Effectiveness of Following Mediterranean Diet Recommendations in the Real World in the Incidence of Gestational Diabetes Mellitus (GDM) and Adverse Maternal-Foetal Outcomes: A Prospective, Universal, Interventional Study with a Single Group. The St Carlos Study
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design
2.2. Setting
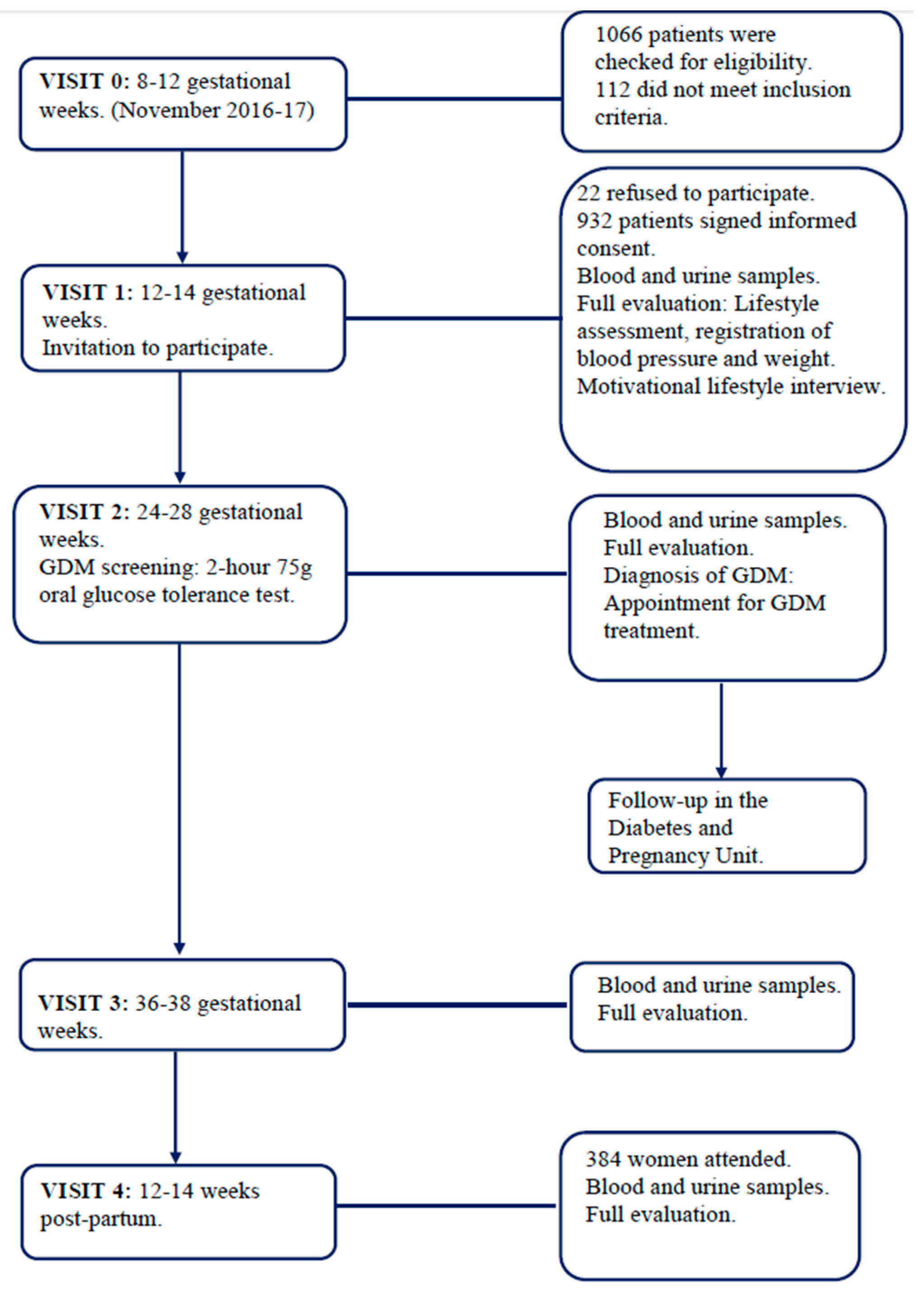
2.3. Participants and Study Conduct
2.4. Nutritional Intervention
2.5. Lifestyle Evaluation
2.6. GDM Screening and Treatment
2.7. Clinical History
2.8. Anthropometric Data
2.9. Biochemical Variables
2.10. Maternal Outcomes
2.11. Neonatal Outcomes
2.12. Sample Size
2.13. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Watson, W.; Dooley, S.L.; Foderaro, M.; Niznik, C.; Bjaloncik, J.; Catalano, P.M.; Dierker, L. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [PubMed]
- Daly, B.; Toulis, K.A.; Thomas, N.; Gokhale, K.; Martin, J.; Webber, J.; Keerthy, D.; Jolly, K.; Saravanan, P.; Nirantharakumar, K. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study. PLoS Med. 2018, 15, e1002488. [Google Scholar] [CrossRef] [PubMed]
- Grunnet, L.G.; Hansen, S.; Hjort, L.; Camilla, M.M.; Kampmann1, F.B.; Thuesen, A.C.B.; Granstrømi, C.; Strøm, M.; Maslova, E.; Frikke-Schmidt, R.; et al. Adiposity, Dysmetabolic Traits, and Earlier Onset of Female Puberty in Adolescent Offspring of Women With Gestational Diabetes Mellitus: A Clinical Study Within the Danish National Birth Cohort. Diabetes Care 2017, 40, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Lowe, W.L.; Scholtens, D.M.; Kuang, A.; Linder, B.; Lawrence, J.M.; Lebenthal, Y.; McCance, D.; Hamilton, J.; Nodzenski, M.; Talbot, O.; et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, D.M.; Kuang, A.; Lowe, L.P.; Hamilton, J.; Lawrence, J.M.; Lebenthal, Y.; Brickman, W.J.; Clayton, P.; Ma, R.C.; McCance, D.; et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Glycemia and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 381–392. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Executive summary: Standards of medical care in Diabetes 2011. Diabetes Care 2011, 34. [Google Scholar] [CrossRef]
- Blumer, I.; Hadar, E.; Hadden, D.R.; Jovanovič, L.; Mestman, J.H.; Murad, M.H.; Yogev, Y. Diabetes and pregnancy: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4227–4249. [Google Scholar] [CrossRef]
