Imbalance between Omega-6 and Omega-3 Polyunsaturated Fatty Acids in Early Pregnancy Is Predictive of Postpartum Depression in a Belgian Cohort
Abstract
:1. Introduction
2. Materials and Methods
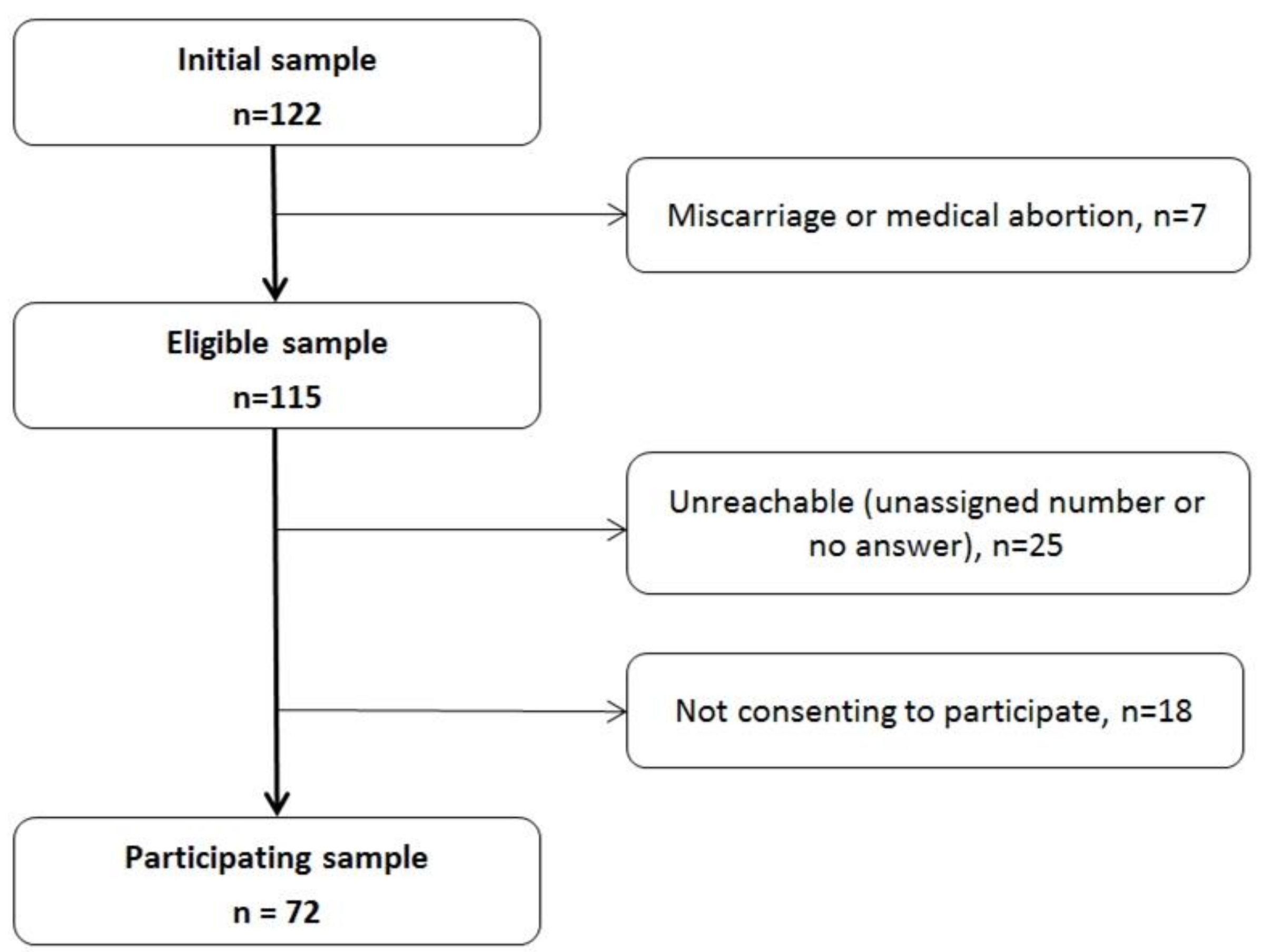
2.1. Study Population and Design
2.2. Blood Sampling and Fatty Acid Analysis
2.3. Postpartum Depression
2.4. Other Covariates
2.5. Statistical Methods
3. Results
3.1. Participants
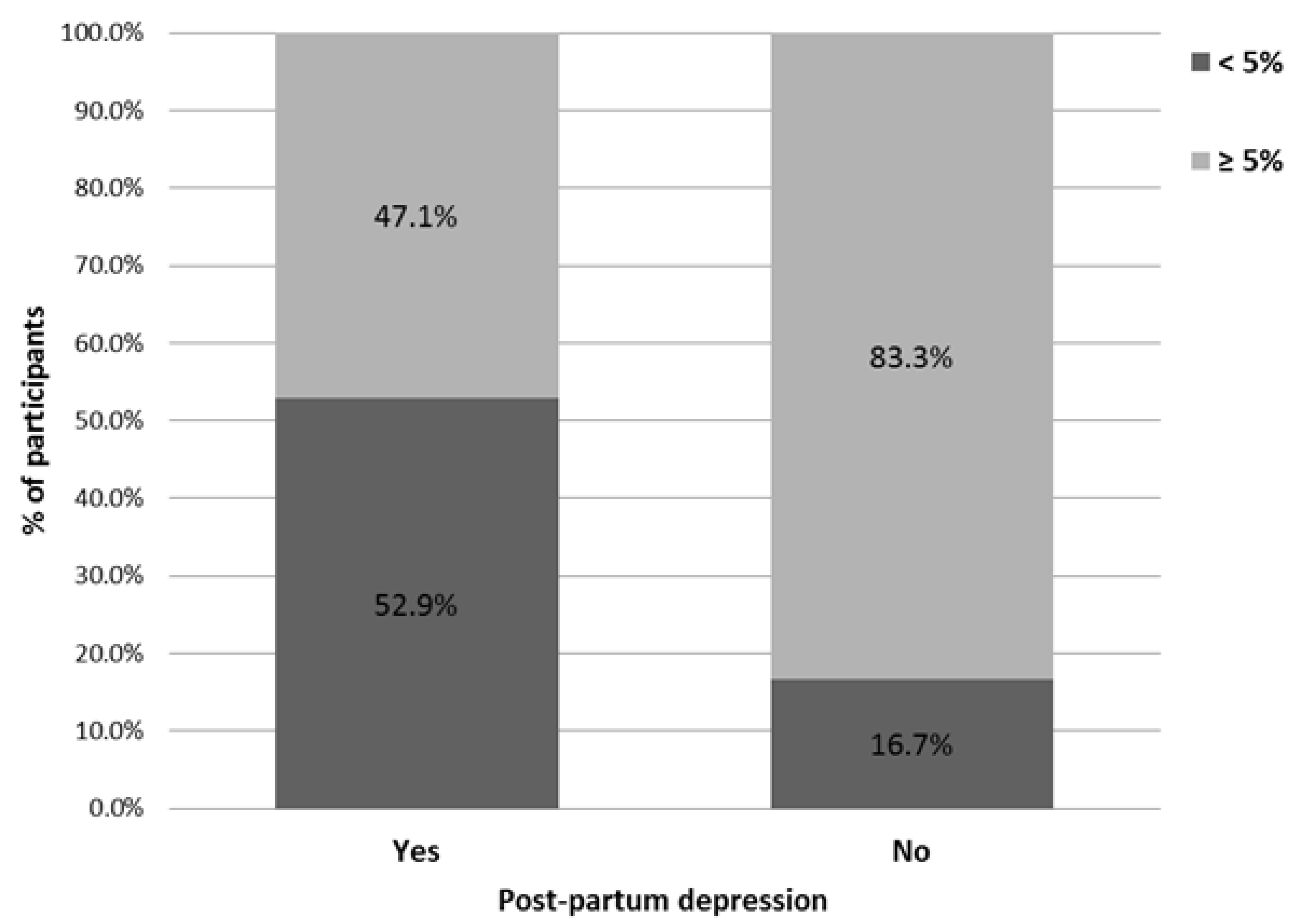
3.2. Determinants of PPD
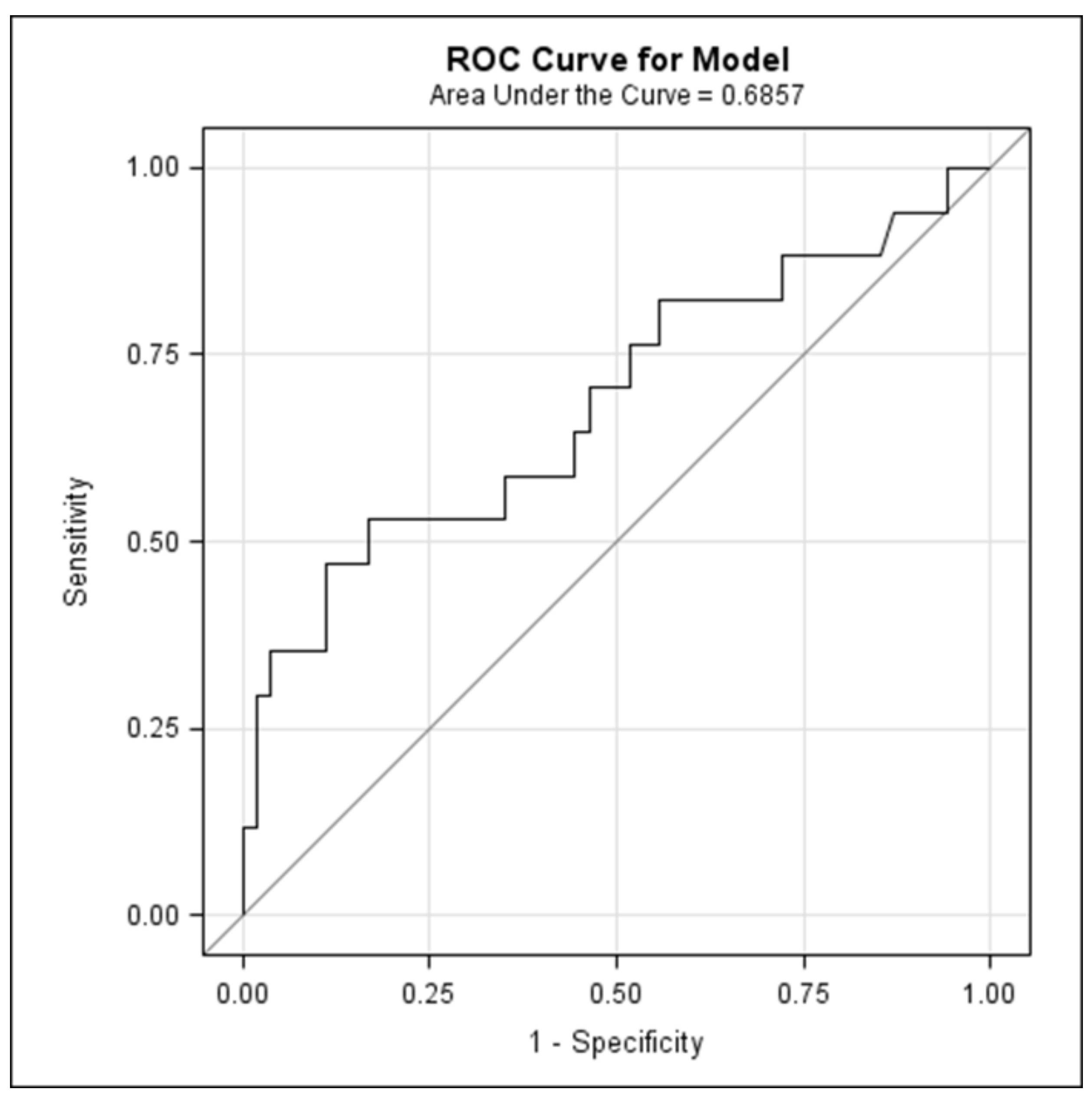
3.3. Association between PUFA and Odds of Postpartum Depression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramakrishnan, U. Fatty acid status and maternal mental health. Matern. Child Nutr. 2011, 7, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Gavin, N.I.; Gaynes, B.N.; Lohr, K.N.; Meltzer-Brody, S.; Gartlehner, G.; Swinson, T. Perinatal Depression. Obstet. Gynecol. 2005, 106, 1071–1083. [Google Scholar] [CrossRef]
