Sea Cucumber (Stichopus japonicas) F2 Enhanced TRAIL-Induced Apoptosis via XIAP Ubiquitination and ER Stress in Colorectal Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Reagents and Antibodies
2.3. Survival Assay
2.4. Transfection of Small Interfering RNA (siRNA)
2.5. Transient Transfection
2.6. Western Blotting
2.7. Apoptosis Assay
2.8. Co-Immunoprecipitation
2.9. Colony Formation Assay
2.10. Reverse Transcriptase Polymerase Chain Reaction
2.11. Real-Time PCR
2.12. ROS Generation
2.13. Tumor Xenograft Experiment
2.14. Statistical Analysis
3. Results
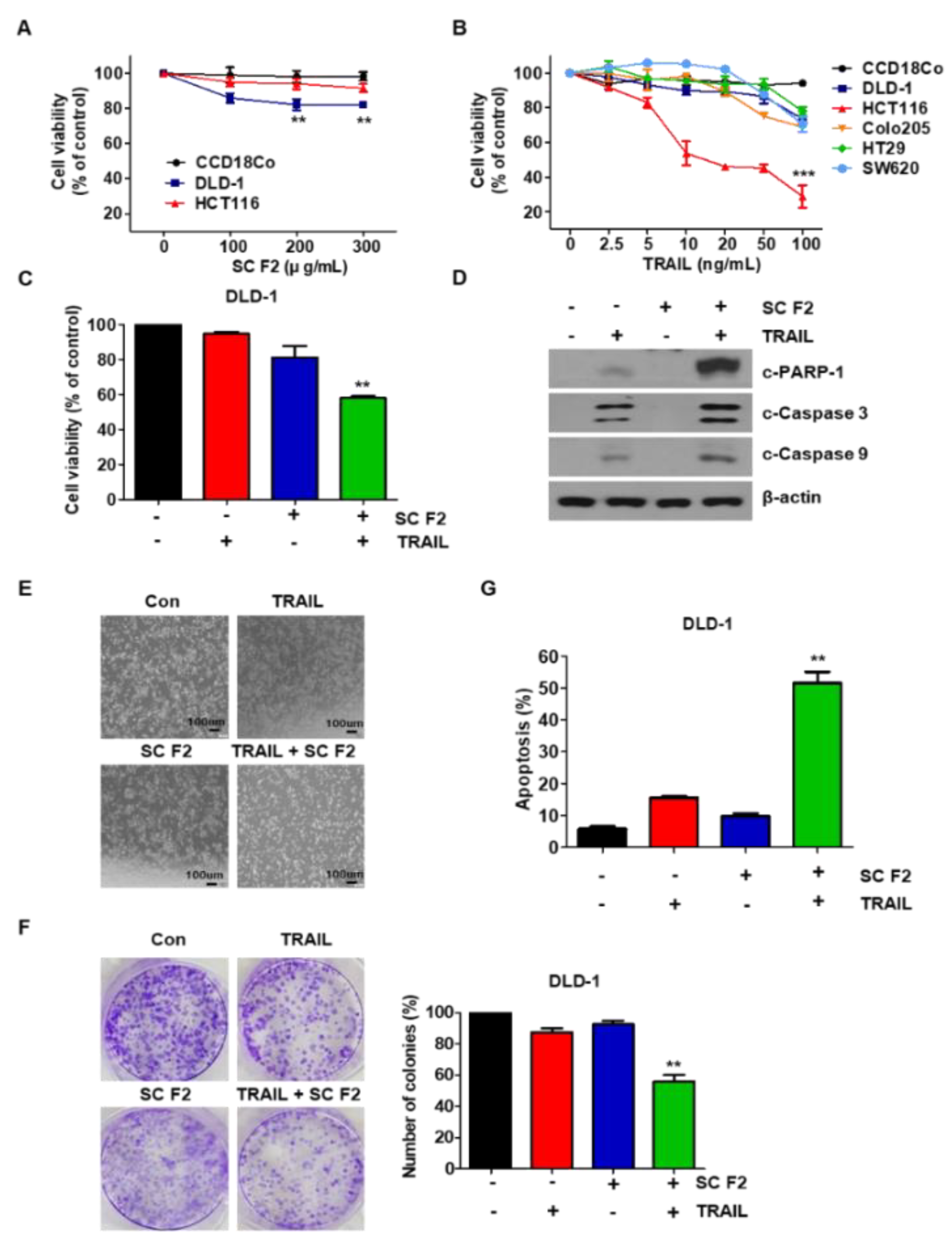
3.1. SC F2 Enhances TRAIL-Induced Apoptosis in CRC Cells
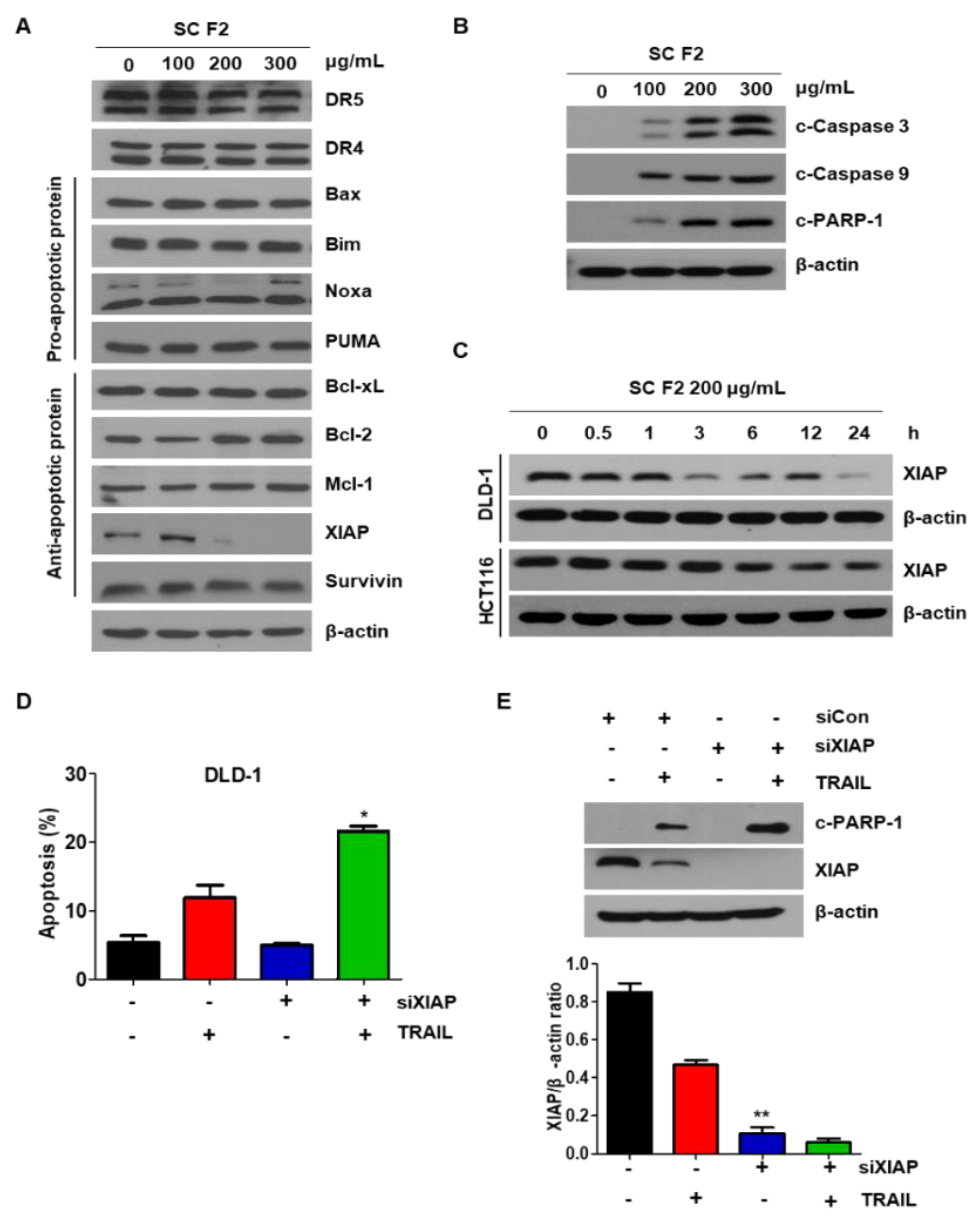
3.2. SC F2 Sensitizes CRC Cells to TRAIL-Induced Apoptosis Via XIAP
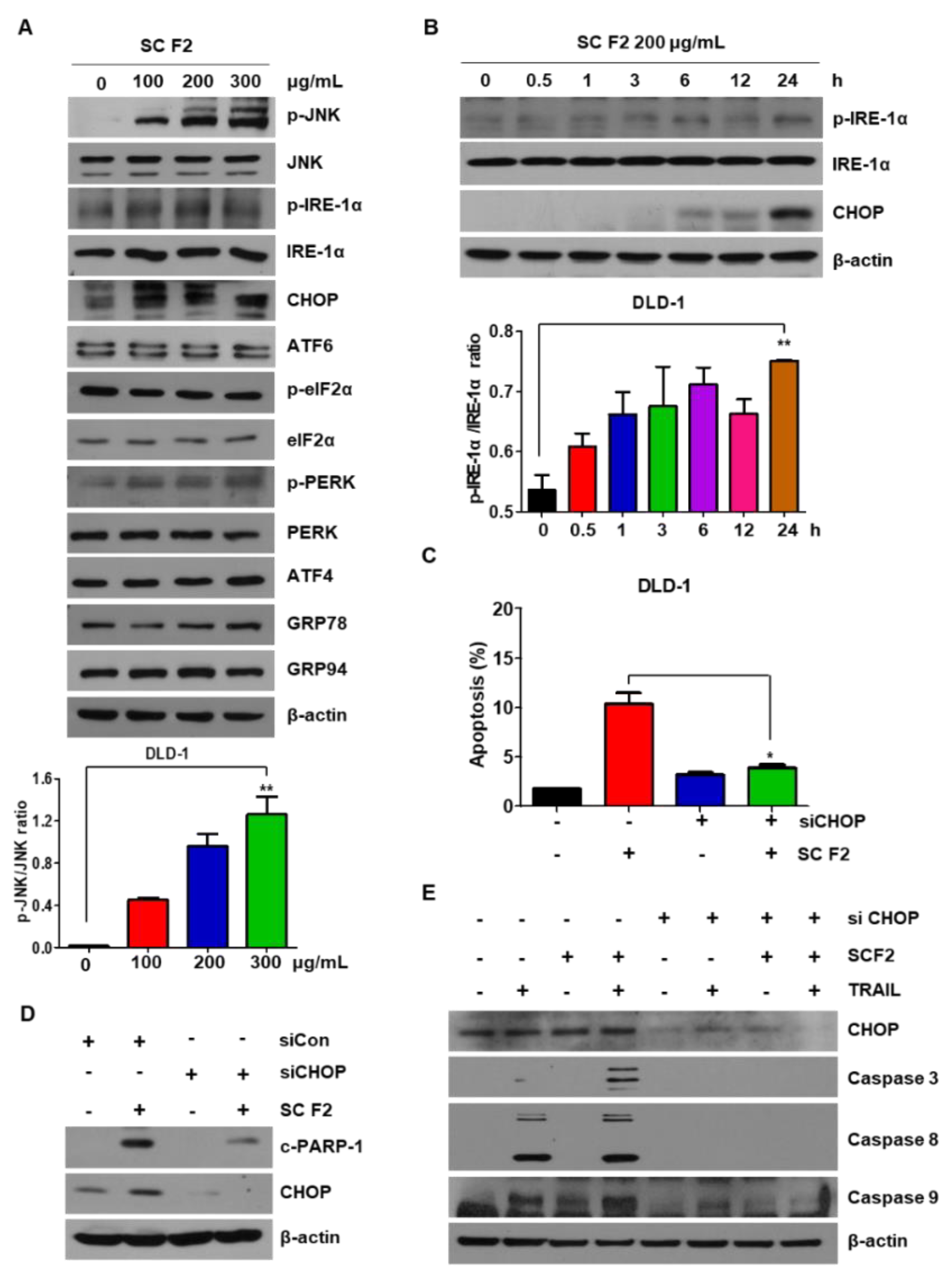
3.3. SC F2-Induced TRAIL Apoptosis of CRC Is Mediated by ER Stress and JNK Phosphorylation
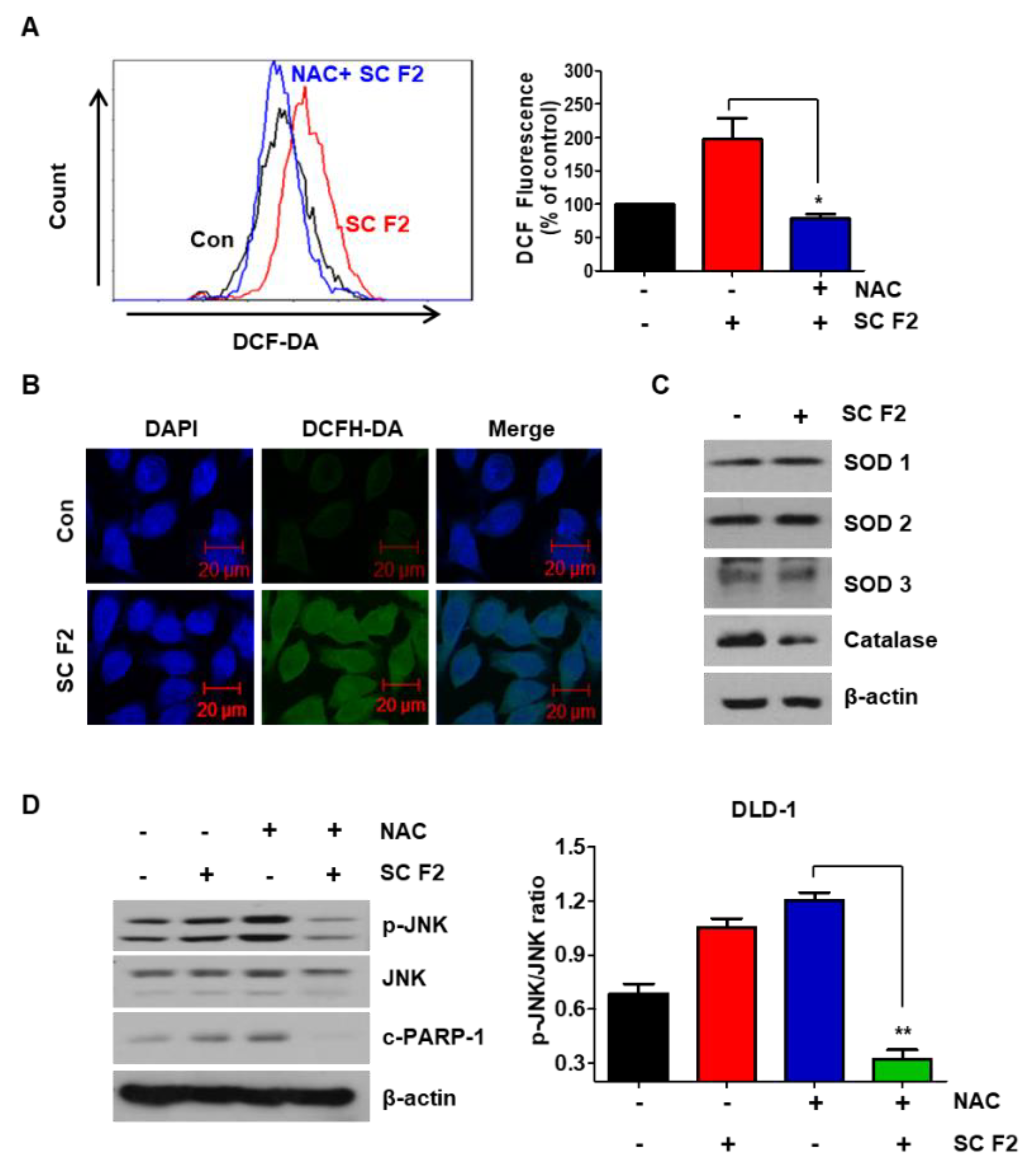
3.4. ROS Generation Mediates SC F2-Activated ER Stress Pathways
3.5. SC F2 Induces XIAP Degradation through the Ubiquitin Signaling Pathway
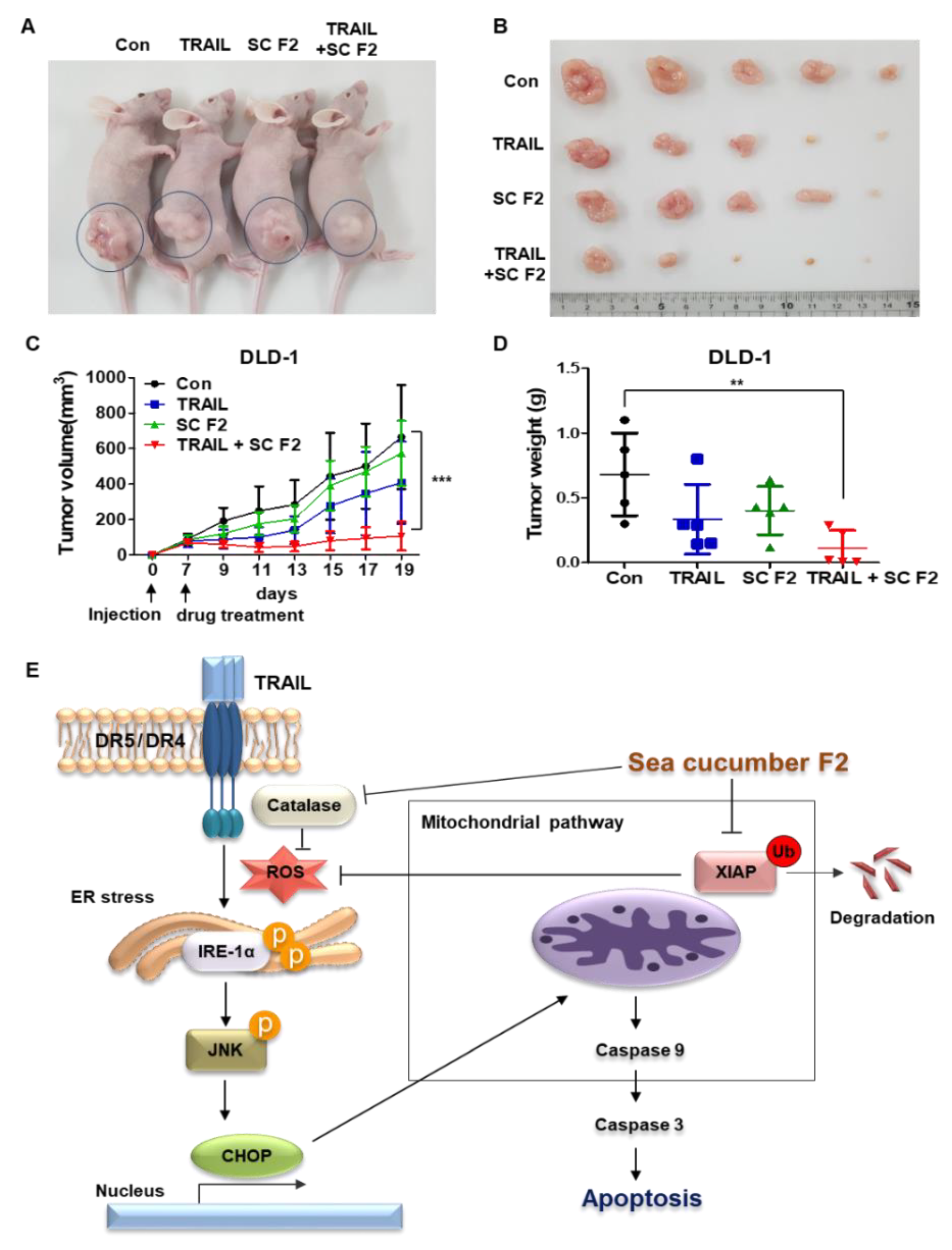
3.6. SC F2 Increases TRAIL-Induced Apoptosis In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TRAIL | TNF-related apoptosis-inducing ligand |
| XIAP | X-linked inhibitor of apoptosis protein |
| CHOP | C/EBP-homologous protein |
| eIF2α | eukaryotic translation initiation factor 2α |
| ER | endoplasmic reticulum |
| FITC | fluorescein isothiocyanate |