- The World Health Organization guideline 2013. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Available online: http://apps.who.int/iris/bitstream/10665/85975/1/WHO_NMH_MND_13.2_eng.pdf (accessed on 12 January 2019).
- Durán, A.; Saénz, S.; Torrejón, M.J.; Bordiú, E.; del Valle, L.; Galindo1, M.; Perez, N.; Herraiz, M.A.; Izquierdo, N.; Rubio, M.; et al. Introduction of IADPSG Criteria for the Screening and Diagnosis of Gestational Diabetes Mellitus Results in Improved Pregnancy Outcomes at a Lower Cost in a Large Cohort of Pregnant Women: The St. Carlos Gestational Diabetes Study. Diabetes Care 2014, 37, 2442–2450. [Google Scholar] [CrossRef]
- Agha-Raffar, R.; Oliver, N.; Jhonston, D.; Robinson, S. Gestational diabetes mellitus: Does an effective prevention strategy exist? Nat. Rev. Endocrinol. 2016, 12, 533–546. [Google Scholar] [CrossRef]
- Salas-Salvado, J.; Bullo, M.; Babio, N.; Martínez-González, M.A.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F.; et al. Reduction in the Incidence of Type 2 Diabetes with the Mediterranean Diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011, 34, 14–19. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; García de la Torre, N.; Durán, A.; Fuentes, M.; Bordiú, E.; del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; Garcia de la Torre, N.; Durán, A.; Fuentes, M.; Bordiú, E.; del Valle, L.; Valerio, J.; Familiar, C.; Jiménez, I.; Herraiz, M.A.; et al. Medical nutrition therapy for gestational diabetes mellitus based on Mediterranean Diet principles: A subanalysis of the St Carlos GDM Prevention Study. BMJ 2018, 6, e000550. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; Garcia de la Torre, N.; Durán, A.; Fuentes, M.; Bordiú, E.; del Valle, L.; Familiar, C.A.; Valerio, J.A.; Jiménez, I.A.; Herraiz, M.A.; et al. A Mediterranean Diet with an Enhanced Consumption of Extra Virgin Olive Oil and Pistachios Improves Pregnancy Outcomes in Women Without Gestational Diabetes Mellitus: A Sub-Analysis of the St. Carlos Gestational Diabetes Mellitus Prevention Study. Ann. Nutr. Metab. 2019, 74, 69–79. [Google Scholar] [CrossRef]
