Elaeagnus glabra f. oxyphylla Attenuates Scopolamine-Induced Learning and Memory Impairments in Mice by Improving Cholinergic Transmission via Activation of CREB/NGF Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation
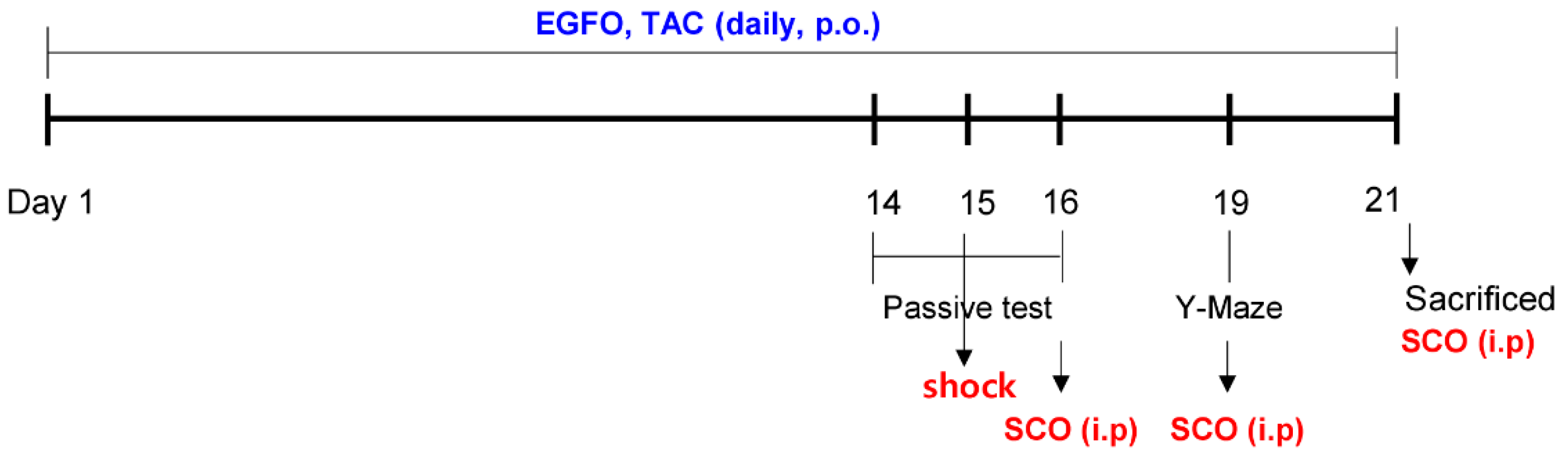
2.2. Experimental Animals and Drug Administration
2.3. Step-through Passive Avoidance Test
2.4. Y-maze Test
2.5. Brain Sectioning and Tissue Preparation
2.6. Immunoblot Analysis
2.7. Determination of Ach Levels and AChE Activity in Brain Tissue
2.8. Nissl and TUNEL Staining
2.9. Statistical Analysis
3. Results
3.1. Effect of EGFO in Mice with SCO-Induced Memory Impairments
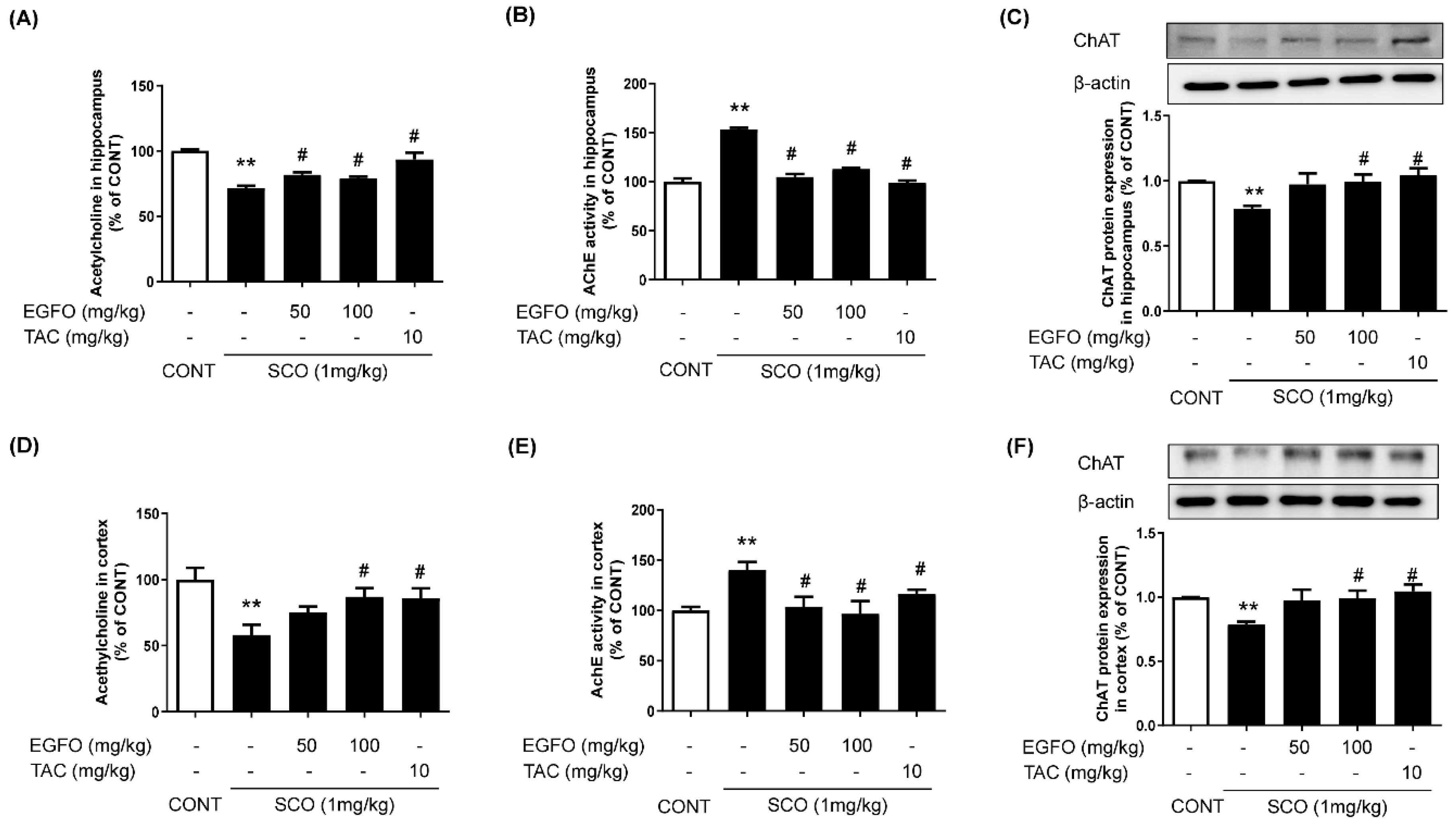
3.2. Effect of EGFO Extract on Cholinergic Dysfunction in Mice with SCO-Induced Memory Impairments
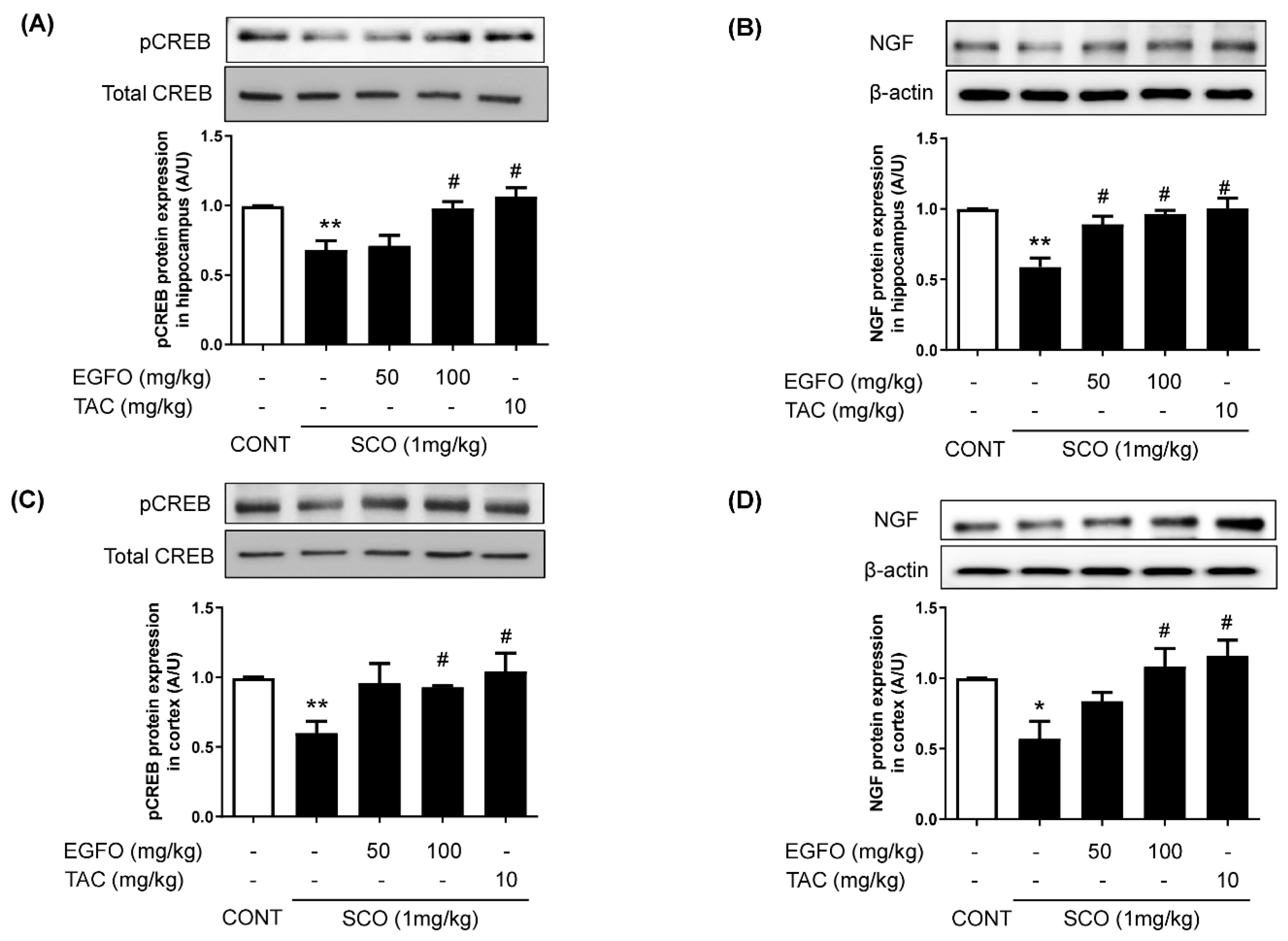
3.3. Effect of EGFO Extract on the Neuronal Markers CREB and NGF in Mice with SCO-Induced Memory Impairments
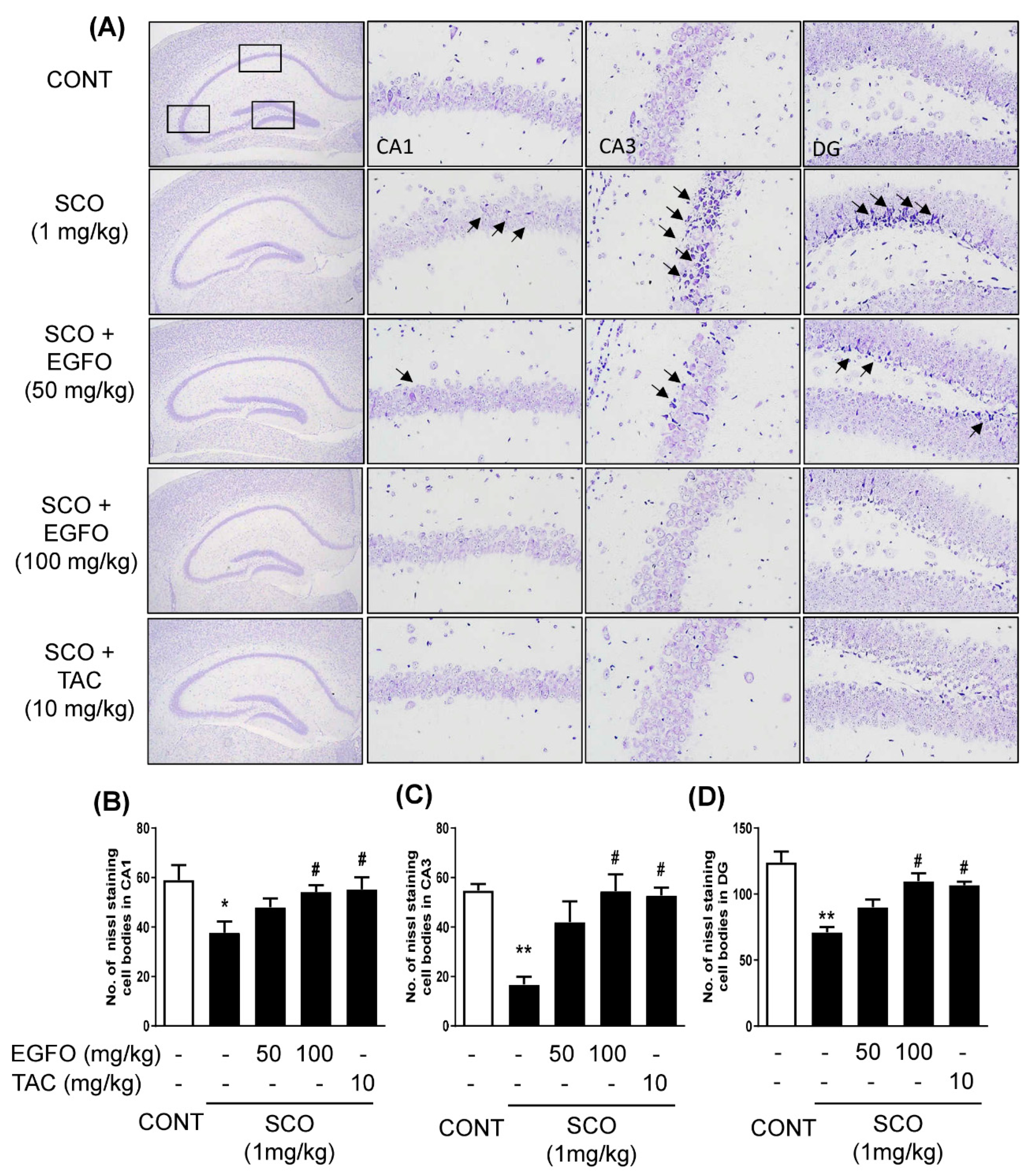
3.4. Protective Effect of EGFO Extract against Neuronal Damage in Mice with SCO-Induced Memory Impairments
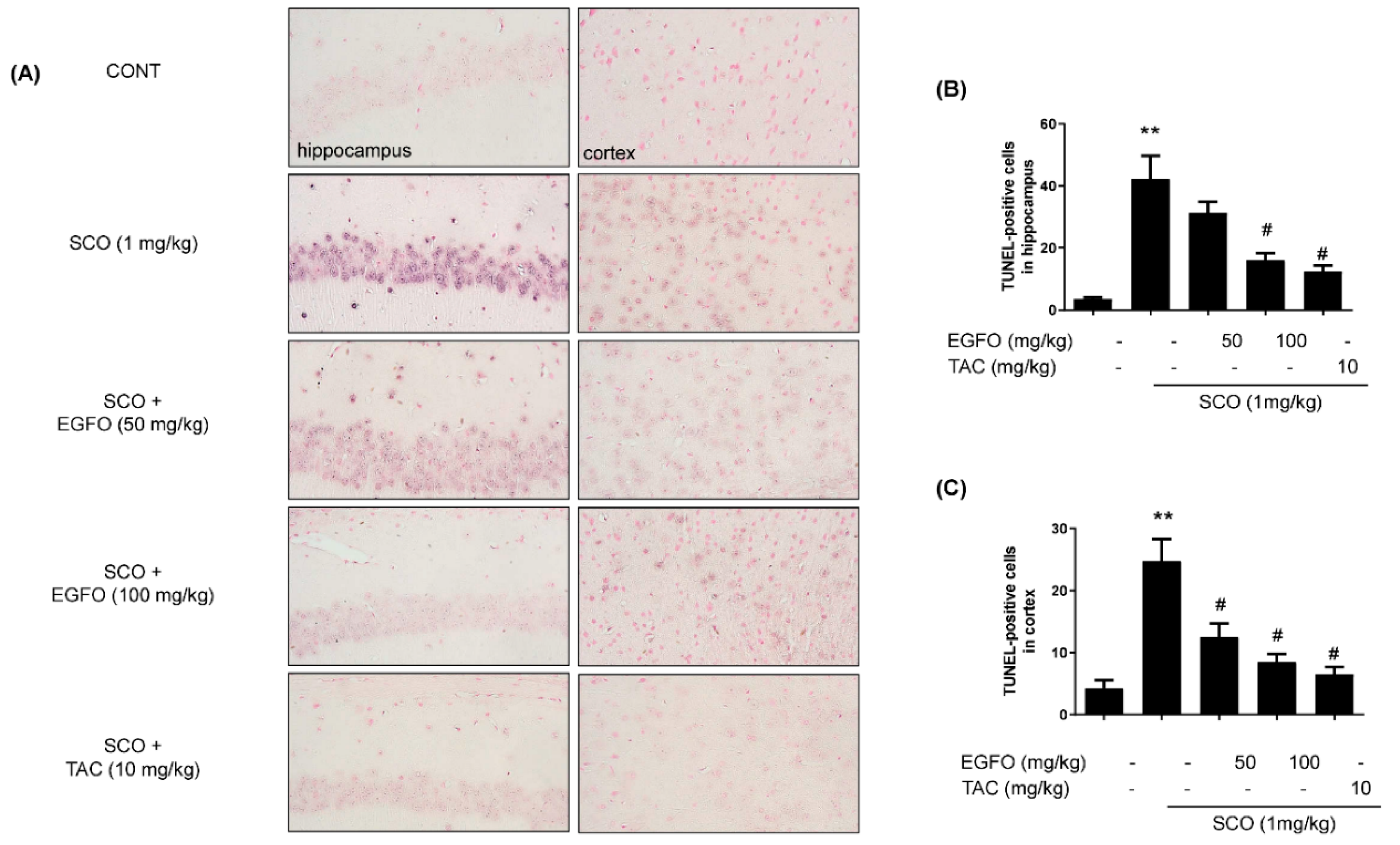
3.5. Effects of EGFO Extract on the Apoptosis Pathway in Mice with SCO-Induced Memory Impairments
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Mufson, E.J. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2006, 27, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhu, Y.; Wang, Y.; Qi, S.; Wang, Y.; Ma, C.; Li, S.; Jiang, B.; Cheng, X.; Wang, Z.; et al. Anti-amnesic effect of extract and alkaloid fraction from aerial parts of Peganum harmala on scopolamine-induced memory deficits in mice. J. Ethnopharmacol. 2017, 204, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Hung, T.M.; Bae, K.H.; Jung, J.W.; Lee, S.; Yoon, B.H.; Cheong, J.H.; Ko, K.H.; Ryu, J.H. Gomisin A improves scopolamine-induced memory impairment in mice. Eur. J. Pharmacol. 