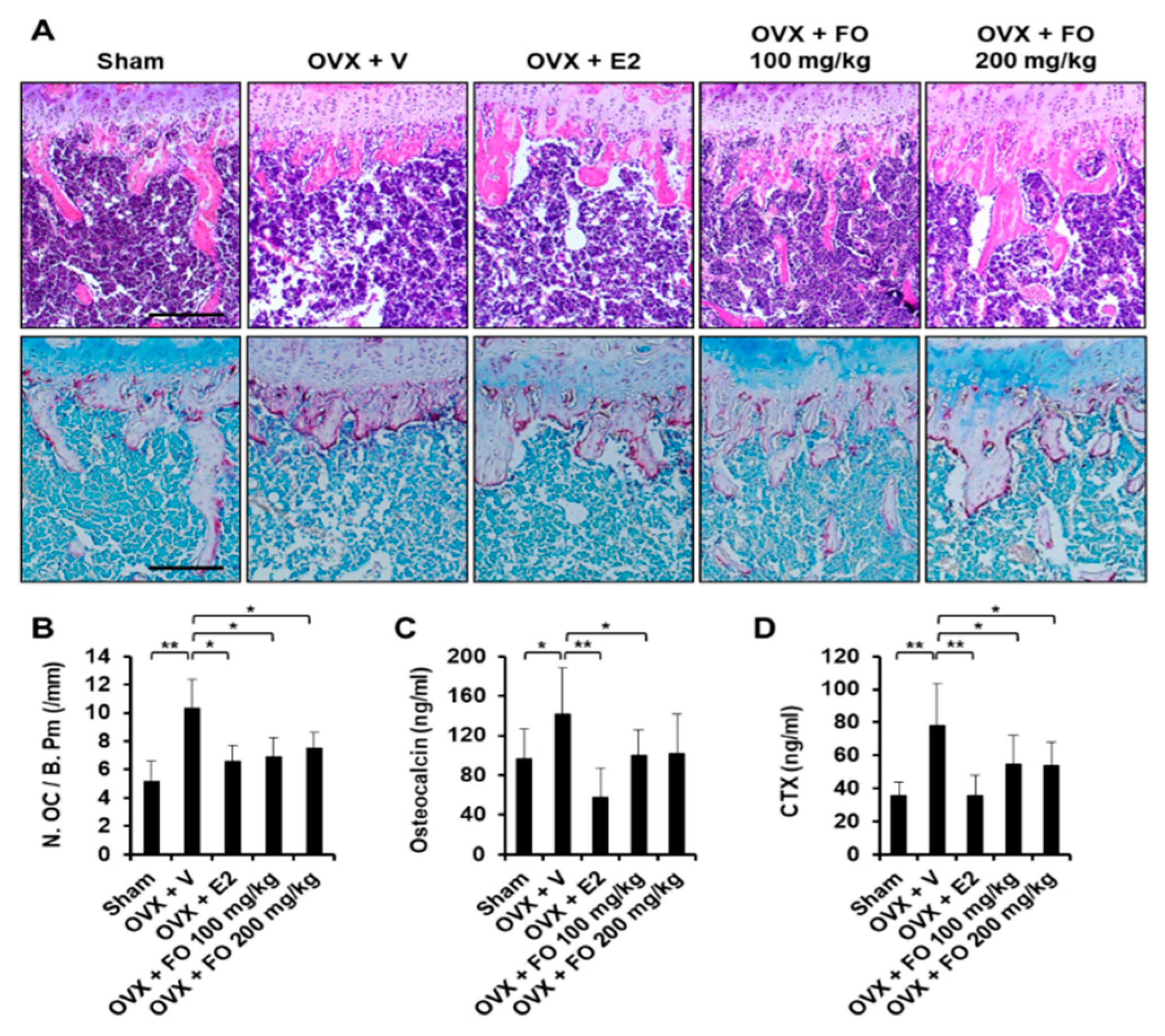
2.1. Administration of FO Mitigates OVX-Induced Bone Loss in Vivo
2.2. FO Suppresses RANKL-Induced Osteoclast Formation in Vitro
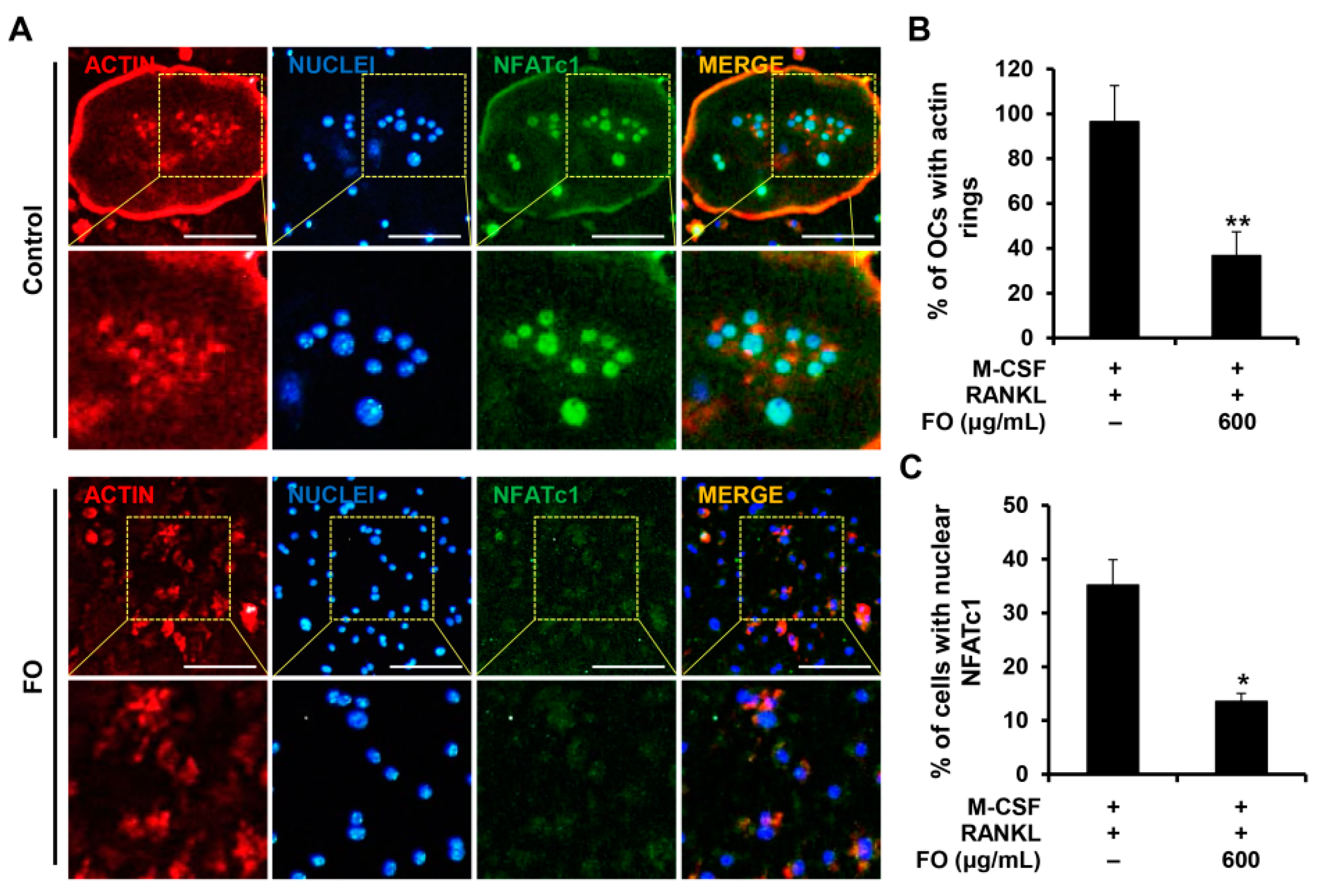
2.3. FO Attenuates the Expression of Osteoclast-Specific Genes and the Formation of Actin Rings
2.4. FO Suppresses the RANKL-Mediated NF-κB Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Preparation of Fermented Oyster Extract (FO)
4.2. Reagents and Antibodies
4.3. Animals and Treatments
4.4. Micro-CT and Histomorphometric Analysis
4.5. Osteocalcin and CTX-1 Measurements
4.6. In Vitro Osteoclastogenesis Assay
4.7. Pit Formation Assay
4.8. Quantitative PCR Assay
4.9. Western Blot Assay
4.10. Immunofluorescence Staining
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sunyecz, J.A. The use of calcium and vitamin d in the management of osteoporosis. Ther. Clin. Risk Manag. 2008, 4, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Huh, J.E.; Lee, J.H.; Park, D.R.; Lee, Y.; Lee, S.G.; Choi, S.; Lee, H.J.; Song, S.W.; Jeong, Y.; et al. A novel pyrazole derivative protects from ovariectomy-induced osteoporosis through the inhibition of nadph oxidase. Sci. Rep. 2016, 6, 22389. [Google Scholar] [CrossRef] [PubMed]
- Compston, J. Clinical and therapeutic aspects of osteoporosis. Eur. J. Radiol. 2009, 71, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Cantley, M.D.; Haynes, D.R. Mechanisms and control of pathologic bone loss in periodontitis. Periodontol 2000 2010, 53, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Jilka, R.L. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N. Engl. J. Med. 1995, 332, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takayanagi, H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr. Opin. Rheumatol. 2006, 18, 419–426. [Google Scholar] [CrossRef]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A review of treatment options. P T 2018, 43, 92–104. [Google Scholar]
- Khosla, S.; Burr, D.; Cauley, J.; Dempster, D.W.; Ebeling, P.R.; Felsenberg, D.; Gagel, R.F.; Gilsanz, V.; Guise, T.; Koka, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the american society for bone and mineral research. J. Bone Miner. Res. 2007, 22, 1479–1491. [Google Scholar] [CrossRef]