- The Diabetes and Nutrition Study Group of the Spanish Diabetes Association. Diabetes Nutrition and Complications Trial: Trends in nutritional pattern between 1993 and 2000 and targets of diabetes treatment in a sample of Spanish people with diabetes. Diabetes Care 2004, 27, 984–987. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Lapetra, M.F.J.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Moses, R.G.; Luebcke, M.; Davis, W.S.; Coleman, K.J.; Tapsell, L.C.; Peter, P.; Brand-Miller, J.C. Effect of a low-glycemic-index diet during pregnancy on obstetric outcomes. Am. J. Clin. Nutr. 2006, 84, 807–812. [Google Scholar] [CrossRef]
- Asbee, S.M.; Jenkins, T.R.; Butler, J.R.; White, J.; Elliot, M.; Rutledge, A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: A randomized controlled trial. Obstet. Gynecol. 2009, 113, 305–311. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; Familiar, C.; García de la Torre, N.; Rubio, M.A.; Bordiú, E.; del Valle1, L.; Lara, M.; Ruiz, T.; Ortolá, A.; Crespo, I.; et al. Gestational diabetes mellitus treatment reduces obesity-induced adverse pregnancy and neonatal outcomes: The St. Carlos gestational study. BMJ 2016, 4, e000314. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; Costa, A.G.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef]
- Imamura, F.; Micha, R.; Wu, J.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffaria, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of Olive Oil on Markers of Inflammation and Endothelial Function-A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [CrossRef]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- Viguiliouk, E.; Kendall, C.W.; Blanco Mejia, S.; Cozma, A.I.; Ha, V.; Mirrahimi, A.; Jayalath, V.H.; Augustin, L.S.A.; Chiavaroli, L.; Leiter, L.A.; et al. Effect of Tree Nuts on Glycemic Control in Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Dietary Trials. PLoS ONE 2014, 9, e103376. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; Garcia de la Torre, N.; Fuentes, M.; Durán, A.; Bordiú, E.; Del Valle, L.; Valerio, J.; Jiménez, I.; Herraiz, M.A.; Izquierdo, N.; et al. A High Adherence to Six Food Targets of the Mediterranean Diet in the Late First Trimester is Associated with a Reduction in the Risk of Materno-Foetal Outcomes: The St. Carlos Gestational Diabetes Mellitus Prevention Study. Nutrients 2019, 11, 66. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, D.; Mao, X.; Xia, Y.; Baker, P.N.; Zhang, H. Maternal Dietary Patterns and Pregnancy Outcome. Nutrients 2016, 8, 351. [Google Scholar] [CrossRef]
