Anti-Photoaging Effects of Four Insect Extracts by Downregulating Matrix Metalloproteinase Expression via Mitogen-Activated Protein Kinase-Dependent Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Insects Extracts
2.2. Experimental Animals and Oral Administration
2.3. UVB Irradiation
2.4. Skin Hydration and Transepidermal Water Loss (TEWL)
2.5. Histological Investigation
2.6. Determination of MMP-1, MMP-9, and Hyaluronic Acid (HA) Secretion Using Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Antioxidant Enzyme Activities
2.8. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
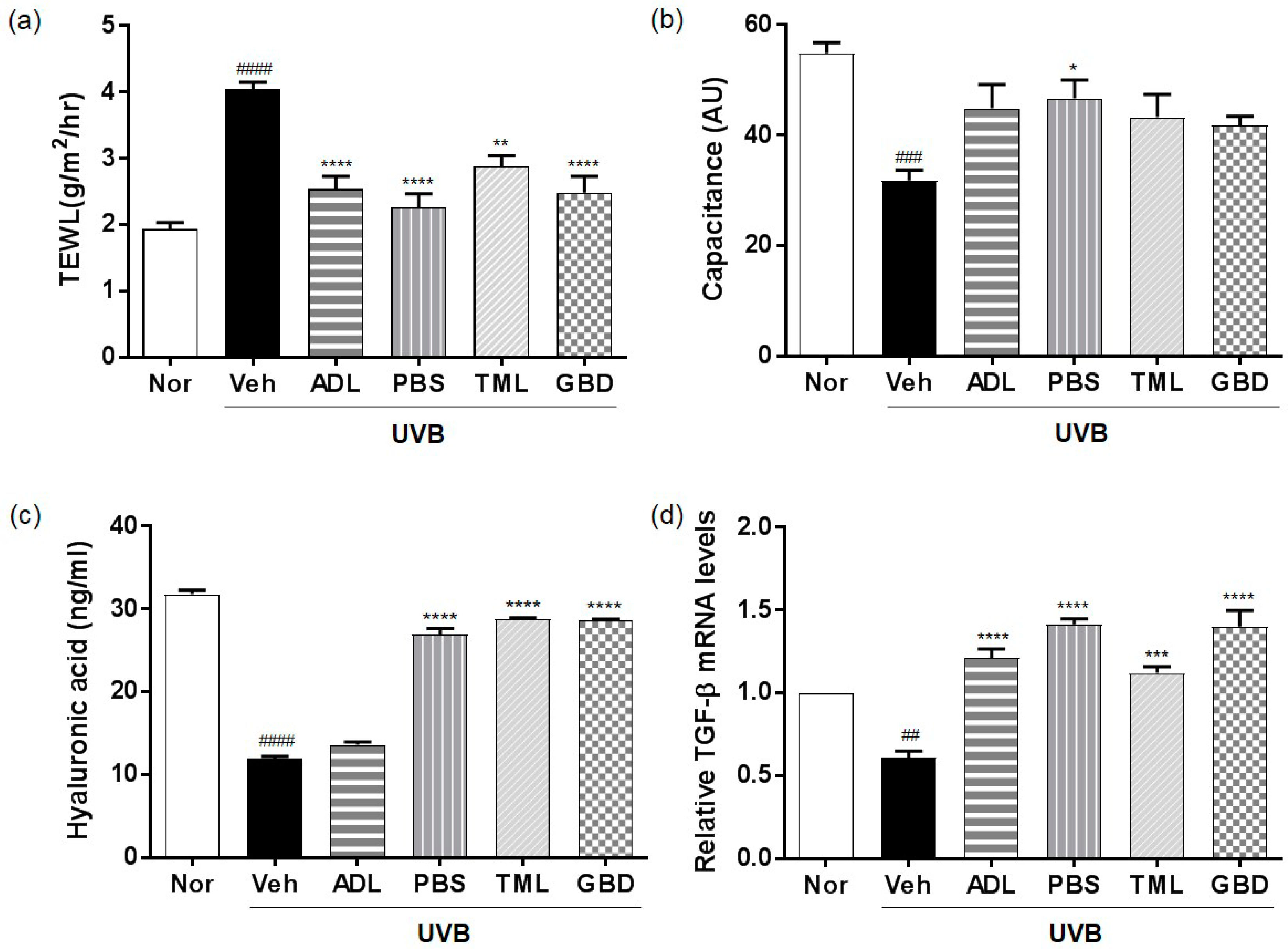
3.1. Effects of Insect Extracts on Skin Hydration Factors in UVB-Irradiated Hairless Mice
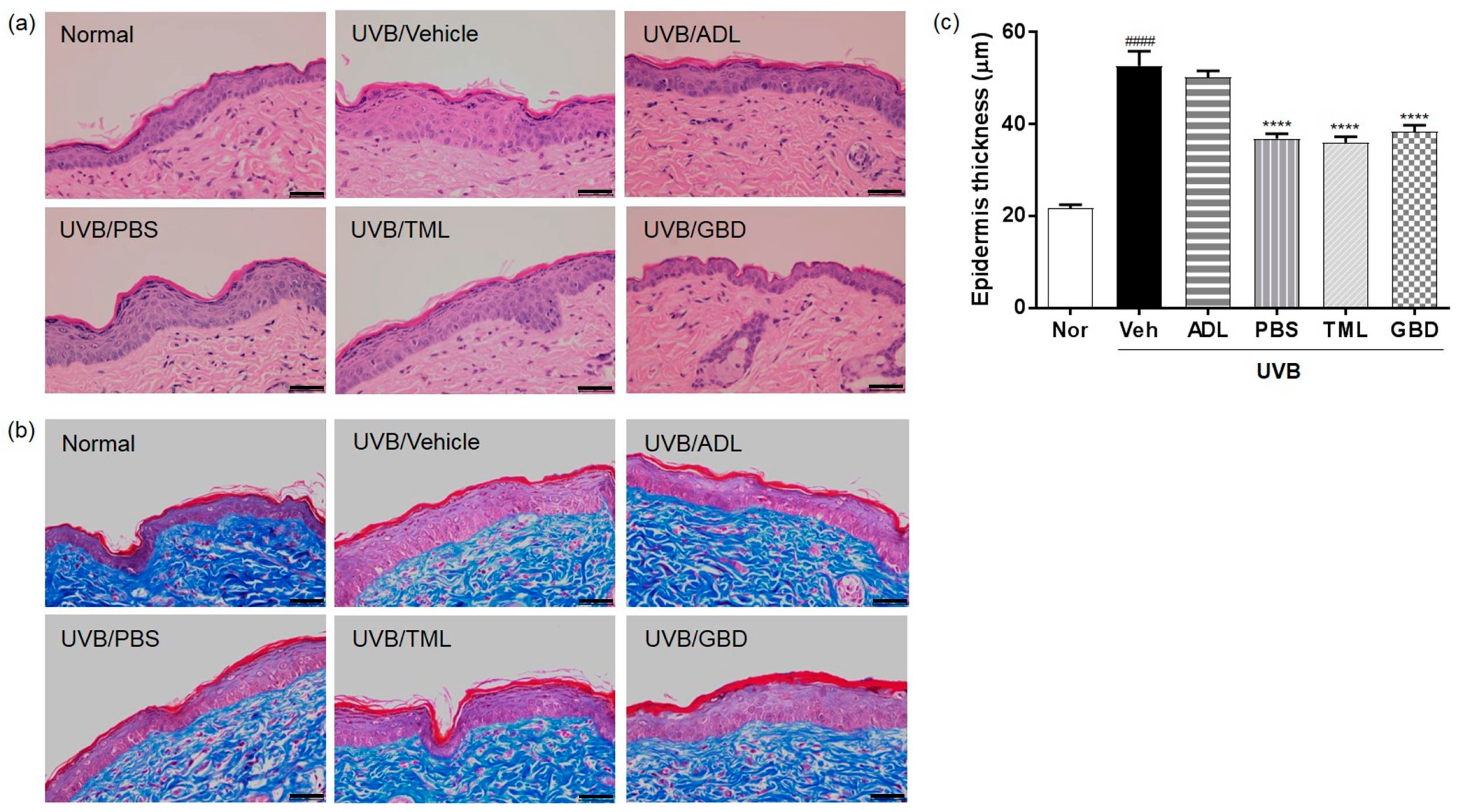
3.2. Effect of Insect Extracts on Restoration of Skin Thickening and Collagen Destruction on UVB-Irradiated Hairless Mice
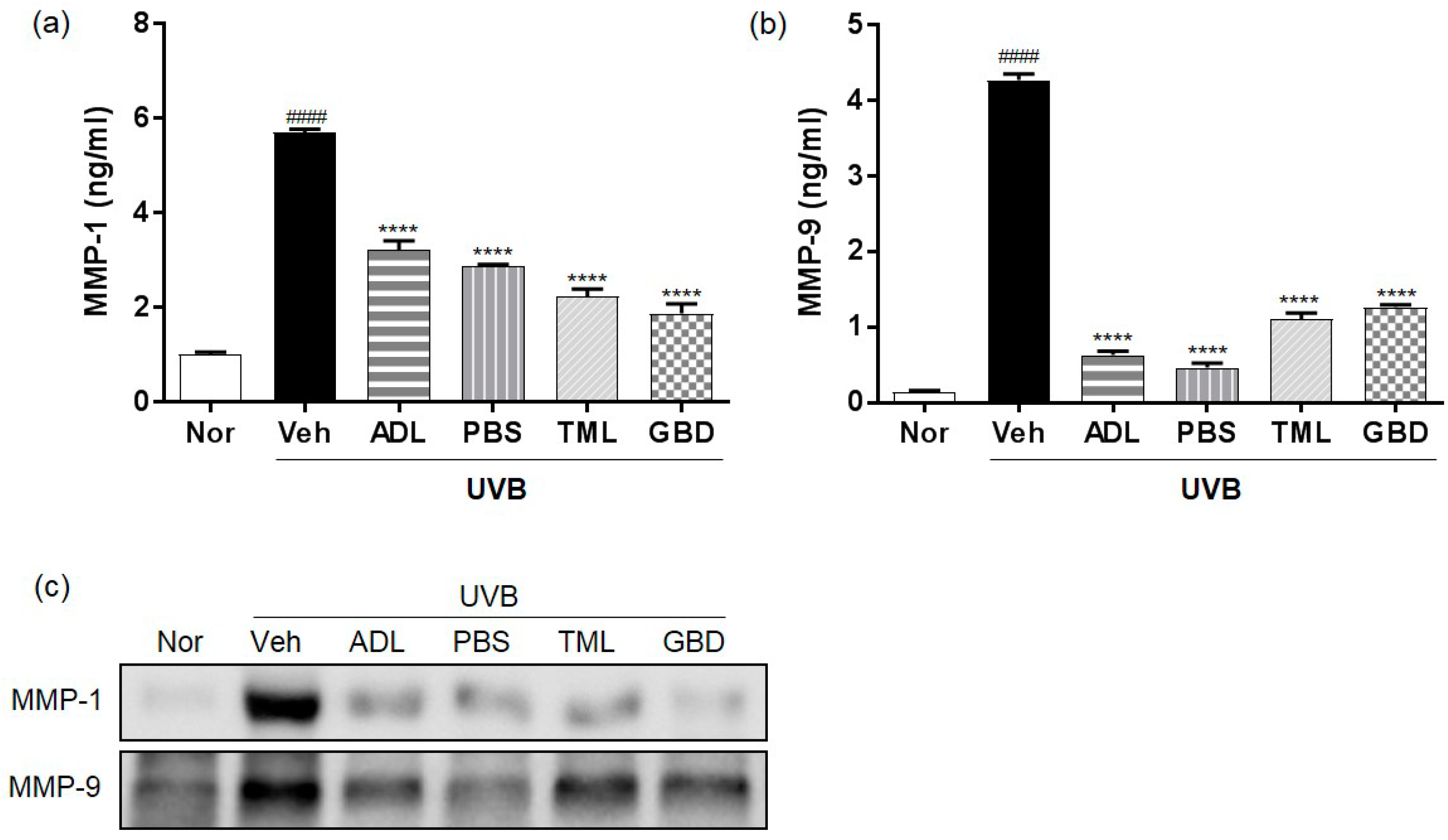
3.3. Insect Extracts Inhibit UVB-Induced MMP-1 and MMP-9 Expression
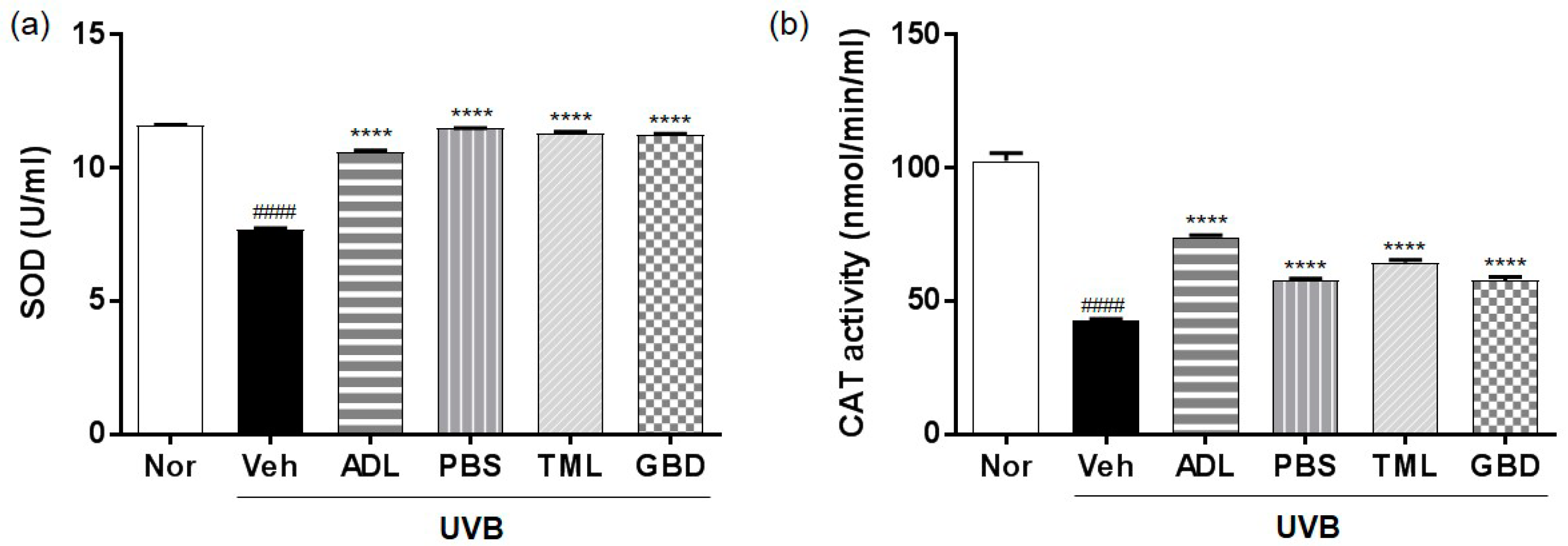
3.4. Effects of Insect Extracts on Antioxidant Enzymes in UVB-Irradiated Hairless Mice