2006, 542, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Bierer, L.M.; Haroutunian, V.; Gabriel, S.; Knott, P.J.; Carlin, L.S.; Purohit, D.P.; Perl, D.P.; Schmeidler, J.; Kanof, P.; Davis, K.L. Neurochemical correlates of dementia severity in Alzheimer’s disease: Relative importance of the cholinergic deficits. J. Neurochem. 1995, 64, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinergic foundations of Alzheimer’s disease therapy. J. Physiol. 1998, 92, 283–287. [Google Scholar] [CrossRef]
- Op den Velde, W.; Stam, F.C. Some cerebral proteins and enzyme systems in Alzheimer’s presenile and senile dementia. J. Am. Geriatr. Soc. 1976, 24, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Gibson, P.H.; Blessed, G.; Perry, R.H.; Tomlinson, B.E. Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. J. Neurol. Sci. 1977, 34, 247–265. [Google Scholar] [CrossRef]
- Bruel-Jungerman, E.; Lucassen, P.J.; Francis, F. Cholinergic influences on cortical development and adult neurogenesis. Behav. Brain Res. 2011, 221, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Sugisaki, E.; Fukushima, Y.; Fujii, S.; Yamazaki, Y.; Aihara, T. The effect of coactivation of muscarinic and nicotinic acetylcholine receptors on LTD in the hippocampal CA1 network. Brain Res. 2016, 1649, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinesterase inhibitors stabilize Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2000, 920, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Nickmahzar, E.G.; Babakordi, F. Effect of Gingko biloba extract on scopolamine-induced apoptosis in the hippocampus of rats. Anat. Sci. Int. 2013, 88, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.Q.; Wu, D.W.; Zhang, C.X.; Yan, R.; Yang, C.; Rong, C.P.; Zhang, L.; Chang, X.; Su, R.Y.; Zhang, S.J.; et al. BushenYizhi formula ameliorates cognition deficits and attenuates