- Kibret, K.T.; Chojenta, C.; Gresham, E.; Tegegne, T.K.; Loxton, D. Maternal dietary patterns and risk of adverse pregnancy (hypertensive disorders of pregnancy and gestational diabetes mellitus) and birth (preterm birth and low birth weight) outcomes: A systematic review and meta-analysis. Public Health Nutr. 2019, 22, 506–520. [Google Scholar] [CrossRef]
| Variables | Mean ± SD or n (%) |
|---|---|
| N | 932 |
| Age (years) | 32.4 ± 5.2 |
| Race/Ethnicity | |
| Caucasian | 612 (66.7) |
| Hispanic | 284 (30.5) |
| Others | 26 (2.8) |
| Family history of Type 2 Diabetes | 260 (27.9) |
| Family history of MetS (>2 components) | 174 (18.7) |
| Previous history of Gestational Diabetes | 37 (4.0) |
| Previous history of Miscarriages | 325 (34.9) |
| Educational status | |
| Elementary education | 58 (6.2) |
| Secondary School | 241 (25.9) |
| University Degree | 621 (66.7) |
| UNK | 12 (1.3) |
| Employment | 743 (79.7) |
| Number of pregnancies | |
| Primiparous | 394 (42.3) |
| Second pregnancy | 276 (29.6) |
| >2 pregnancies | 262 (28.1) |
| Never Smoker | 531 (57.0) |
| Current Smoker | 77 (8.3) |
| Gestational Age at baseline (weeks) | 12.1 ± 0.5 |
| Prepregnancy Body Weight (Kg) | 59.6 ± 9.7 |
| At entry Body Weight (Kg) | 61.4 ± 9.9 |
| Weight gain (Kg) | 1.8 ± 3.4 |
| Prepregnancy BMI (kg/m2) | 22.5 ± 3.5 |
| At baseline BMI (kg/m2) | 23.4 ± 3.7 |
| Systolic Blood Pressure (mm Hg) | 108 ± 10 |
| Diastolic Blood Pressure (mm Hg) | 67 ± 8 |
| Fasting Blood Glucose (mg/dL) | 80 ± 6 |
| mmol/dL | 4.4 ± 0.3 |
| HbA1c % | 5.1 ± 0.2 |
| mmol/mol | 32 ± 0.8 |
| Cholesterol mg/dL | 169 ± 32 |
| mmol/L | 4.39 ± 0.83 |
| Triglycerides mg/dl | 79 ± 34 |
| mmol/L | 0.9 ± 0.4 |
| TSH mcUI/mL | 2.1 ± 2.9 |
| T4L ng/dL | 8.8 ± 1.6 |
| MEDAS Score | 4.3 ± 1.7 |
| Nutrition Score | 0.4 ± 3.1 |
| Variables | NGT Group (n = 802) | GDM Group (n = 130), (13.9%) | Differences (95% CI) | p |
|---|---|---|---|---|
| 75 g-OGTT 24–28 GW | ||||
| FBG mg/dL mmol/dL | 82.9 ± 4.9 4.6 ± 0.3 | 92.4 ± 8.6 5.2 ± 0.4 | 9.5 (8.4;10.5) 0.56 (0.51;0.61) | 0.0001 |
| 1 h Blood Glucose mg/dL mmol/dL | 118.3 ± 26.4 6.6 ± 1.5 | 164.1 ± 33.5 8.9 ± 2.0 | 45.7 (39.4;52.1) 2.3 (2.0;2.6) | 0.0001 |
| 2 h Blood Glucose mg/dL mmol/dL | 101.3 ± 19.8 5.6 ± 1.1 | 137.6 ± 31.6 7.6 ± 1.8 | 36.2 (31.3;41.2) 1.9 (1.7;2.1) | 0.0001 |
| HbA1c % (mmol/mol) 24–28 GW | 4.9 ± 0.3 (30± 0.9) | 5.0 ± 0.3 (31± 0.9) | 0.15 (0.10;0.20) | 0.0001 |
