Effect of Topically Applied Wikstroemia dolichantha Diels on the Development of Atopic Dermatitis-Like Skin Symptoms in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Animals
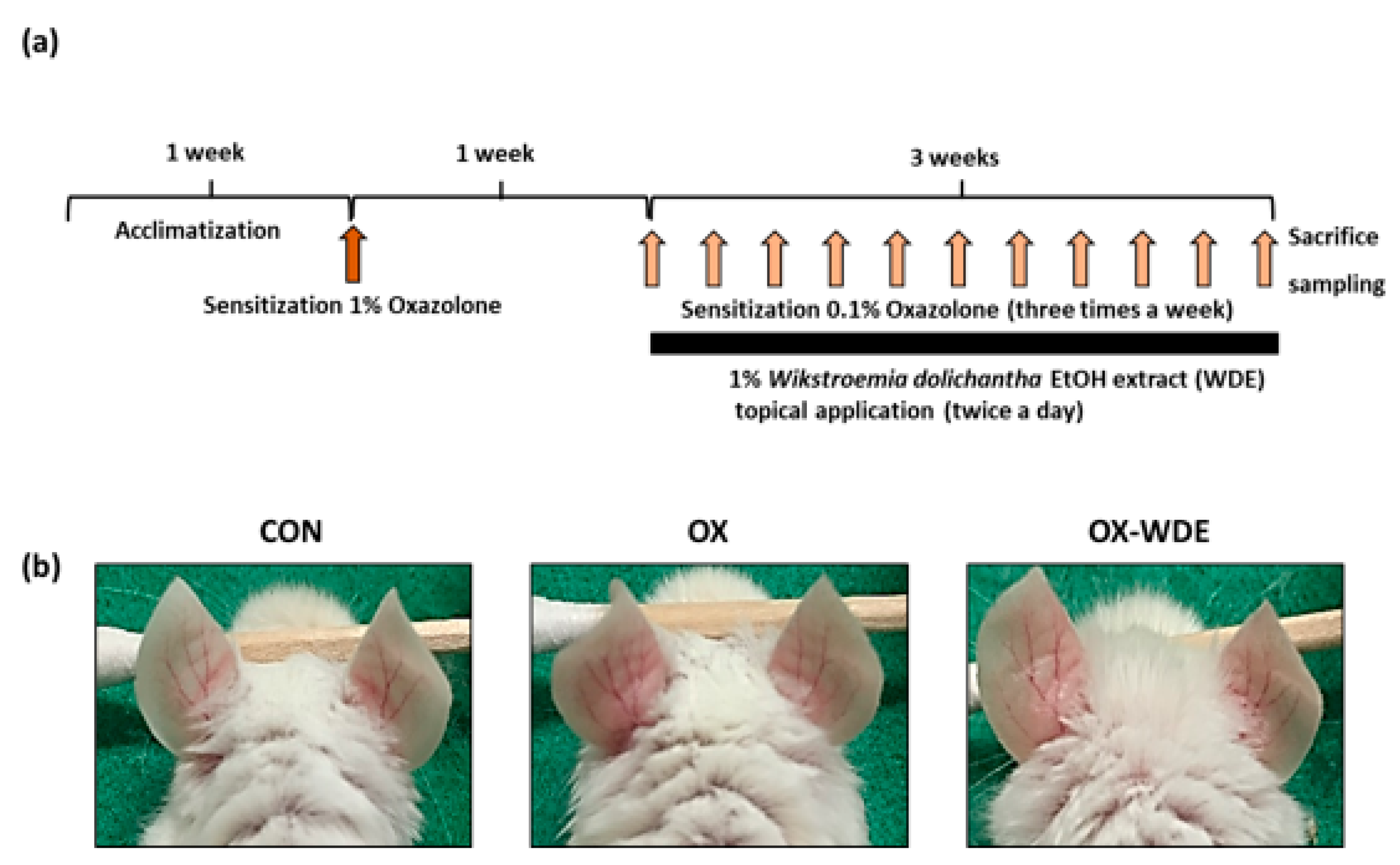
2.3. Measurements of Skin Inflammation in The Oxazolone-Induced Model and WDE Treatment
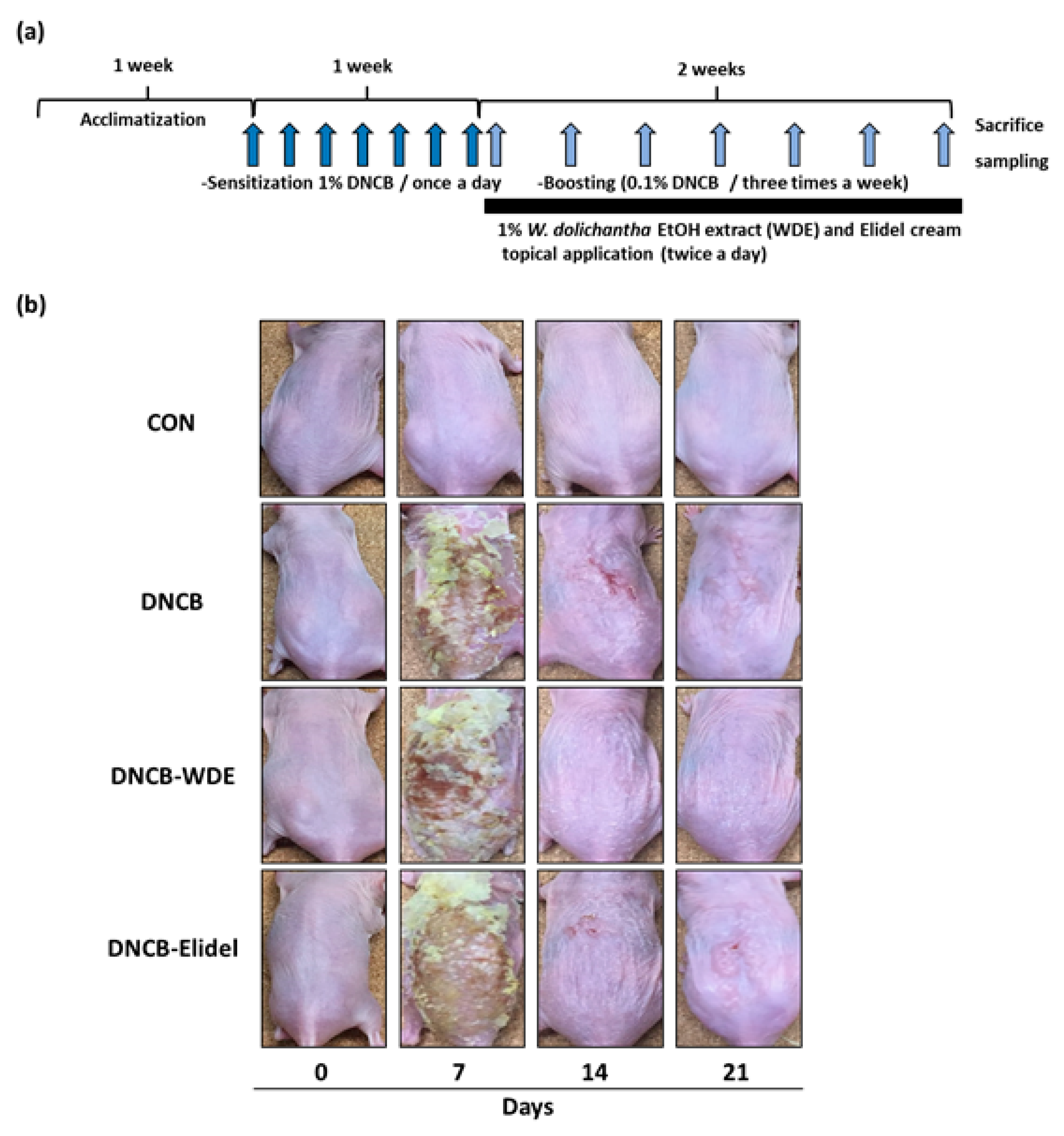
2.4. Measurement of Skin Severity in The DNCB-Induced Model and Treatment with WDE
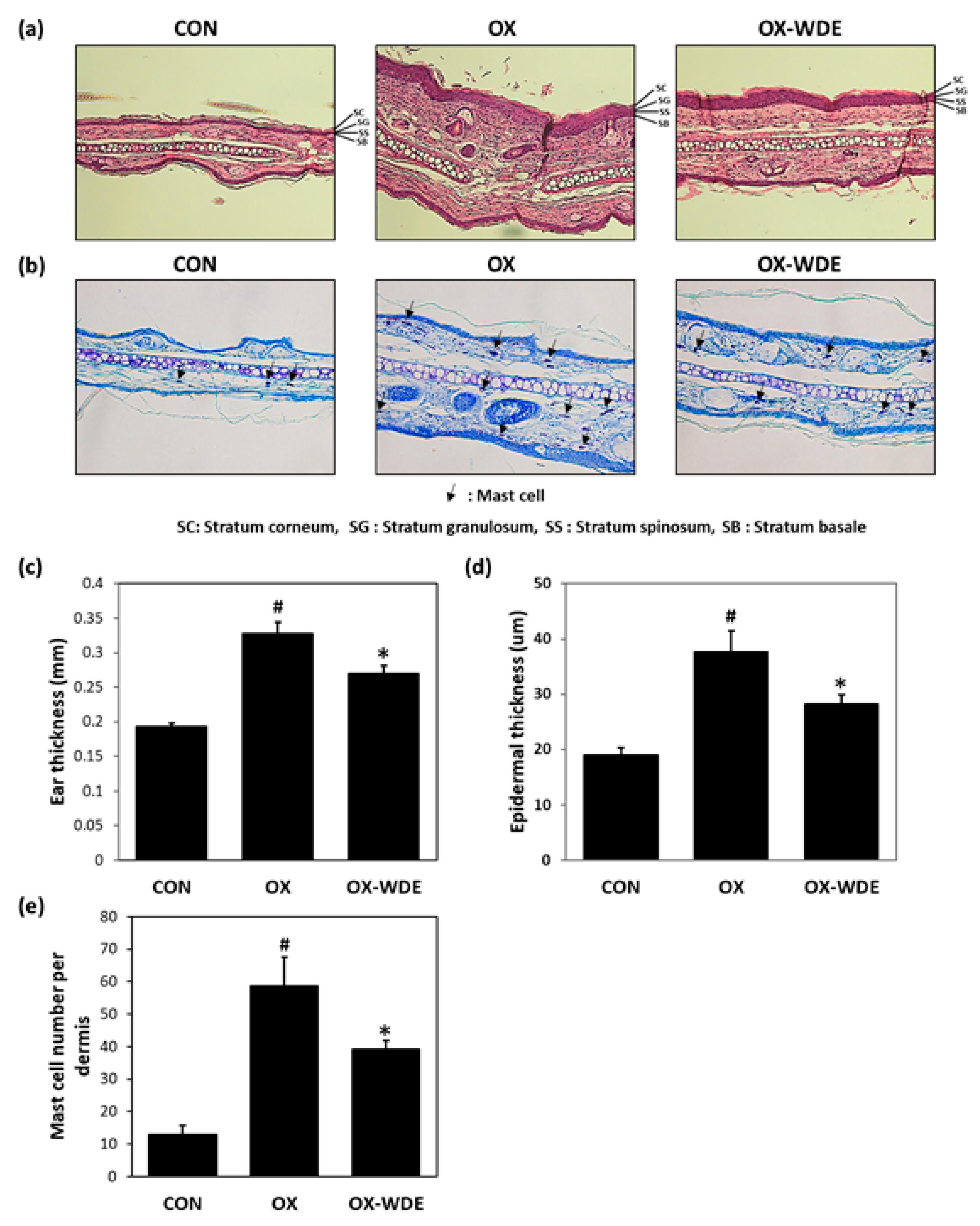
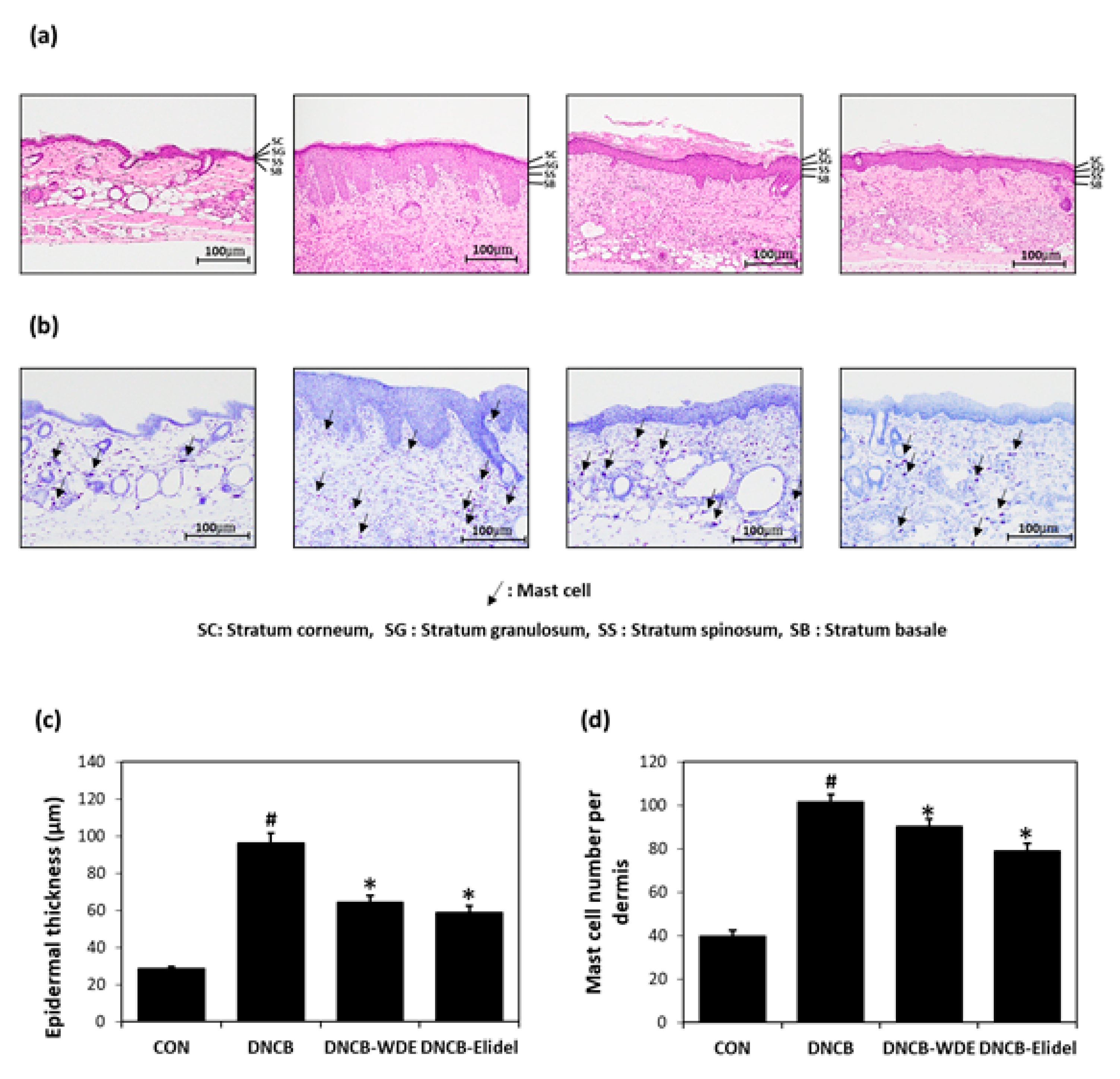
2.5. Histological Examinations
2.6. Transepidermal Water Loss (TEWL) and Skin Hydration
2.7. Total Serum IgE and IL-4 Levels
2.8. HPLC/MS Analysis of WDE
2.9. Statistical Analysis
3. Results
3.1. Effects of WDE on Oxazolone-Induced AD Mice
3.2. Histopathological Analysis of Oxazolone-Treated Skins
3.3. Effects of WDE on Skin Severity in The DNCB-Induced Model and Histopathological Analysis
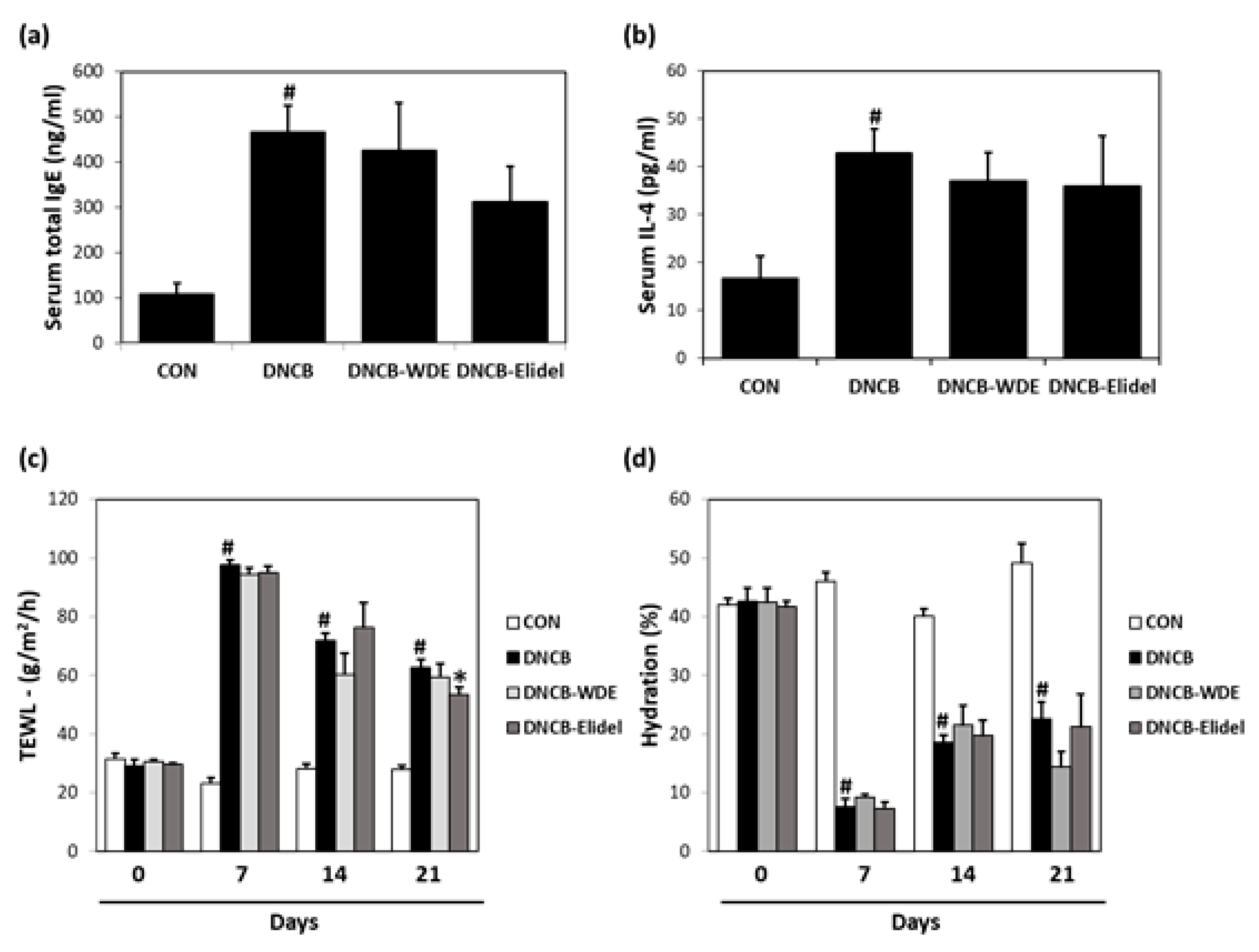
3.4. Effects of WDE on Blood IL-4 and IgE Levels in The DNCB Model
3.5. Effects of WDE on Skin Barrier Function in DNCB-Treated SKH-1 mice