oxidative stressrelated neuronal apoptosis in scopolamineinduced senescence in mice. Int. J. Mol. Med. 2014, 34, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, S.; Rabiei, Z.; Alibabaei, Z.; Mokhtari, S.; Rafieian-Kopaei, M.; Deris, F. Anti-amnesic activity of Citrus aurantium flowers extract against scopolamine-induced memory impairments in rats. Neurol. Sci. 2015, 36, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jimenez, R.; Groeneveld, G.J.; van Gerven, J.M.; Goulooze, S.C.; Baakman, A.C.; Hay, J.L.; Stevens, J. Model-based exposure-response analysis to quantify age related differences in the response to scopolamine in healthy subjects. Br. J. Clin. Pharmacol. 2016, 82, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.A.; Gartlehner, G.; Webb, A.P.; Morgan, L.C.; Moore, C.G.; Jonas, D.E. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. Clin. Interv. Aging 2008, 3, 211–225. [Google Scholar] [PubMed]
- Mimica, N.; Presecki, P. Side effects of approved antidementives. Psychiatr. Danub. 2009, 21, 108–113. [Google Scholar] [PubMed]
- Li, L.H.; Baek, I.K.; Kim, J.H.; Kang, K.H.; Koh, Y.S.; Jung, Y.D.; Cho, C.K.; Choi, S.Y.; Shin, B.A. Methanol extract of Elaeagnus glabra, a Korean medicinal plant, inhibits HT1080 tumor cell invasion. Oncol. Rep. 2009, 21, 559–563. [Google Scholar]
- Nishino, C.; Enoki, N.; Tawata, S.; Mori, A.; Kobayashi, K.; Fukushima, M. Antibacterial activity of flavonoids against Staphy;ococcus epidermids, a skin bacterium. Agric. Biol. Chem. 1987, 51, 139–143. [Google Scholar]
- Ahmadiani, A.; Hosseiny, J.; Semnanian, S.; Javan, M.; Saeedi, F.; Kamalinejad, M.; Saremi, S. Antinociceptive and anti-inflammatory effects of Elaeagnus angustifolia fruit extract. J. Ethnopharmacol. 2000, 72, 287–292. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Ramezani, M.; Namjo, N. Muscle relaxant activity of Elaeagnus angustifolia L. fruit seeds in mice. J. Ethnopharmacol. 2003, 84, 275–278. [Google Scholar] [CrossRef]
