Identification of N-Acetyl-S-(3-Cyano-2-(Methylsulfanyl)Propyl-Cysteine as a Major Human Urine Metabolite from the Epithionitrile 1-Cyano-2,3-Epithiopropane, the Main Glucosinolate Hydrolysis Product from Cabbage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Buffers
2.2. Initial Experiment for the Identification of Epithionitrile Metabolites
2.3. Preparation of Urine Samples and UHPLC-ESI-(Q)ToF-MS
2.4. Preparation of Potential Metabolites
2.5. Plant Material For Human Pilot Intervention
2.6. Human Intervention and Quantitation of the CETP-Metabolite in Urine Samples
2.7. Analysis of Glucosinolate Hydrolysis Products in Plant Samples
3. Results
3.1. Epithionitriles and Other Glucosinolate Hydrolysis Products in Plant Material for Human Consumption
3.2. Identification of the CETP Metabolite
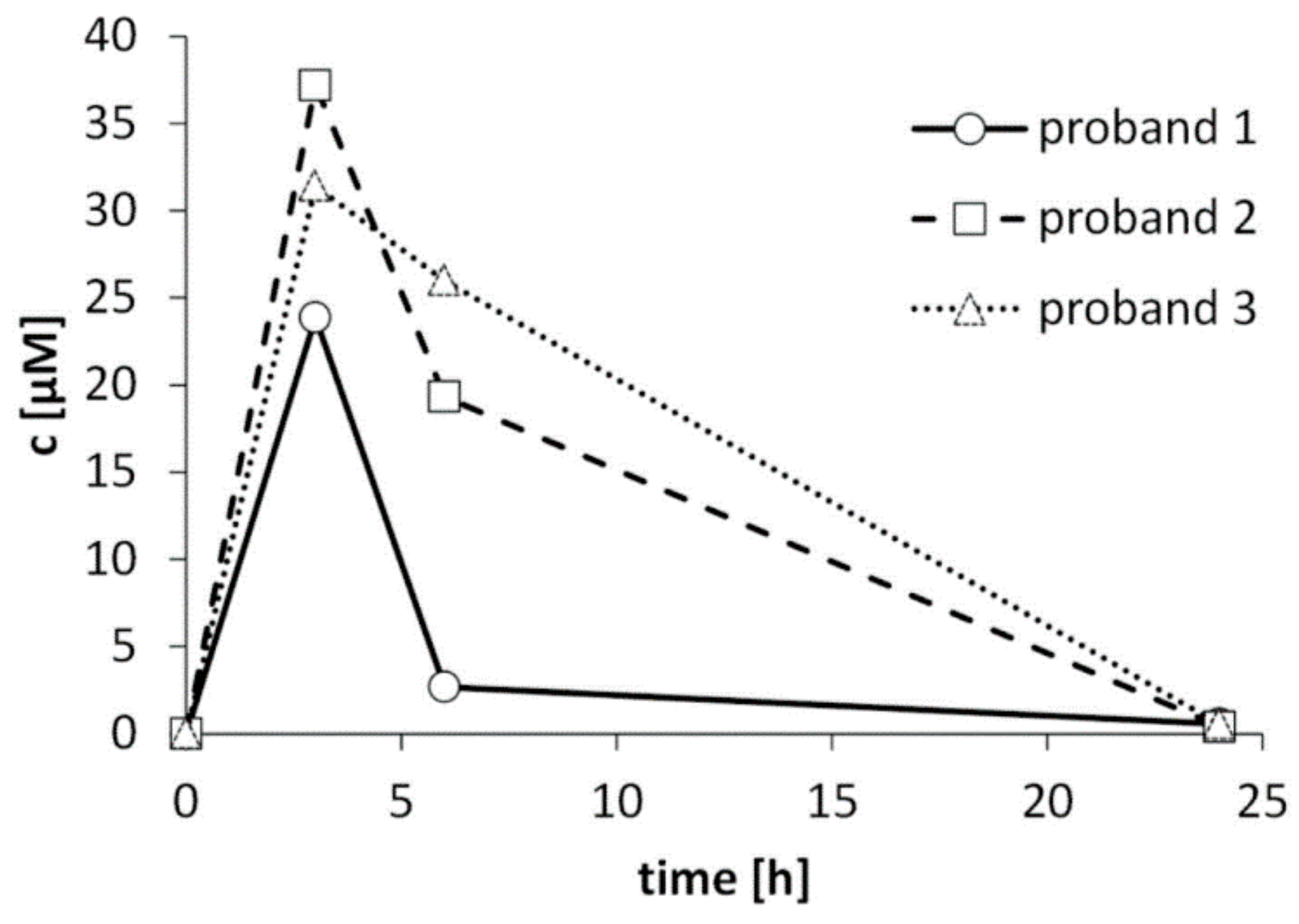
3.3. Excretion Kinetics of CETP in Three Volunteers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, nitriles, and epithionitriles from glucosinolates are affected by genotype and developmental stage in Brassica oleracea varieties. Front. Plant Sci. 2017, 8, 1095. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E. Glucosinolate structures in evolution. Phytochemistry 2012, 77, 16–45. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and stability of glucosinolates and their breakdown products in foods. Angew. Chem. Int. Ed. 2014, 53, 11430–11450. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Herz, C.; Schlotz, N.; Kupke, F.; Bartolomé Rodríguez, M.M.; Schreiner, M.; Rohn, S.; Lamy, E. The Brassica epithionitrile 1-cyano-2,3-epithiopropane triggers cell death in human liver cancer cells in vitro. Mol. Nutr. Food Res. 2015, 59, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Klopsch, R.; Witzel, K.; Artemyeva, A.; Ruppel, S.; Hanschen, F.S. Genotypic variation of glucosinolates and their breakdown products in leaves of Brassica rapa. J. Agric. Food Chem. 2018, 66, 5481–5490. [Google Scholar] [CrossRef] [PubMed]
- Klopsch, R.; Witzel, K.; Borner, A.; Schreiner, M.; Hanschen, F.S. Metabolic profiling of glucosinolates and their hydrolysis products in a germplasm collection of Brassica rapa turnips. Food Res. Int. 2017, 100, 392–403. [Google Scholar] [CrossRef]
- Daxenbichler, M.E.; Spencer, G.F.; Carlson, D.G.; Rose, G.B.; Brinker, A.M.; Powell, R.G. Glucosinolate composition of seeds from 297 species of wild plants. Phytochemistry 1991, 30, 2623–2638. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Steinbrecher, A.; Linseisen, J. Dietary intake of individual glucosinolates in participants of the EPIC-Heidelberg cohort study. Ann. Nutrand. Metab. 2009, 54, 87–96. [Google Scholar] [CrossRef]