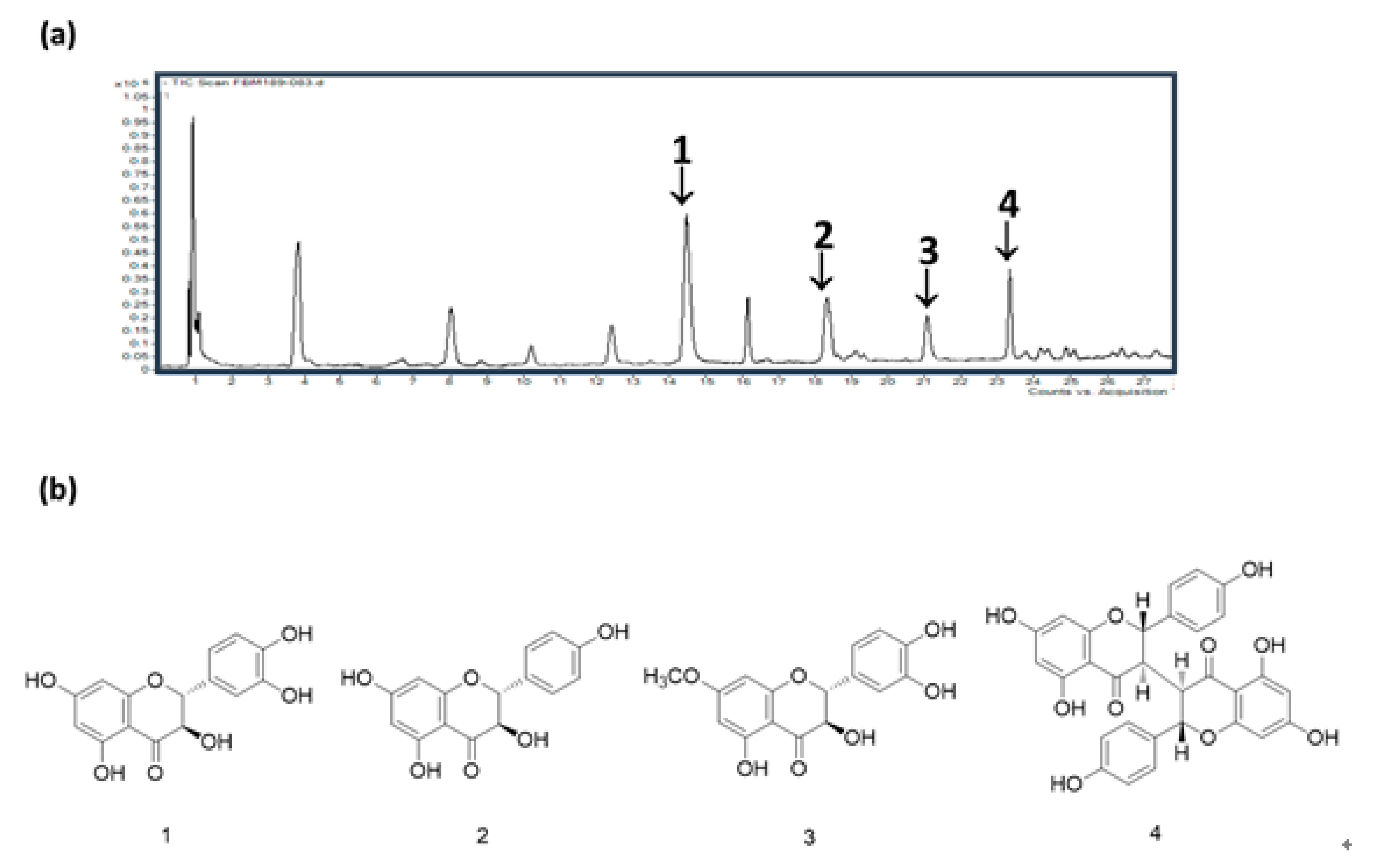
3.6. HPLC/MS of WDE
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Nograles, K.E.; Krueger, J.G. Contrasting pathogenesis of atopic dermatitis and psoriasis—Part I: Clinical and pathologic concepts. J. Allergy Clin. Immunol. 2011, 127, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.L.; Balkrishnan, R.; Feldman, S.R.; Fleischer, A.B., Jr.; Manuel, J.C. The Burden of Atopic Dermatitis: Impact on the Patient, Family, and Society. Pediatr. Dermatol. 2005, 22, 192–199. [Google Scholar] [CrossRef]
- Boccardi, D.; D’Auria, E.; Turati, F.; DI, M.V.; Sortino, S.; Riva, E.; Cerri, A. Disease severity and quality of life in children with atopic dermatitis: PO-SCORAD in clinical practice. Minerva Pediatr. 2017, 69, 373–380. [Google Scholar]
- Wollenberg, A.; Kraft, S.; Oppel, T.; Bieber, T. Atopic dermatitis: Pathogenetic mechanisms. Clin. Exp. Dermatol. 2000, 25, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Jujo, K.; Bradley, K.L.; Domenico, J.; Gelfand, E.W.; Leung, D.Y. Enhanced IL-4 production and IL-4 receptor expression in atopic dermatitis and their modulation by interferon-gamma. J. Investig. Dermatol. 1992, 99, 403–438. [Google Scholar] [CrossRef]