| HbA1c % (mmol/mol) 36–38 GW | 5.2 ± 0.3 (33± 1) | 5.2 ± 0.3 (34± 1) | 0.07 (0.00;0.14) | 0.051 |
| FBG 36–38 GW mg/dL mmol/dL | 77.6 ± 10.4 4.2 ± 0.4 | 80.1 ± 10.0 4.4 ± 0.4 | 2.5 (0.3;4.7) 0.2 (0.1;0.3) | 0.024 |
| Fasting Serum Insulin mcUI/mL | ||||
| 24–28 GW | 7.9 ± 5.3 | 10.0 ± 6.5 | 2.1 (0.7;3.5) | 0.003 |
| 36–38 GW | 13.3 ± 19.6 | 12.0 ± 16.1 | −1.3 (−5.7;3.2) | 0.182 |
| HOMA-IR | ||||
| 24–28 GW | 1.9 ± 2.7 | 2.3 ± 1.5 | 0.4 (−0.2;1.1) | 0.558 |
| 36–38 GW | 3.1 ± 5.5 | 2.7 ± 4.6 | −0.4 (−1.6;0.9) | 0.231 |
| Cholesterol mg/dL (mmol/dL) | ||||
| 24–28 GW | 243 ± 42 (6.32 ± 1.09) | 238 ± 43 (6.19 ± 1.12) | −4.7 (−12.9;3.5) | 0.952 |
| 36–38 GW | 272 ± 49 (7.07 ± 1.27) | 261 ± 50 (6.79 ± 1.30) | −10.3 (−20.8;0.2) | 0.054 |
| Triglycerides mg/dL (mmol/dL) | ||||
| 24–28 GW | 155 ± 52 (1.77 ± 0.59) | 167 ± 57 (1.90 ± 0.65) | 11.8 (0.7;23.1) | 0.038 |
| 36–38 GW | 228 ± 74 (2.60 ± 0.84) | 223 ± 69 (2.54 ± 0.79) | −4.5 (−20.5;11.5) | 0.577 |
| TSH (mcUI/mL) | ||||
| 24–28 GW | 2.0 ± 1.0 | 1.9 ± 1.3 | −0.1 (−0.4;0.1) | 0.215 |
| 36–38 GW | 1.6 ± 1.0 | 1.7 ± 1.0 | 0.1 (−0.2;0.3) | 0.602 |
| T4L(ng/dL) | ||||
| 24–28 GW | 7.0 ± 1.2 | 7.0 ± 1.3 | −0.0 (−0.2;0.2) | 0.999 |
| 36–38 GW | 7.1 ± 1.3 | 7.2 ± 1.3 | 0.2 (−0.1;0.5) | 0.188 |
| Treatment of GDM | ||||
| Nutritional | 100 (72.8) | |||
| Insulin (total) | 30 (27.2) | |||
| Bolus | 5 (14.9) | |||
| Basal | 18 (74.5) | |||
| Basal/Bolus | 7 (10.6) | |||
| Body Weight (kg) | ||||
| 24–28 GW | 66.5 ± 10.0 | 66.3 ± 10.3 | −0.2 (−2.1;1.7) | 0.807 |
| 36–38 GW | 71.4 ± 9.9 | 70.3 ± 11.3 | −1.1 (−3.1;0.9) | 0.281 |
| Weight gain (kg) pregestation to 24–28 GW | 7.02 ± 4.27 | 7.13 ± 4.91 | 0.11 (−0.72;0.94) | 0.804 |
| Weight gain (kg) pregestation to 36v38 GW | 12.30 ± 5.42 | 10.88 ± 6.46 | −1.42 (−2.54; −0.30) | 0.013 |
| EWG | 371 (46.3) | 34 (26.2) | ||
| AWG | 296 (36.9) | 61 (46.9) | ||
| IWG | 135 (16.8) | 35 (26.9) | 0.001 | |
| Systolic BP (mm Hg) 24–28 GW | 105 ± 11 | 106 ± 11 | 1.8 (0.3;3.4) | 0.098 |
| Diastolic BP (mm Hg) 24–28 GW | 63 ± 8 | 65 ± 9 | 1.6 (−0.5;3.7) | 0.819 |
| Systolic BP (mm Hg) 36–38 GW | 115 ± 14 | 114 ± 11 | −1.1 (−3.0;0.8) | 0.661 |
| Diastolic BP (mm Hg) 36–38 GW | 71 ± 10 | 70 ± 10 | −0.8 (−3.4;1.8) | 0.019 |
| MEDAs SCORE | ||||
| 24–28 GW | 5.2 + 1.8 | 5.4 + 1.6 | 0.2 (−0.2;0.7) | 0.292 |
| 36–38 GW | 5.4 + 1.8 | 7.7 + 2.2 | 2.2 (1.6;2.9) | 0.001 |
| NUTRITION SCORE | ||||