- Ramezani, M.; Hosseinzadeh, H.; Daneshmand, N. Antinociceptive effect of Elaeagnus angustifolia fruit seeds in mice. Fitoterapia 2001, 72, 255–262. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M.; Sohraby, M.; Shahlari, N.; Hamidpour, R. Russian olive (Elaeagnus angustifolia L.): From a variety of traditional medicinal applications to its novel roles as active antioxidant, anti-inflammatory, anti-mutagenic and analgesic agent. J. Tradit. Complement. Med. 2016, 7, 24–29. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Taghizadeh, M.; Takhtfiroozeh, S.M.; Talaei, S.A.R. The effect of Elaeagnus angustifolia water extract on scopolamine-induced memory impairment in rats. Zanjan Univ. Med. Sci. J. ZUMS J. 2014, 22, 101–111. [Google Scholar]
- Lee, Y.S.; Chang, Z.Q.; Oh, B.C.; Park, S.C.; Shin, S.R.; Kim, N.W. Antioxidant activity, anti-inflammatory activity, and whitening effects of extracts of Elaeagnus multiflora Thunb. J. Med. Food. 2007, 10, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Luo, D.; Li, L.; Tan, R.R.; Xu, Q.Q.; Qin, J.; Zhu, L.; Luo, N.C.; Xu, T.T.; Zhang, R.; et al. Ethyl acetate extract components of bushen-yizhi formula provides neuroprotection against scopolamine-induced cognitive impairment. Sci. Rep. 2017, 7, 9824. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hong, S.S.; Kim, H.W.; Lee, S.K.; Son, C.G. Gongjin-Dan Enhances Hippocampal Memory in a Mouse Model of Scopolamine-Induced Amnesia. PLoS ONE. 2016, 11, e0159823. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharmacol. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- Qizilbash, N.; Whitehead, A.; Higgins, J.; Wilcock, G.; Schneider, L.; Farlow, M. Cholinesterase inhibition for Alzheimer disease: A meta-analysis of the tacrine trials. Dementia Trialists’ Collaboration. Jama 1998, 280, 1777–1782. [Google Scholar] [CrossRef]