- Jujo, K.; Renz, H.; Abe, J.; Gelfand, E.W.; Leung, D.Y. Decreased interferon gamma and increased interleukin-4 production in atopic dermatitis promotes IgE synthesis. J. Allergy Clin. Immunol. 1992, 90, 323–331. [Google Scholar] [CrossRef]
- D’Auria, E.; Banderali, G.; Barberi, S.; Gualandri, L.; Pietra, B.; Riva, E.; Cerri, A. Atopic dermatitis: Recent insight on pathogenesis and novel therapeutic target. Asian Pac. J. Allergy Immunol. 2016, 34, 98–108. [Google Scholar] [PubMed]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Medina, F.S.D. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Flavonoids-chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef]
- Robak, J.; Gryglewski, R. Bioactivity of flavonoids. Pol. J. Pharmacol. 1996, 48, 555–564. [Google Scholar]
- Jo, B.G.; Park, N.J.; Jegal, J.; Choi, S.; Lee, S.W.; Jin, H.; Kim, S.N.; Yang, M.H. A new flavonoid from Stellera chamaejasme L., stechamone, alleviated 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in a murine model. Int. Immunopharmacol. 2018, 59, 113–119. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Watanabe, K. Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov. Today 2016, 21, 632–639. [Google Scholar] [CrossRef]
- Choi, J.K.; Jang, Y.H.; Lee, S.; Lee, S.R.; Choi, Y.A.; Jin, M.; Choi, J.H.; Park, J.H.; Park, P.H.; Choi, H.; et al. Chrysin attenuates atopic dermatitis by suppressing inflammation of keratinocytes. Food Chem. Toxicol. 2017, 110, 142–150. [Google Scholar] [CrossRef]
- Peterson, B. Johan emanuel wikstrom, with historical notes on the genus Wikstroemia. Pac. Sci. 1996, 50, 77–83. [Google Scholar]
- Lu, C.L.; Li, Y.M.; Fu, G.Q.; Yang, L.; Jiang, J.G.; Zhu, L.; Lin, F.L.; Chen, J.; Lin, Q.S. Extraction optimisation of daphnoretin from root bark of Wikstroemia indica (L.) C.A. and its anti-tumour activity tests. Food Chem. 2011, 124, 1500–1506. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, W.W.; Sun, L.X.; Wang, Q.; Qi, W.; Yu, H. A new coumarin from Wikstroemia indica (L.) C.A. Mey. Chin. Chem. Lett. 2009, 20, 592–594. [Google Scholar] [CrossRef]