| JNK | c-Jun NH2-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| MTT | 3-(4,5-dimethylthiazol-2-ly)-2,5-diphenyl tetrazolium bromide |
| PARP | poly(ADP-ribose) polymerase |
| PBS | phosphate-buffered saline |
| PI | propidium iodide |
| ROS | reactive oxygen species |
References
- The World Health Organisation. Global battle against cancer won’t be won with treatment alone--effective prevention measures urgently needed to prevent cancer crisis. Cent. Eur. J. Public Health 2014, 22, 23–28. [Google Scholar]
- Refaat, A.; Abd-Rabou, A.; Reda, A. Trail combinations: The new ‘trail’ for cancer therapy (review). Oncol. Lett. 2014, 7, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, F.C.; Lawrence, D.A.; Tinel, A.; LeBlanc, H.; Virmani, A.; Schow, P.; Gazdar, A.; Blenis, J.; Arnott, D.; Ashkenazi, A. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J. Biol. Chem. 2001, 276, 46639–46646. [Google Scholar] [CrossRef]
- Fulda, S. Safety and tolerability of trail receptor agonists in cancer treatment. Eur. J. Clin. Pharm. 2015, 71, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Stuckey, D.W.; Shah, K. Trail on trial: Preclinical advances in cancer therapy. Trends Mol. Med. 2013, 19, 685–694. [Google Scholar] [CrossRef]
- Trivedi, R.; Mishra, D.P. Trailing trail resistance: Novel targets for trail sensitization in cancer cells. Front. Oncol. 2015, 5, 69. [Google Scholar] [CrossRef]
- Minamino, T.; Komuro, I.; Kitakaze, M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ. Res. 2010, 107, 1071–1082. [Google Scholar] [CrossRef]