| 24–28 GW | 2.4 + 3.5 | 2.8 + 3.5 | 0.4 (−0.3;1.1) | 0.257 |
| 36–38 GW | 3.2 + 3.5 | 7.6 + 3.2 | 4.5 (3.6;5.4) | 0.001 |
| NGT Group (n = 802) | GDM Group (n = 130) | p | Crude RR (95% CI) | |
|---|---|---|---|---|
| Maternal outcomes | ||||
| IWG/AWG | 135/296 (31.3) | 35/61 (36.5) | 0.196 | 1.21 (0.83–1.75) |
| EWG/AWG | 371/296 (55.6) | 34/61 (35.8) | 0.001 | 0.91 (0.86–0.96) |
| Pregnancy-induced hypertension | 15 (1.9) | 3 (2.3) | 0.470 | 1.20 (0.42–3.41) |
| Preeclampsia | 9 (1.1) | 1 (0.8) | 0.583 | 0.72 (0.11–4.62) |
| Albuminuria | 4 (0.5) | 2 (1.5) | 0.199 | 2.41 (0.77–7.56) |
| Bacteriuria | 120 (15.0) | 35 (26.9) | 0.001 | 1.85 (1.31–2.61) |
| Urinary Tract Infection | 31 (3.9) | 7 (5.4) | 0.271 | 1.34 (0.67–2.67) |
| Delivery | ||||
| Vaginal | 503 (62.7) | 76 (58.5) | 0.140 | 0.86 (0.43–1.71) |
| Instrumental | 129 (16.1) | 30 (23.1) | ||
| Cesarean section | 170 (21.2) | 24 (18.5) | ||
| Emergency-CS | 22 (13.5) | 3 (13.6) | 0.739 | 0.84 (0.52–1.35) |
| Induction | 490 (61.1) | 78 (60.0) | 0.442 | 0.96 (0.69–1.33) |
| Analgesia | 661 (82.4) | 106 (81.5) | 0.445 | 0.95 (0.63–1.42) |
| Episiotomy | 256 (31.9) | 43 (33.1) | 0.443 | 1.05 (0.75–1.47) |
| Perineal Trauma | 265 (33.0) | 36 (27.7) | 0.133 | 0.80 (0.56–1.15) |
| Dystocia | 10 (1.2) | 2 (1.5) | 0.516 | 1.20 (0.34–4.29) |
| Neonatal outcomes | ||||
| Gestational Age at birth (weeks) | 39.5 ± 2.0 | 39.5 ± 1.7 | 0.716 | |
| <37 GW | 46 (5.7) | 7 (5.4) | 0.535 | 0.94 (0.46–1.92) |
| Birthweight (g) | 3273 ± 468 | 3126 ± 465 | 0.002 | |
| Percentile | 50.0 ± 30.3 | 42.7 ± 28.3 | 0.005 | |
| Length (cm) | 49.4 ± 2.1 | 49.1 ± 1.9 | 0.202 | |
| Percentile | 42.7 ± 27.4 | 38.7 ± 26.6 | 0.263 | |
| LGA > 90 percentile | 31 (3.9) | 1 (0.8) | 0.048 | 0.21 (0.03–1.51) |
| >4500 g | 3 (0.4) | 1 (0.8) | NA | |
| SGA < 10 percentile | 30 (3.7) | 5 (3.8) | 0.553 | 0.99 (0.87–1.14) |
| Ph Cord Blood | 7.28 ± 0.13 | 7.27 ± 0.08 | 0.405 | |
| <7.1 | 19 (2.4) | 6 (4.6) | 0.122 | 1.76 (0.86–3.59) |
| Apgar Score at 1min | 8.8 ± 1.0 | 8.8 ± 0.8 | 0.846 | |
| <5 | 11 (1.4) | 1 (0.8) | 0.484 | 0.59 (0.09–3.91) |
| Apgar Score at 5 min | 9.8 ± 0.7 | 9.9 ± 0.3 | 0.196 | |
| <7 | 5 (0.6) | 0 (0) | 0.471 | NA |
| Hypoglycemia | 7 (0.9) | 1 (0.8) | 0.691 | 0.89 (0.14–5.64) |
| Respiratorydistress | 8 (1.0) | 0 (0) | 0.299 | NA |
| Hyperbilurrubinemia | 14 (1.7) | 1 (0.8) | 0.358 | 0.47 (0.07–3.17) |