- Geng, L.D.; Zhang, C.; Xiao, Y.Q. Studies on the chemical constituents in stem rind of Wikstroemia indica. Zhongguo Zhong Yao Za Zhi 2006, 31, 817–819. [Google Scholar]
- Wang, L.Y.; Unehara, T.; Kitanaka, S. Anti-inflammatory activity of new guaiane type sesquiterpene from Wikstroemia indica. Chem. Pharm. Bull. 2005, 53, 137–139. [Google Scholar] [CrossRef]
- Hu, K.; Kobayashi, H.; Dong, A.; Iwasaki, S.; Yao, X. Antifungal, antimitotic and anti-hiv-1 agents from the roots of Wikstroemia indica. Planta Med. 2000, 66, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, J.C.; Park, N.J.; Bong, S.K.; Lee, S.; Jegal, H.; Jin, L.T.; Kim, S.M.; Kim, Y.K.; Kim, S.N. Eupatilin, an activator of PPARα, inhibits the development of oxazolone-induced atopic dermatitis symptoms in Balb/c mice. Biochem. Biophys. Res. Commun. 2018, 496, 508–514. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Kesharwani, P.; Khan, S.; Hussain, F. Phytotherapeutic potential of natural herbal medicines for the treatment of mild-to-severe atopic dermatitis: A review of human clinical studies. Biomed. Pharmacother. 