- Thuerauf, D.J.; Marcinko, M.; Gude, N.; Rubio, M.; Sussman, M.A.; Glembotski, C.C. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ. Res. 2006, 99, 275–282. [Google Scholar] [CrossRef]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, M.; Zou, P.; Kanchana, K.; Weng, Q.; Chen, W.; Zhong, P.; Ji, J.; Zhou, H.; He, L.; et al. Curcumin analog wz35 induced cell death via ros-dependent er stress and g2/m cell cycle arrest in human prostate cancer cells. BMC Cancer 2015, 15, 866. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, C.; Su, J.; Xie, Q.; Ma, L.; Zeng, L.; Yu, Y.; Liu, S.; Li, S.; Li, Z.; et al. Tolerance to endoplasmic reticulum stress mediates cisplatin resistance in human ovarian cancer cells by maintaining endoplasmic reticulum and mitochondrial homeostasis. Oncol. Rep. 2015, 34, 3051–3060. [Google Scholar] [CrossRef]
- Zhu, H.; Abulimiti, M.; Liu, H.; Su, X.J.; Liu, C.H.; Pei, H.P. Rita enhances irradiation-induced apoptosis in p53-defective cervical cancer cells via upregulation of ire1alpha/xbp1 signaling. Oncol. Rep. 2015, 34, 1279–1288. [Google Scholar] [CrossRef][Green Version]
- Kong, B.; Cheng, T.; Wu, W.; Regel, I.; Raulefs, S.; Friess, H.; Erkan, M.; Esposito, I.; Kleeff, J.; Michalski, C.W. Hypoxia-induced endoplasmic reticulum stress characterizes a necrotic phenotype of pancreatic cancer. Oncotarget 2015, 6, 32154–32160. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Mohammed, A.; Rao, C.V. Sea cucumbers metabolites as potent anti-cancer agents. Mar. Drugs 2015, 13, 2909–2923. [Google Scholar] [CrossRef]