- Kida, S. A functional role for CREB as a positive regulator of memory formation and LTP. Exp. Neurobiol. 2012, 21, 136–140. [Google Scholar] [CrossRef]
- Walton, M.R.; Dragunow, I. Is CREB a key to neuronal survival? Trends Neurosci. 2000, 23, 48–53. [Google Scholar] [CrossRef]
- Deogracias, R.; Espliguero, G.; Iglesias, T.; Rodriguez-Pena, A. Expression of the neurotrophin receptor trkB is regulated by the cAMP/CREB pathway in neurons. Mol. Cell. Neurosci. 2004, 26, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Niccolini, F.; Pagano, G.; Politis, M. Cholinergic imaging in dementia spectrum disorders. Eur. J. Nucl. Med. Mol. Imaging. 2016, 43, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Whishaw, I.Q. Cholinergic receptor blockade in the rat impairs locale but not taxon strategies for place navigation in a swimming pool. Behav. Neurosci. 1985, 99, 979–1005. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Park, J.; Kwon, S.; Park, M.W.; Oh, S.M.; Yeom, M.J.; Shim, I.; Lee, H.J.; Hahm, D.H. Effect of wild ginseng on scopolamine-induced acetylcholine depletion in the rat hippocampus. J. Pharm. Pharmacol. 2010, 62, 263–271. [Google Scholar] [CrossRef]
- Puzzo, D.; Lee, L.; Palmeri, A.; Calabrese, G.; Arancio, O. Behavioral assays with mouse models of Alzheimer’s disease: Practical considerations and guidelines. Biochem. Pharmacol. 2014, 88, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Goodrich-Hunsaker, N.J.; Hopkins, R.O. Spatial memory deficits in a virtual radial arm maze in amnesic participants with hippocampal damage. Behav. Neurosci. 2010, 124, 405–413. [Google Scholar] [CrossRef]
- Giovannini, M.G.; Lana, D.; Pepeu, G. The integrated role of ACh, ERK and mTOR in the mechanisms of hippocampal inhibitory avoidance memory. Neurobiol. Learn. Mem. 2015, 119, 18–33. [Google Scholar] [CrossRef]