- Sun, L.M.; Lin, M.C.; Muo, C.H.; Liang, J.A.; Kao, C.H. Calcitonin nasal spray and increased cancer risk: A population-based nested case-control study. J. Clin. Endocrinol. Metab. 2014, 99, 4259–4264. [Google Scholar] [CrossRef]
- Yoshimura, H.; Ohba, S.; Yoshida, H.; Saito, K.; Inui, K.; Yasui, R.; Ichikawa, D.; Aiki, M.; Kobayashi, J.; Matsuda, S.; et al. Denosumab-related osteonecrosis of the jaw in a patient with bone metastases of prostate cancer: A case report and literature review. Oncol. Lett. 2017, 14, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Singhal, S.S.; Reddy, A.B. Therapeutic potential of natural pharmacological agents in the treatment of human diseases. Biomed. Res. Int. 2014, 2014, 573452. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Alvarino, R.; Leiros, M.; Tabudravu, J.N.; Feussner, K.; Dam, M.A.; Rateb, M.E.; Jaspars, M.; Botana, L.M. Evaluation of the antioxidant activity of the marine pyrroloiminoquinone makaluvamines. Mar. Drugs 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.A.; Clarke, S.A. Bioactive compounds from marine organisms: Potential for bone growth and healing. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Yamaguchi, M. Anabolic effect of marine alga sargassum horneri extract on bone components in the femoral-diaphyseal and -metaphyseal tissues of young and aged rats in vivo. J. Health Sci. 2002, 48, 325–330. [Google Scholar] [CrossRef]
- Chaugule, S.R.; Indap, M.M.; Chiplunkar, S.V. Marine natural products: New avenue in treatment of osteoporosis. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Moon, P.D.; Kim, M.H.; Lim, H.S.; Oh, H.A.; Nam, S.Y.; Han, N.R.; Kim, M.J.; Jeong, H.J.; Kim, H.M. Taurine, a major amino acid of oyster, enhances linear bone growth in a mouse model of protein malnutrition. Biofactors 2015, 41, 190–197. [Google Scholar] [CrossRef]
- Ma, J.Y.; Wong, K.L.; Xu, Z.Y.; Au, K.Y.; Lee, N.L.; Su, C.; Su, W.W.; Li, P.B.; Shaw, P.C. N16, a nacreous protein, inhibits osteoclast differentiation and enhances osteogenesis. J. Nat. Prod. 2016, 79, 204–212. [Google Scholar] [CrossRef]