- Wargasetia, T.L.; Widodo. Mechanisms of cancer cell killing by sea cucumber-derived compounds. Investig. New Drugs 2017, 35, 820–826. [Google Scholar] [CrossRef]
- Wargasetia, T.L.; Permana, S.; Widodo, N. Potential use of compounds from sea cucumbers as mdm2 and cxcr4 inhibitors to control cancer cell growth. Exp. Ther. Med. 2018, 16, 2985–2991. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.A.; Surayot, U.; You, S. Structural characterization of immunostimulating protein-sulfated fucan complex extracted from the body wall of a sea cucumber, stichopus japonicus. Int. J. Biol. Macromol. 2017, 99, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, J.L.; Jeong, S.; Kim, B.R.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A.; Kim, D.Y.; Kim, B.G.; et al. Codium fragile f2 sensitize colorectal cancer cells to trail-induced apoptosis via c-flip ubiquitination. Biochem. Biophys. Res. Commun. 2019, 508, 1–8. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Huang, H.; Lin, F.; Wu, D.; Sun, A.; Chang, H.; Feng, Y. Combination of DNA methylation inhibitor 5-azacytidine and arsenic trioxide has synergistic activity in myeloma. Eur. J. Haematol. 2009, 82, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kim, J.L.; Lee, D.H.; Jeong, S.; Kim, B.R.; Na, Y.J.; Park, S.H.; Jo, M.J.; Jeong, Y.A.; Oh, S.C. Imatinibinduced apoptosis of gastric cancer cells is mediated by endoplasmic reticulum stress. Oncol. Rep. 2019, 41, 1616–1626. [Google Scholar]