2017, 93, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Nedoszytko, B.; Sokołowska-Wojdyło, M.; Ruckemann-Dziurdzińska, K.; Roszkiewicz, J.; Nowicki, R.J. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: Atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatol. Alergol. 2014, 31, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Egawa, G.; Kabashima, K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J. Allergy Clin. Immunol. 2016, 138, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Boguniewicz, M.; Leung, D.Y. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.H. Epidermal permeability barrier defects and barrier repair therapy in atopic dermatitis. Allergy Asthma Immunol. Res. 2014, 6, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Chalmers, J.R.; Hanifin, J.M.; Thomas, K.S.; Cork, M.J.; McLean, W.I.; Brown, S.J.; Chen, Z.; Chen, Y.; Williams, H.C. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J. Allergy Clin. Immunol. 2014, 134, 818–823. [Google Scholar] [CrossRef]
- Hvid, M.; Vestergaard, C.; Kemp, K.; Christensen, G.B.; Deleuran, B.; Deleuran, M. IL-25 in atopic dermatitis: A possible link between inflammation and skin barrier dysfunction? J. Allergy Clin. Immunol. 2011, 131, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Nagao, K.; Amagai, M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J. Clin. Investig. 2012, 122, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Campos, M.M.; Otuki, M.F.; Santos, A.R. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004, 70, 93–103. [Google Scholar] [PubMed]
- Werner, Y.; Lindberg, M. Transepidermal water loss in dry and clinically normal skin in patients with atopic dermatitis. Acta Derm. Venereol. 1985, 65, 102–105. [Google Scholar] [PubMed]
- Marsella, R.; Olivry, T.; Carlotti, D.N. Current evidence of skin barrier dysfunction in human and canine atopic dermatitis. Vet. Dermatol. 2011, 22, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E. Biological properties of plant flavonoids: An overview. Int. J. Pharmacogn. 1996, 34, 344–348. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jegal, J.; Park, N.-J.; Kim, T.-Y.; Choi, S.; Lee, S.W.; Hang, J.; Kim, S.