- Hughes, R.N. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 2004, 28, 497–505. [Google Scholar] [CrossRef]
- Reid, S.N.S.; Ryu, J.K.; Kim, Y.; Jeon, B.H. GABA-enriched fermented Laminaria japonica improves cognitive impairment and neuroplasticity in scopolamine- and ethanol-induced dementia model mice. Nutr. Res. Pract. 2018, 12, 199–207. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Paleari, L.; Grozio, A.; Cesario, A.; Russo, P. The cholinergic system and cancer. Semin. Cancer Biol. 2008, 18, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Villaca, Y.; Filgueiras, C.C.; Manhaes, A.C. Developmental aspects of the cholinergic system. Behav. Brain Res. 2011, 221, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Falsafi, S.K.; Deli, A.; Hoger, H.; Pollak, A.; Lubec, G. Scopolamine administration modulates muscarinic, nicotinic and NMDA receptor systems. PLoS ONE 2012, 7, e32082. [Google Scholar] [CrossRef]
- Ravichandran, V.A.; Kim, M.; Han, S.K.; Cha, Y.S. Stachys sieboldii extract supplementation attenuates memory deficits by modulating BDNF-CREB and its downstream molecules, in animal models of memory impairment. Nutrients 2018, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.M.; Franks, K.M.; Titterness, A.K.; Russell, K.; Merrill, D.A.; Christie, B.R.; Sejnowski, T.J.; Tuszynski, M.H. NGF is essential for hippocampal plasticity and learning. J. Neurosci. 2009, 29, 10883–10889. [Google Scholar] [CrossRef]
- Scott Bitner, R. Cyclic AMP response element-binding protein (CREB) phosphorylation: A mechanistic marker in the development of memory enhancing Alzheimer’s disease therapeutics. Biochem. Pharmacol. 2012, 83, 705–714. [Google Scholar] [CrossRef]
- Ao, H.; Ko, S.W.; Zhuo, M. CREB activity maintains the survival of cingulate cortical pyramidal neurons in the adult mouse brain. Mol. Pain 2006, 2, 15. [Google Scholar] [CrossRef]