- Rathore, R.; McCallum, J.E.; Varghese, E.; Florea, A.M.; Busselberg, D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 2017, 22, 898–919. [Google Scholar] [CrossRef] [PubMed]
- Selvarajoo, K. A systems biology approach to overcome trail resistance in cancer treatment. Apoptosis Int. J. Progr. Cell Death 2017, 128, 142–154. [Google Scholar] [CrossRef]
- Adrian, T.E.; Collin, P. The anti-cancer effects of frondoside a. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef]
- Hossain, Z.; Sugawara, T.; Hirata, T. Sphingoid bases from sea cucumber induce apoptosis in human hepatoma hepg2 cells through p-akt and dr5. Oncol. Rep. 2013, 29, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Zhu, K.Q. A novel antitumor compound nobiliside d isolated from sea cucumber (holothuria nobilis selenka). Exp. Ther. Med. 2017, 14, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Amidi, S.; Hashemi, Z.; Motallebi, A.; Nazemi, M.; Farrokhpayam, H.; Seydi, E.; Pourahmad, J. Identification of (z)-2,3-diphenylacrylonitrileas anti-cancer molecule in persian gulf sea cucumber holothuria parva. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef]
- Surayot, U.; Lee, S.; You, S. Effects of sulfated fucan from the sea cucumber stichopus japonicus on natural killer cell activation and cytotoxicity. Int. J. Biol. Macromol. 2018, 108, 177–184. [Google Scholar] [CrossRef]