- Jeong, J.W.; Choi, S.H.; Han, M.H.; Kim, G.Y.; Park, C.; Hong, S.H.; Lee, B.J.; Park, E.K.; Kim, S.O.; Leem, S.H.; et al. Protective effects of fermented oyster extract against rankl-induced osteoclastogenesis through scavenging ros generation in raw 264.7 cells. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor nfatc1 (nfat2) integrate rankl signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Abu-Amer, Y. Nf-kappab signaling and bone resorption. Osteoporos Int. 2013, 24, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.B.; Kim, Y.S.; Hwang, J.W.; Park, P.J. Immunomodulatory properties of shellfish derivatives associated with human health. RSC Adv. 2016, 6, 26163–26177. [Google Scholar] [CrossRef]
- Lee, Y.K.; Jung, S.K.; Chang, Y.H.; Kwak, H.S. Highly bioavailable nanocalcium from oyster shell for preventing osteoporosis in rats. Int. J. Food Sci. Nutr. 2017, 68, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L. The mechanisms of estrogen regulation of bone resorption. J. Clin. Investig. 2000, 106, 1203–1204. [Google Scholar] [CrossRef] [PubMed]
- Inada, M.; Matsumoto, C.; Miyaura, C. Animal models for bone and joint disease. Ovariectomized and orchidectomized animals. Clin. Calcium 2011, 21, 164–170. [Google Scholar] [PubMed]
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013, 9, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Darnay, B.G.; Haridas, V.; Ni, J.; Moore, P.A.; Aggarwal, B.B. Characterization of the intracellular domain of receptor activator of nf-kappab (rank). Interaction with tumor necrosis factor receptor-associated factors and activation of nf-kappab and c-jun n-terminal kinase. J. Biol. Chem. 1998, 273, 20551–20555. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Signaling to nf-kappab. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef]
- Iotsova, V.; Caamano, J.; Loy, J.; Yang, Y.; Lewin, A.; Bravo, R. Osteopetrosis in mice lacking nf-kappab1 and nf-kappab2. Nat. Med. 1997, 3, 1285–1289. [Google Scholar] [CrossRef]