- Sakamoto, K.; Karelina, K.; Obrietan, K. CREB: A multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 2011, 116, 1–9. [Google Scholar] [CrossRef]
- Brightwell, J.J.; Smith, C.A.; Neve, R.L.; Colombo, P.J. Long-term memory for place learning is facilitated by expression of cAMP response element-binding protein in the dorsal hippocampus. Learn. Mem. 2007, 14, 195–199. [Google Scholar] [CrossRef]
- Riccio, A.; Ahn, S.; Davenport, C.M.; Blendy, J.A.; Ginty, D.D. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 1999, 286, 2358–2361. [Google Scholar] [CrossRef] [PubMed]
- Mantamadiotis, T.; Lemberger, T.; Bleckmann, S.C.; Kern, H.; Kretz, O.; Martin Villalba, A.; Tronche, F.; Kellendonk, C.; Gau, D.; Kapfhammer, J.; et al. Disruption of CREB function in brain leads to neurodegeneration. Nat. Genet. 2002, 31, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kotani, S.; Yamauchi, T.; Teramoto, T.; Ogura, H. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience 2006, 142, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Ibach, B.; Haen, E. Acetylcholinesterase inhibition in Alzheimer’s Disease. Curr. Pharm. Des. 2004, 10, 231–251. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.G.; Lee, H.W.; Han, J.M.; Lee, S.K.; Kim, D.W.; Saravanakumar, A.; Son, C.G. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci. Rep. 2015, 5, 9651. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.S.; Jin, D.E.; Park, S.K.; Park, C.H.; Seung, T.W.; Bae, D.W.; Kim, D.O.; Heo, H.J. Antiamnesic effect of Actinidia arguta extract intake in a mouse model of TMT-Induced learning and memory dysfunction. Evid. Based Complement. Alternat. Med. 2015, 2015, 876484. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Jackson, P.A.; Forster, J.; Khan, J.; Grothe, T.; Perrinjaquet-Moccetti, T.; Haskell-Ramsay, C.F. Acute effects of a wild green-oat (Avena sativa) extract on cognitive function in middle-aged adults: A double-blind, placebo-controlled, within-subjects trial. Nutr. Neurosci. 2017, 20, 135–151. [Google Scholar] [CrossRef]