- Raymond, E.; Buquet-Fagot, C.; Djelloul, S.; Mester, J.; Cvitkovic, E.; Allain, P.; Louvet, C.; Gespach, C. Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor ag337 in human colon, breast and ovarian cancers. Anti-Cancer Drugs 1997, 8, 876–885. [Google Scholar] [CrossRef]
- Al Marzouqi, N.; Iratni, R.; Nemmar, A.; Arafat, K.; Ahmed Al Sultan, M.; Yasin, J.; Collin, P.; Mester, J.; Adrian, T.E.; Attoub, S. Frondoside a inhibits human breast cancer cell survival, migration, invasion and the growth of breast tumor xenografts. Eur. J. Pharm. 2011, 668, 25–34. [Google Scholar] [CrossRef]
- Al Shemaili, J.; Mensah-Brown, E.; Parekh, K.; Thomas, S.A.; Attoub, S.; Hellman, B.; Nyberg, F.; Adem, A.; Collin, P.; Adrian, T.E. Frondoside a enhances the antiproliferative effects of gemcitabine in pancreatic cancer. Eur. J. Cancer 2014, 50, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Attoub, S.; Arafat, K.; Khalaf, T.; Sulaiman, S.; Iratni, R. Frondoside a enhances the anti-cancer effects of oxaliplatin and 5-fluorouracil on colon cancer cells. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.K.; Yadav, N.; Bhat, T.A.; O’Malley, J.; Kumar, S.; Chandra, D. A potential role of x-linked inhibitor of apoptosis protein in mitochondrial membrane permeabilization and its implication in cancer therapy. Drug Discov. Today 2016, 21, 38–47. [Google Scholar] [CrossRef]