- Vaira, S.; Alhawagri, M.; Anwisye, I.; Kitaura, H.; Faccio, R.; Novack, D.V. Rela/p65 promotes osteoclast differentiation by blocking a rankl-induced apoptotic jnk pathway in mice. J. Clin. Investig. 2008, 118, 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- Aliprantis, A.O.; Ueki, Y.; Sulyanto, R.; Park, A.; Sigrist, K.S.; Sharma, S.M.; Ostrowski, M.C.; Olsen, B.R.; Glimcher, L.H. Nfatc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J. Clin. Investig. 2008, 118, 3775–3789. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.C.; Reid, S.N.S.; Ryu, J.K.; Kim, Y.; Jo, Y.H.; Jeon, B.H. Effects of gamma-aminobutyric acid-enriched fermented sea tangle (laminaria japonica) on brain derived neurotrophic factor-related muscle growth and lipolysis in middle aged women. Algae-Seoul 2016, 31, 175–187. [Google Scholar] [CrossRef]
- Kim, T.H.; Jung, J.W.; Ha, B.G.; Hong, J.M.; Park, E.K.; Kim, H.J.; Kim, S.Y. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. Nutr. Biochem. 2011, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Jun, A.Y.; Kim, H.J.; Park, K.K.; Son, K.H.; Lee, D.H.; Woo, M.H.; Kim, Y.S.; Lee, S.K.; Chung, W.Y. Extract of magnoliae flos inhibits ovariectomy-induced osteoporosis by blocking osteoclastogenesis and reducing osteoclast-mediated bone resorption. Fitoterapia 2012, 83, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Choi, Y.Y.; Han, J.M.; Lee, H.S.; Hong, S.B.; Lee, S.G.; Yang, W.M. Ameliorative effects of schizandra chinensis on osteoporosis via activation of estrogen receptor (er)-alpha/-beta. Food Funct. 2014, 5, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Ihn, H.J.; Lee, D.; Lee, T.; Kim, S.H.; Shin, H.I.; Bae, Y.C.; Hong, J.M.; Park, E.K. Inhibitory effects of kp-a159, a thiazolopyridine derivative, on osteoclast differentiation, function, and inflammatory bone loss via suppression of rankl-induced map kinase signaling pathway. PLoS ONE 2015, 10, e0142201. [Google Scholar] [CrossRef]
- Ihn, H.J.; Lee, T.; Lee, D.; Bae, J.S.; Kim, S.H.; Jang, I.H.; Bae, Y.C.; Shin, H.I.; Park, E.K. Inhibitory effect of kp-a038 on osteoclastogenesis and inflammatory bone loss is associated with downregulation of blimp1. Front. Pharmacol 2019, 10, 367. [Google Scholar] [CrossRef]
- Ihn, H.J.; Lee, T.; Lee, D.; Kim, J.A.; Kim, K.; Lim, S.; Kim, J.Y.; Lee, Y.; Kim, S.H.; Lee, H.S.; et al. A novel benzamide derivative protects ligature-induced alveolar bone erosion by inhibiting nfatc1-mediated osteoclastogenesis. Toxicol Appl. Pharmacol. 2018, 355, 9–17. [Google Scholar] [CrossRef]