-N.; Yang, M.H. Effect of Topically Applied Wikstroemia dolichantha Diels on the Development of Atopic Dermatitis-Like Skin Symptoms in Mice. Nutrients 2019, 11, 914. https://doi.org/10.3390/nu11040914
Jegal J, Park N-J, Kim T-Y, Choi S, Lee SW, Hang J, Kim S-N, Yang MH. Effect of Topically Applied Wikstroemia dolichantha Diels on the Development of Atopic Dermatitis-Like Skin Symptoms in Mice. Nutrients. 2019; 11(4):914. https://doi.org/10.3390/nu11040914
Chicago/Turabian StyleJegal, Jonghwan, No-June Park, Tae-Young Kim, Sangho Choi, Sang Woo Lee, Jin Hang, Su-Nam Kim, and Min Hye Yang. 2019. "Effect of Topically Applied Wikstroemia dolichantha Diels on the Development of Atopic Dermatitis-Like Skin Symptoms in Mice" Nutrients 11, no. 4: 914. https://doi.org/10.3390/nu11040914
APA StyleJegal, J., Park, N.-J., Kim, T.-Y., Choi, S., Lee, S. W., Hang, J., Kim, S.-N., & Yang, M. H. (2019). Effect of Topically Applied Wikstroemia dolichantha Diels on the Development of Atopic Dermatitis-Like Skin Symptoms in Mice. Nutrients, 11(4), 914. https://doi.org/10.3390/nu11040914