| Body Weight (g) | CONT | SCO | SCO + EGFO-50 | SCO + EGFO-100 | SCO + TAC |
|---|---|---|---|---|---|
| Initial | 35.35 ± 0.32 | 34.54 ± 0.60 | 35.66 ± 0.31 | 34.21 ± 0.69 | 35.39 ± 0.54 |
| End | 37.66 ± 0.60 | 37.32 ± 0.73 | 37.00 ± 0.52 | 36.05 ± 0.62 | 36.59 ± 0.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohn, E.; Lim, H.-S.; Kim, Y.J.; Kim, B.-Y.; Kim, J.-H.; Jeong, S.-J. Elaeagnus glabra f. oxyphylla Attenuates Scopolamine-Induced Learning and Memory Impairments in Mice by Improving Cholinergic Transmission via Activation of CREB/NGF Signaling. Nutrients 2019, 11, 1205. https://doi.org/10.3390/nu11061205
Sohn E, Lim H-S, Kim YJ, Kim B-Y, Kim J-H, Jeong S-J. Elaeagnus glabra f. oxyphylla Attenuates Scopolamine-Induced Learning and Memory Impairments in Mice by Improving Cholinergic Transmission via Activation of CREB/NGF Signaling. Nutrients. 2019; 11(6):1205. https://doi.org/10.3390/nu11061205
Chicago/Turabian StyleSohn, Eunjin, Hye-Sun Lim, Yu Jin Kim, Bu-Yeo Kim, Joo-Hwan Kim, and Soo-Jin Jeong. 2019. "Elaeagnus glabra f. oxyphylla Attenuates Scopolamine-Induced Learning and Memory Impairments in Mice by Improving Cholinergic Transmission via Activation of CREB/NGF Signaling" Nutrients 11, no. 6: 1205. https://doi.org/10.3390/nu11061205
APA StyleSohn, E., Lim, H.-S., Kim, Y. J., Kim, B.-Y., Kim, J.-H., & Jeong, S.-J. (2019). Elaeagnus glabra f. oxyphylla Attenuates Scopolamine-Induced Learning and Memory Impairments in Mice by Improving Cholinergic Transmission via Activation of CREB/NGF Signaling. Nutrients, 11(6), 1205. https://doi.org/10.3390/nu11061205