| Brachial plexus | 1 (0.1) | 0 (0) | 0.861 | NA |
| NICU/observation | 9(1.1)/17 (2.1) | 0 (0)/2 (1.5) | 0.257/0.492 | NA/0.75 (0.20-2.81) |
| ALL | NGT | GDM | p | |
|---|---|---|---|---|
| N | 384 | 314 | 70 | |
| Age (year) | 33.2 ± 4.9 | 32.7 ± 4.9 | 35.2 ± 4.3 | 0.001 |
| Pregravid BW (Kg) | 59.8 ± 9.3 | 59.5 ± 8.8 | 60.9 ± 11.2 | 0.265 |
| PG-BMI (Kg/m2) | 22.6 ± 3.4 | 22.4 ± 3.2 | 23.3 ± 4.2 | 0.036 |
| Gestational Weight Gain | 12.06 ± 5.42 | 12.6 ± 5.2 | 10.0 ± 5.7 | 0.001 |
| Postdelivery BW (Kg) | 64.1 ± 10.4 | 64.2 ± 17.4 | 63.6 ± 9.2 | 0.702 |
| PD-BMI (Kg/m2) | 24.2 ± 4.1 | 24.2 ± 4.0 | 24.6 ± 4.1 | 0.518 |
| BWPostD-Pregravid (Kg) | 4.82 ± 5.66 | 5.2 ± 5.7 | 3.1 ± 5.1 | 0.022 |
| WC (cm) | 84.0 ± 9.0 | 83.9 ± 9.1 | 84.8 ± 8.6 | 0.545 |
| >89.5 cm | 71 (18.5%) | 54 (17.2%) | 17 (24.3%) | 0.119 |
| FP Glucose (mg/dL) mmol/dL | 84.6 ± 7.6. 4.7 ± 0.4 | 84.3 + 7.6 4.7 ± 0.4 | 86.0 + 7.6 4.8 ± 0.4 | 0.093 |
| >100 mg/dL | 8 (2.1%) | 6 (1.9%) | 2 (2.9%) | 0.443 |
| FP insulin (mcUI/mL) | 6.4 ± 5.3 | 6.6 ± 5.6 | 5.6 ± 4.0 | 0.172 |
| HOMA-IR | 1.6 ± 1.4 | 1.6 ± 1.3 | 1.6 ± 1.6 | 0.892 |
| >3.5 | 20 (5.2%) | 17 (5.4%) | 3 (4.3%) | 0.534 |
| SBP (mm Hg) | 111 ± 13 | 111 ± 13 | 111 ± 12 | 0.923 |
| DBP (mm Hg) | 73 ± 10 | 73 ± 11 | 74 ± 10 | 0.501 |
| T-Cholesterol mg/dL mmol/L | 196 ± 39 5.11 ± 1.01 | 195± 38 5.07 ± 0.99 | 205 ± 42 5.33 ± 1.09 | 0.055 |
| HDL-C mg/dL mmol/L | 64 ± 15 1.66 ± 0.39 | 63 ± 15 1.64 ± 0.39 | 68 ± 13 1.77 ± 0.34 | 0.199 |
| LDL-C mg/dL mmol/L | 122 ± 35 3.17 ± 0.91 | 120 ± 32 3.12 ± 0.83 | 130 ± 44 3.38 ± 1.14 | 0.314 |
| Triglycerides mg/dL mmol/L | 80 ± 44 0.91 ± 0.50 | 80 ± 42 0.91 ± 0.48 | 80 ± 52 0.91 ± 0.59 | 0.981 |
| Apolipoprotein B (mg/dL) | 91 ± 28 | 89 ± 28 | 97 ± 28 | 0.342 |
| CPR (mg/dL) | 2.0 ± 4.1 | 2.3 ± 4.1 | 1.2 ± 4.0 | 0.390 |
| Albumin/creatinine ratio (mg/g) | 11 ± 22 | 12 ± 24 | 7 ± 8 | 0.462 |
| HbA1c-IFCC % (mmol/mol) | 5.2 ± 0.3 33 ± 3 | 5.2 ± 0.3 33 ± 3 | 5.3 ± 0.3 34 ± 3 | 0.001 |
| >5.7% | 17 (4.4%) | 10 (3.2%) | 7 (10%) | 0.034 |
| TSH mcUI/mL | 2.2 ± 4.6 | 2.2 ± 5.0 | 2.1 ± 1.8 | 0.975 |
| FT4 (ng/dL) | 8.3 ± 3.0 | 8.3 ± 3.3 | 8.0 ± 1.2 | 0.409 |
| Nutrition Score | ||||
| Pregestational | 0.8 ± 2.9 | 0.8 ± 2.9 | 0.8 ± 3.0 | 0.976 |
| 36–38 GW | 4.8 ± 3.4 | 3.9 ± 3.0 | 7.8 ± 2.8 | 0.001 |
| PostDelivery | 3.7 ± 3.8 | 3.4 ± 3.8 | 5.2 ± 3.4 | 0.002 |
| MedDiet Score | ||||