- Wittstock, U.; Meier, K.; Dörr, F.; Ravindran, B.M. NSP-Dependent simple nitrile formation dominates upon breakdown of major aliphatic glucosinolates in roots, seeds, and seedlings of Arabidopsis thaliana Columbia-0. Front. Plant. Sci. 2016, 7, 1821. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, U.; Burow, M. Glucosinolate breakdown in Arabidopsis: Mechanism, regulation and biological significance. Arab. Book 2010, 8, e0134. [Google Scholar] [CrossRef]
- Burow, M.; Markert, J.; Gershenzon, J.; Wittstock, U. Comparative biochemical characterization of nitrile-forming proteins from plants and insects that alter myrosinase-catalysed hydrolysis of glucosinolates. Febs J. 2006, 273, 2432–2446. [Google Scholar] [CrossRef]
- Kissen, R.; Hyldbakk, E.; Wang, C.W.V.; Sørmo, C.G.; Rossiter, J.T.; Bones, A.M. Ecotype dependent expression and alternative splicing of epithiospecifier protein (ESP) in Arabidopsis thaliana. Plant. Mol. Biol. 2012, 78, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, W.; Liu, Z.; Xie, Y.; Wang, H.; Mu, Y.; Huang, Y.; Feng, Y. Crystal structure of the epithiospecifier protein, ESP from Arabidopsis thaliana provides insights into its product specificity. Biochem. Biophys. Res. Commun. 2016, 478, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Veeranki, O.; Bhattacharya, A.; Tang, L.; Marshall, J.; Zhang, Y. Cruciferous vegetables, isothiocyanates, and prevention of bladder cancer. Curr. Pharm. Rep. 2015, 1, 272–282. [Google Scholar] [CrossRef]
- Gould, D.H.; Gumbmann, M.R.; Daxenbichler, M.E. Pathological changes in rats fed the crambe meal-glucosinolate hydrolytic products, 2S-1-cyano-2-hydroxy-3,4-epithiobutanes (erythro and threo) for 90 days. Food Cosmet. Toxicol. 1980, 18, 619–625. [Google Scholar] [CrossRef]
- VanSteenhouse, J.L.; Fettman, M.J.; Gould, D.H. Sequential changes in hepatic and renal glutathione and development of renal karyomegaly in 1-cyano-3,4-epithiobutane toxicity in rats. Food Chem. Toxicol. 1989, 27, 731–739. [Google Scholar] [CrossRef]
- Wallig, M.A.; Gould, D.H.; Fettman, M.J.; Willhite, C.C. Comparative toxicities of the naturally occurring nitrile 1-cyano-3,4-epithiobutane and the synthetic nitrile n-valeronitrile in rats: Differences in target organs, metabolism and toxic mechanisms. Food Chem. Toxicol. 1988, 26, 149–157. [Google Scholar] [CrossRef]