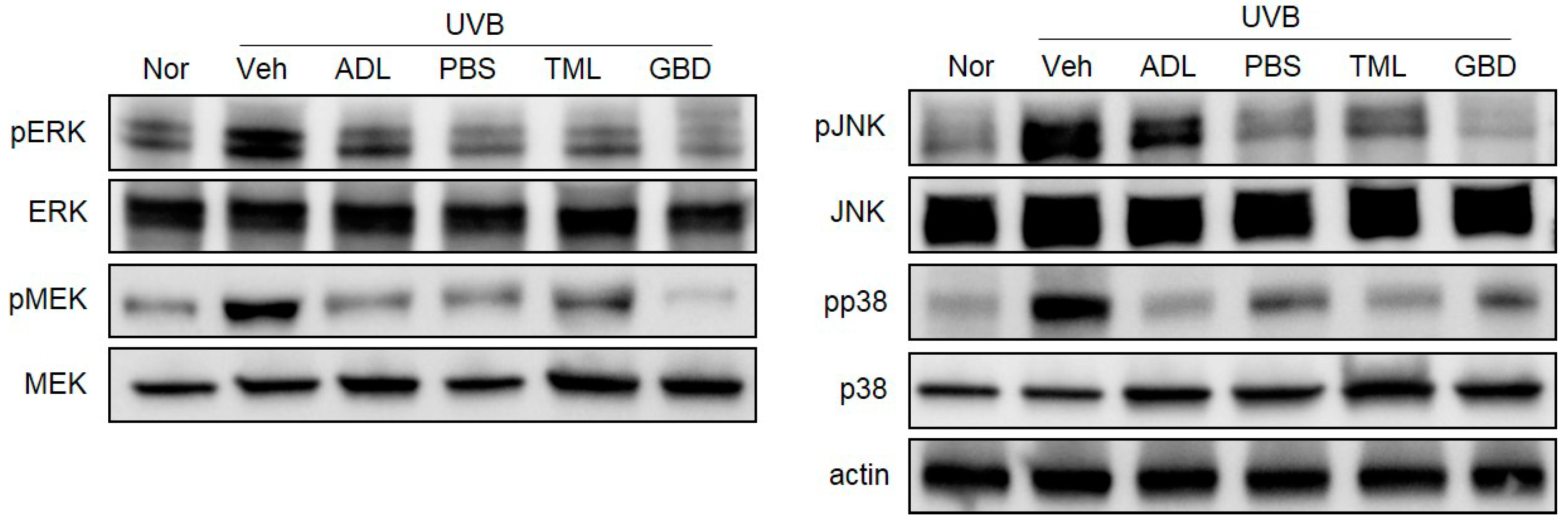
3.5. Effects of Insect Extracts on MAPK Phosphorylation in UVB-Irradiated Hairless Mice
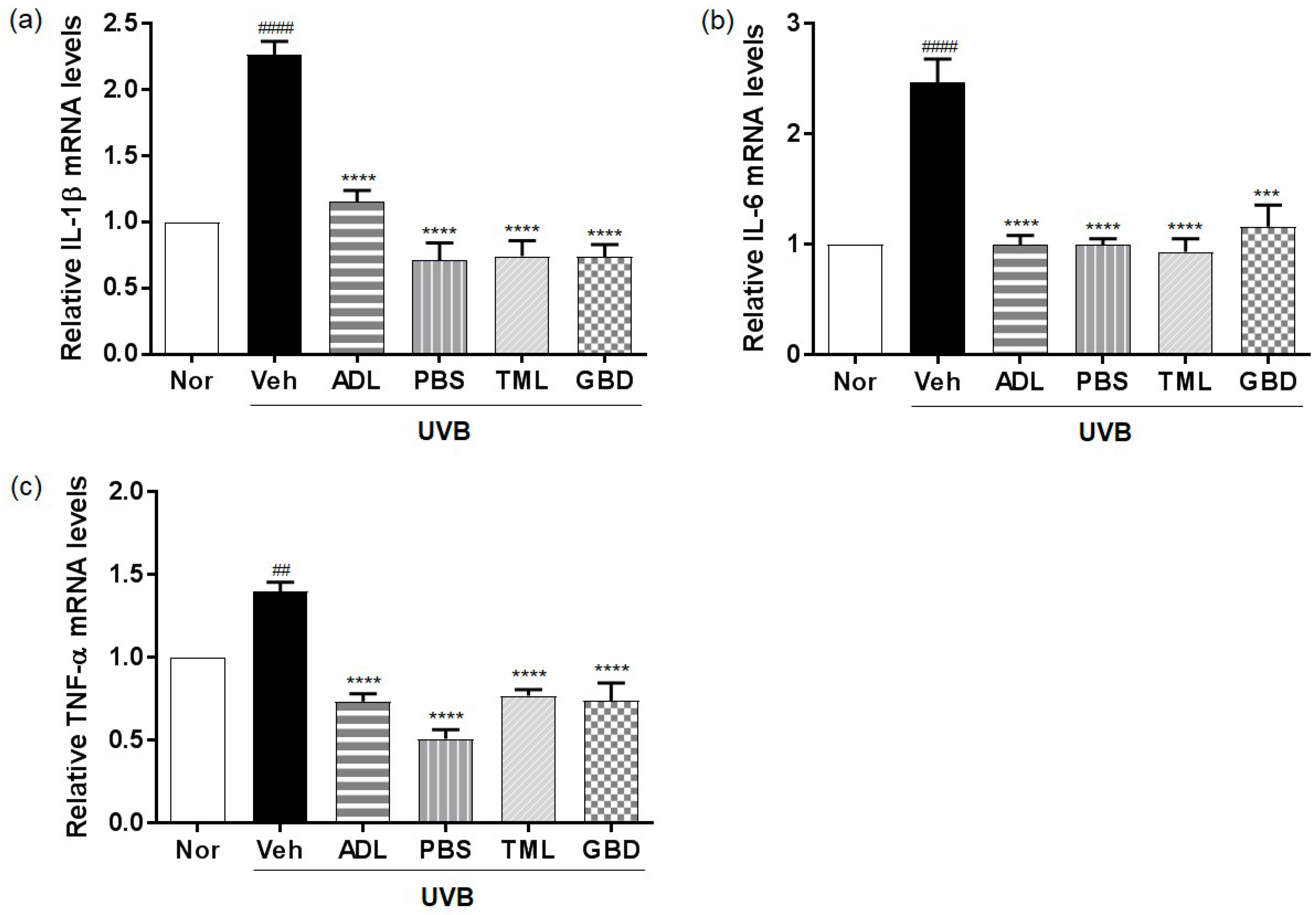
3.6. Effects of Insect Extracts on the mRNA Expression of Inflammatory Cytokines in UVB-Irradiated Hairless Mice
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, D.; Xu, M.L.; Shi, S.S.; Xiong, J.F. Toxicological characteristics of edible insects in China: A historical review. Food Chem. Toxicol. 2018, 119, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.A.; Schluter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Poma, G.; Cuykx, M.; Amato, E.; Calaprice, C.; Focant, F.J.; Covaci, A. Evaluation of hazardous chemicals in edible insects and insect-based food intended for human consumption. Food Chem. Toxicol. 2017, 100, 70–79. [Google Scholar] [CrossRef]
- Shantibala, T.; Lokeshwari, R.K.; Debaraj, H. Nutritional and antinutritional composition of the five species of aquatic edible insects consumed in Manipur, India. J. Insect Sci. 2014, 14, 14. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Dupont, E.; Gomez, J.; Bilodeau, D. Beyond UV radiation: A skin under challenge. Int. J. Cosmet. Sci. 2013, 35, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Oba, C.; Ohara, H.; Morifuji, M.; Ito, K.; Ichikawa, S.; Kawahata, K.; Koga, J. Collagen hydrolysate intake improves the loss of epidermal barrier function and skin elasticity induced by UVB irradiation in hairless mice. Photodermatol. Photoimmunol. Photomed. 2013, 29, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Yasuda, R.; Sayo, T.; Ishikawa, O.; Inoue, S. Hyaluronan exists in the normal stratum corneum. J. Investig. Dermatol. 2000, 114, 1184–1187. [Google Scholar] [CrossRef]
- Kawada, C.; Kimura, M.; Masuda, Y.; Nomura, Y. Oral administration of hyaluronan prevents skin dryness and epidermal thickening in ultraviolet irradiated hairless mice. J. Photochem. Photobiol. B Biol. 2015, 153, 215–221. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermato-endocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Little, E.; Quan, H.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: Impact of altered extracellular matrix microenvironment on dermal fibroblast function. J. Investig. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, S.M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia-Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Scharffetter-Kochanek, K.; Wlaschek, M.; Brenneisen, P.; Schauen, M.; Blaudschun, R.; Wenk, J. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol. Chem. 1997, 378, 1247–1257. [Google Scholar]
- Wlaschek, M.; Tantcheva-Poor, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schuller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B Biol. 2001, 63, 41–51. [Google Scholar] [CrossRef]
- McCullough, J.L.; Kelly, K.M. Prevention and treatment of skin aging. Ann. N. Y. Acad. Sci. 2006, 1067, 323–331. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, I.H.; Kim, J.H.; Kim, M.A.; Hwang, J.S.; Kim, Y.H.; Na, M. Quinoxaline-, dopamine-, and amino acid-derived metabolites from the edible insect Protaetia brevitarsis seulensis. Arch. Pharm Res. 2017, 40, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, W.; Kim, M.A.; Hwang, J.S.; Na, M.; Bae, J.S. Inhibition of platelet aggregation and thrombosis by indole alkaloids isolated from the edible insect Protaetia brevitarsis seulensis (Kolbe). J. Cell. Mol. Med. 2017, 21, 1217–1227. [Google Scholar] [CrossRef]