- Ihn, H.J.; Lee, T.; Kim, J.A.; Lee, D.; Kim, N.D.; Shin, H.I.; Bae, Y.C.; Park, E.K. Ocli-023, a novel pyrimidine compound, suppresses osteoclastogenesis in vitro and alveolar bone resorption in vivo. PLoS ONE 2017, 12, e0170159. [Google Scholar] [CrossRef]
- Ihn, H.J.; Kim, J.A.; Cho, H.S.; Shin, H.I.; Kim, G.Y.; Choi, Y.H.; Jeon, Y.J.; Park, E.K. Diphlorethohydroxycarmalol from ishige okamurae suppresses osteoclast differentiation by downregulating the nf-kappab signaling pathway. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Ihn, H.J.; Kim, K.; Cho, H.S.; Park, E.K. Pentamidine inhibits titanium particle-induced osteolysis in vivo and receptor activator of nuclear factor-κB ligand-mediated osteoclast differentiation in vitro. Tissue Eng. Regen. Med. 2019, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ihn, H.J.; Kim, J.A.; Lim, S.; Nam, S.-H.; Hwang, S.H.; Lim, J.; Kim, G.-Y.; Choi, Y.H.; Jeon, Y.-J.; Lee, B.-J.; et al. Fermented Oyster Extract Prevents Ovariectomy-Induced Bone Loss and Suppresses Osteoclastogenesis. Nutrients 2019, 11, 1392. https://doi.org/10.3390/nu11061392
Ihn HJ, Kim JA, Lim S, Nam S-H, Hwang SH, Lim J, Kim G-Y, Choi YH, Jeon Y-J, Lee B-J, et al. Fermented Oyster Extract Prevents Ovariectomy-Induced Bone Loss and Suppresses Osteoclastogenesis. Nutrients. 2019; 11(6):1392. https://doi.org/10.3390/nu11061392
Chicago/Turabian StyleIhn, Hye Jung, Ju Ang Kim, Soomin Lim, Sang-Hyeon Nam, So Hyeon Hwang, Jiwon Lim, Gi-Young Kim, Yung Hyun Choi, You-Jin Jeon, Bae-Jin Lee, and et al. 2019. "Fermented Oyster Extract Prevents Ovariectomy-Induced Bone Loss and Suppresses Osteoclastogenesis" Nutrients 11, no. 6: 1392. https://doi.org/10.3390/nu11061392
APA StyleIhn, H. J., Kim, J. A., Lim, S., Nam, S.-H., Hwang, S. H., Lim, J., Kim, G.-Y., Choi, Y. H., Jeon, Y.-J., Lee, B.-J., Bae, J.-S., Kim, Y. H., & Park, E. K. (2019). Fermented Oyster Extract Prevents Ovariectomy-Induced Bone Loss and Suppresses Osteoclastogenesis. Nutrients, 11(6), 1392. https://doi.org/10.3390/nu11061392