| Pregestational | 4.5 ± 1.6 | 4.5 ± 1.6 | 4.5 ± 1.4 | 0.830 |
| 36–38 GW | 6.0 ± 2.1 | 5.5 ± 1.8 | 7.9 ± 2.2 | 0.001 |
| PostDelivery | 4.5 ± 3.2 | 4.5 ± 3.1 | 4.7 ± 3.6 | 0.625 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García de la Torre, N.; Assaf-Balut, C.; Jiménez Varas, I.; del Valle, L.; Durán, A.; Fuentes, M.; del Prado, N.; Bordiú, E.; Valerio, J.J.; Herraiz, M.A.; et al. Effectiveness of Following Mediterranean Diet Recommendations in the Real World in the Incidence of Gestational Diabetes Mellitus (GDM) and Adverse Maternal-Foetal Outcomes: A Prospective, Universal, Interventional Study with a Single Group. The St Carlos Study. Nutrients 2019, 11, 1210. https://doi.org/10.3390/nu11061210
García de la Torre N, Assaf-Balut C, Jiménez Varas I, del Valle L, Durán A, Fuentes M, del Prado N, Bordiú E, Valerio JJ, Herraiz MA, et al. Effectiveness of Following Mediterranean Diet Recommendations in the Real World in the Incidence of Gestational Diabetes Mellitus (GDM) and Adverse Maternal-Foetal Outcomes: A Prospective, Universal, Interventional Study with a Single Group. The St Carlos Study. Nutrients. 2019; 11(6):1210. https://doi.org/10.3390/nu11061210
Chicago/Turabian StyleGarcía de la Torre, Nuria, Carla Assaf-Balut, Inés Jiménez Varas, Laura del Valle, Alejandra Durán, Manuel Fuentes, Náyade del Prado, Elena Bordiú, Johanna Josefina Valerio, Miguel A. Herraiz, and et al. 2019. "Effectiveness of Following Mediterranean Diet Recommendations in the Real World in the Incidence of Gestational Diabetes Mellitus (GDM) and Adverse Maternal-Foetal Outcomes: A Prospective, Universal, Interventional Study with a Single Group. The St Carlos Study" Nutrients 11, no. 6: 1210. https://doi.org/10.3390/nu11061210
APA StyleGarcía de la Torre, N., Assaf-Balut, C., Jiménez Varas, I., del Valle, L., Durán, A., Fuentes, M., del Prado, N., Bordiú, E., Valerio, J. J., Herraiz, M. A., Izquierdo, N., Torrejón, M. J., Cuadrado, M. A., de Miguel, P., Familiar, C., Runkle, I., Barabash, A., Rubio, M. A., & Calle-Pascual, A. L. (2019). Effectiveness of Following Mediterranean Diet Recommendations in the Real World in the Incidence of Gestational Diabetes Mellitus (GDM) and Adverse Maternal-Foetal Outcomes: A Prospective, Universal, Interventional Study with a Single Group. The St Carlos Study. Nutrients, 11(6), 1210. https://doi.org/10.3390/nu11061210