- VanSteenhouse, J.L.; Fettman, M.J.; Gould, D.H. The effect of glutathione depletion by buthionine sulphoximine on 1-cyano-3,4-epithiobutane toxicity. Food Chemtoxicol. 1991, 29, 153–157. [Google Scholar] [CrossRef]
- VanSteenhouse, J.L.; Prescott, J.S.; Swenson, D.H. Protection from 1-cyano-3,4-epithiobutane nephrotoxicity by aminooxyacetic acid and effect on xenobiotic-metabolizing enzymes in male fischer 344 rats. J. Appl. Toxicol. 1999, 19, 237–249. [Google Scholar] [CrossRef]
- Kelleher, M.O.; McMahon, M.; Eggleston, I.M.; Dixon, M.J.; Taguchi, K.; Yamamoto, M.; Hayes, J.D. 1-Cyano-2,3-epithiopropane is a novel plant-derived chemopreventive agent which induces cytoprotective genes that afford resistance against the genotoxic α,β-unsaturated aldehyde acrolein. Carcinogenesis 2009, 30, 1754–1762. [Google Scholar] [CrossRef]
- Brocker, E.R.; Benn, M.H.; Lüthy, J.; von Däniken, A. Metabolism and distribution of 3,4-epithiobutanenitrile in the rat. Food Chem. Toxicol. 1984, 22, 227–232. [Google Scholar] [CrossRef]
- VanSteenhouse, J.L.; Prescott, J.S.; Barker, S.A. Identification of the 1-cyano-3,4-epithiobutane-derived urinary mercapturic acid N-acetyl-S-(4-cyano-2-thio-1-butyl)-cysteine in male fischer 344 rats. J. Appl. Toxicol. 2000, 20, 1–10. [Google Scholar] [CrossRef]
- Platz, S.; Kühn, C.; Schiess, S.; Schreiner, M.; Mewis, I.; Kemper, M.; Pfeiffer, A.; Rohn, S. Determination of benzyl isothiocyanate metabolites in human plasma and urine by LC-ESI-MS/MS after ingestion of nasturtium (Tropaeolum majus L.). Anal. Bioanal. Chem. 2013, 405, 7427–7436. [Google Scholar] [CrossRef]
- Kyung, K.H.; Fleming, H.P.; Young, C.T.; Haney, C.A. 1-Cyano-2,3-epithiopropane as the primary sinigrin hydrolysis product of fresh cabbage. J. Food Sci. 1995, 60, 157–159. [Google Scholar] [CrossRef]
- Bremer, J.; Greenberg, D.M. Enzymic methylation of foreign sulfhydryl compounds. Biochim. Biophys. Acta 1961, 46, 217–224. [Google Scholar] [CrossRef]
- Weisiger, R.A.; Pinkus, L.M.; Jakoby, W.B. Thiol S-methyltransferase: Suggested role in detoxication of intestinal hydrogen sulfide. Biochem. Pharm. 1980, 29, 2885–2887. [Google Scholar] [CrossRef]
- Weinshilboum, R. Methyltransferase pharmacogenetics. Pharm 1989, 43, 77–90. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef]
- Commandeur, J.N.; Stijntjes, G.J.; Vermeulen, N.P. Enzymes and transport systems involved in the formation and disposition of glutathione S-conjugates. Role in bioactivation and detoxication mechanisms of xenobiotics. Pharm. Rev. 1995, 47, 271–330. [Google Scholar]