- Obexer, P.; Ausserlechner, M.J. X-linked inhibitor of apoptosis protein—A critical death resistance regulator and therapeutic target for personalized cancer therapy. Front. Oncol. 2014, 4, 197. [Google Scholar] [CrossRef]
- Vogler, M.; Walczak, H.; Stadel, D.; Haas, T.L.; Genze, F.; Jovanovic, M.; Bhanot, U.; Hasel, C.; Moller, P.; Gschwend, J.E.; et al. Small molecule xiap inhibitors enhance trail-induced apoptosis and antitumor activity in preclinical models of pancreatic carcinoma. Cancer Res. 2009, 69, 2425–2434. [Google Scholar] [CrossRef]
- Yano, K.; Horinaka, M.; Yoshida, T.; Yasuda, T.; Taniguchi, H.; Goda, A.E.; Wakada, M.; Yoshikawa, S.; Nakamura, T.; Kawauchi, A.; et al. Chetomin induces degradation of xiap and enhances trail sensitivity in urogenital cancer cells. Int. J. Oncol. 2011, 38, 365–374. [Google Scholar] [PubMed]
- Rosato, R.R.; Dai, Y.; Almenara, J.A.; Maggio, S.C.; Grant, S. Potent antileukemic interactions between flavopiridol and trail/apo2l involve flavopiridol-mediated xiap downregulation. Leukemia 2004, 18, 1780–1788. [Google Scholar] [CrossRef]
- Hiramatsu, N.; Messah, C.; Han, J.; LaVail, M.M.; Kaufman, R.J.; Lin, J.H. Translational and posttranslational regulation of xiap by eif2alpha and atf4 promotes er stress-induced cell death during the unfolded protein response. Mol. Biol. Cell 2014, 25, 1411–1420. [Google Scholar] [CrossRef]
- Clarke, H.J.; Chambers, J.E.; Liniker, E.; Marciniak, S.J. Endoplasmic reticulum stress in malignancy. Cancer Cell 2014, 25, 563–573. [Google Scholar] [CrossRef]