- Kim, J.; Yun, E.Y.; Park, S.W.; Goo, T.W.; Seo, M. Allomyrina dichotoma larvae regulate food intake and body weight in high fat diet-induced obese mice through mTOR and MAPK signaling pathways. Nutrients 2016, 8, 100. [Google Scholar] [CrossRef]
- Kim, S.W.; Suh, H.W.; Yoo, B.K.; Kwon, K.; Yu, K.; Choi, J.Y.; Kwon, O.Y. Larval hemolymph of rhinoceros beetle, Allomyrina dichotoma, enhances insulin secretion through ATF3 gene expression in INS-1 pancreatic beta-cells. Z. Naturforsch. C 2018, 73, 391–396. [Google Scholar] [CrossRef]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Kang, M.C.; Yumnam, S.; Kim, S.Y. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int. J. Mol. Sci. 2018, 19, 3551. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Freudenberger, T.; Zipper, P.; Melchior, A.; Grether-Beck, S.; Rabausch, B.; de Groot, J.; Twarock, S.; Hanenberg, H.; Homey, B.; et al. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am. J. Pathol. 2007, 171, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef]
- Sjerobabski Masnec, I.; Poduje, S. Photoaging. Coll. Antropol. 2008, 32, 177–180. [Google Scholar]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation-a review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Pandel, R.; Poljsak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef]
- Munshi, A.; Ramesh, R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer 2013, 4, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.M.; Sharma, M.R.; Werth, V.P. UVB and proinflammatory cytokines synergistically activate TNF-alpha production in keratinocytes through enhanced gene transcription. J. Investig. Dermatol. 2009, 129, 994–1001. [Google Scholar] [CrossRef]
| Scientific Name | Family Name | Order Name |
|---|---|---|
| Allomyrina dichotoma larva (ADL) | Scarabaeidae | Coleoptera |
| Protaetia brevitarsis seulensis (PBS) | Scarabaeidae | Coleoptera |
| Tenebrio molitor Linnaeus (TML) | Tenebrionidae | Coleoptera |
| Gryllus bimaculatus De Geer (GBD) | Gryllidae | Orthoptera |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, A.-R.; Ji, K.