- Lock, E.A.; Reed, C.J. Xenobiotic metabolizing enzymes of the kidney. Toxicol. Pathol. 1998, 26, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Hinchman, C.A.; Ballatori, N. Glutathione conjugation and conversion to mercapturic acids can occur as an intrahepatic process. J. Toxicol. Environ. Health 1994, 41, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.L.; Krasnikov, B.F.; Niatsetskaya, Z.V.; Pinto, J.T.; Callery, P.S.; Villar, M.T.; Artigues, A.; Bruschi, S.A. Cysteine S-conjugate β-lyases: Important roles in the metabolism of naturally occurring sulfur and selenium-containing compounds, xenobiotics and anticancer agents. Amino Acids 2011, 41, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Platz, S.; Kühn, C.; Schiess, S.; Schreiner, M.; Kemper, M.; Pivovarova, O.; Pfeiffer, A.F.H.; Rohn, S. Bioavailability and metabolism of benzyl glucosinolate in humans consuming Indian cress (Tropaeolum majus L.). Mol. Nutr. Food Res. 2016, 60, 652–660. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanschen, F.S.; Baldermann, S.; Brobrowski, A.; Maikath, A.; Wiesner-Reinhold, M.; Rohn, S.; Schreiner, M. Identification of N-Acetyl-S-(3-Cyano-2-(Methylsulfanyl)Propyl-Cysteine as a Major Human Urine Metabolite from the Epithionitrile 1-Cyano-2,3-Epithiopropane, the Main Glucosinolate Hydrolysis Product from Cabbage. Nutrients 2019, 11, 908. https://doi.org/10.3390/nu11040908
Hanschen FS, Baldermann S, Brobrowski A, Maikath A, Wiesner-Reinhold M, Rohn S, Schreiner M. Identification of N-Acetyl-S-(3-Cyano-2-(Methylsulfanyl)Propyl-Cysteine as a Major Human Urine Metabolite from the Epithionitrile 1-Cyano-2,3-Epithiopropane, the Main Glucosinolate Hydrolysis Product from Cabbage. Nutrients. 2019; 11(4):908. https://doi.org/10.3390/nu11040908
Chicago/Turabian StyleHanschen, Franziska S., Susanne Baldermann, Adrian Brobrowski, Andrea Maikath, Melanie Wiesner-Reinhold, Sascha Rohn, and Monika Schreiner. 2019. "Identification of N-Acetyl-S-(3-Cyano-2-(Methylsulfanyl)Propyl-Cysteine as a Major Human Urine Metabolite from the Epithionitrile 1-Cyano-2,3-Epithiopropane, the Main Glucosinolate Hydrolysis Product from Cabbage" Nutrients 11, no. 4: 908. https://doi.org/10.3390/nu11040908
APA StyleHanschen, F. S., Baldermann, S., Brobrowski, A., Maikath, A., Wiesner-Reinhold, M., Rohn, S., & Schreiner, M. (2019). Identification of N-Acetyl-S-(3-Cyano-2-(Methylsulfanyl)Propyl-Cysteine as a Major Human Urine Metabolite from the Epithionitrile 1-Cyano-2,3-Epithiopropane, the Main Glucosinolate Hydrolysis Product from Cabbage. Nutrients, 11(4), 908. https://doi.org/10.3390/nu11040908