- Nishitoh, H. Chop is a multifunctional transcription factor in the er stress response. J. Biochem. 2012, 151, 217–219. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.L.; Park, S.H.; Jeong, S.; Kim, B.R.; Na, Y.J.; Jo, M.J.; Jeong, Y.A.; Yun, H.K.; Kim, D.Y.; Kim, B.G.; et al. Sea Cucumber (Stichopus japonicas) F2 Enhanced TRAIL-Induced Apoptosis via XIAP Ubiquitination and ER Stress in Colorectal Cancer Cells. Nutrients 2019, 11, 1061. https://doi.org/10.3390/nu11051061
Kim JL, Park SH, Jeong S, Kim BR, Na YJ, Jo MJ, Jeong YA, Yun HK, Kim DY, Kim BG, et al. Sea Cucumber (Stichopus japonicas) F2 Enhanced TRAIL-Induced Apoptosis via XIAP Ubiquitination and ER Stress in Colorectal Cancer Cells. Nutrients. 2019; 11(5):1061. https://doi.org/10.3390/nu11051061
Chicago/Turabian StyleKim, Jung Lim, Seong Hye Park, Soyeon Jeong, Bo Ram Kim, Yoo Jin Na, Min Jee Jo, Yoon A Jeong, Hye Kyeong Yun, Dae Yeong Kim, Bu Gyeom Kim, and et al. 2019. "Sea Cucumber (Stichopus japonicas) F2 Enhanced TRAIL-Induced Apoptosis via XIAP Ubiquitination and ER Stress in Colorectal Cancer Cells" Nutrients 11, no. 5: 1061. https://doi.org/10.3390/nu11051061
APA StyleKim, J. L., Park, S. H., Jeong, S., Kim, B. R., Na, Y. J., Jo, M. J., Jeong, Y. A., Yun, H. K., Kim, D. Y., Kim, B. G., You, S., Oh, S. C., & Lee, D.-H. (2019). Sea Cucumber (Stichopus japonicas) F2 Enhanced TRAIL-Induced Apoptosis via XIAP Ubiquitination and ER Stress in Colorectal Cancer Cells. Nutrients, 11(5), 1061. https://doi.org/10.3390/nu11051061