-Y.; Park, I.; Lee, J.Y.; Kim, K.M.; Na, M.; Chae, S. Anti-Photoaging Effects of Four Insect Extracts by Downregulating Matrix Metalloproteinase Expression via Mitogen-Activated Protein Kinase-Dependent Signaling. Nutrients 2019, 11, 1159. https://doi.org/10.3390/nu11051159
Im A-R, Ji K-Y, Park I, Lee JY, Kim KM, Na M, Chae S. Anti-Photoaging Effects of Four Insect Extracts by Downregulating Matrix Metalloproteinase Expression via Mitogen-Activated Protein Kinase-Dependent Signaling. Nutrients. 2019; 11(5):1159. https://doi.org/10.3390/nu11051159
Chicago/Turabian StyleIm, A-Rang, Kon-Young Ji, InWha Park, Joo Young Lee, Ki Mo Kim, MinKyun Na, and Sungwook Chae. 2019. "Anti-Photoaging Effects of Four Insect Extracts by Downregulating Matrix Metalloproteinase Expression via Mitogen-Activated Protein Kinase-Dependent Signaling" Nutrients 11, no. 5: 1159. https://doi.org/10.3390/nu11051159
APA StyleIm, A.-R., Ji, K.-Y., Park, I., Lee, J. Y., Kim, K. M., Na, M., & Chae, S. (2019). Anti-Photoaging Effects of Four Insect Extracts by Downregulating Matrix Metalloproteinase Expression via Mitogen-Activated Protein Kinase-Dependent Signaling. Nutrients, 11(5), 1159. https://doi.org/10.3390/nu11051159