- Woody, C.A.; Ferrari, A.J.; Siskind, D.J.; Whiteford, H.A.; Harris, M.G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord. 2017, 219, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Posmontier, B. Functional status outcomes in mothers with and without postpartum depression. J. Midwifery Women’s Health 2008, 53, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Letourneau, N.L.; Dennis, C.-L.; Benzies, K.; Duffett-Leger, L.; Stewart, M.; Tryphonopoulos, P.D.; Este, D.; Watson, W. Postpartum Depression is a Family Affair: Addressing the Impact on Mothers, Fathers, and Children. Issues Ment. Health Nurs. 2012, 33, 445–457. [Google Scholar] [PubMed]
- Field, T. Postpartum depression effects on early interactions, parenting, and safety practices: A review. Infant Behav. Dev. 2010, 33, 1–6. [Google Scholar] [CrossRef]
- Righetti-Veltema, M.; Conne-Perréard, E.; Bousquet, A.; Manzano, J. Postpartum depression and mother-infant relationship at 3 months old. J. Affect. Disord. 2002, 70, 291–306. [Google Scholar] [CrossRef]
- Closa-Monasterolo, R.; Gispert-Llaurado, M.; Canals, J.; Luque, V.; Zaragoza-Jordana, M.; Koletzko, B.; Grote, V.; Weber, M.; Gruszfeld, D.; Szott, K.; et al. The Effect of Postpartum Depression and Current Mental Health Problems of the Mother on Child Behaviour at Eight Years. Matern. Child Health J. 2017, 21, 1563–1572. [Google Scholar] [CrossRef]
- Kingston, D.; Tough, S. Prenatal and Postnatal Maternal Mental Health and School-Age Child Development: A Systematic Review. Matern. Child Health J. 2014, 18, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Faucett, J.; Lichtenberg, P.; Kirsch, I.; Brown, W.A. A Systematic Review of Comparative Efficacy of Treatments and Controls for Depression. PLoS ONE 2012, 7, e41778. [Google Scholar] [CrossRef]
- Fournier, J.C.; DeRubeis, R.J.; Hollon, S.D.; Dimidjian, S.; Amsterdam, J.D.; Shelton, R.C.; Fawcett, J. Antidepressant Drug Effects and Depression Severity. JAMA 2010, 303, 47. [Google Scholar] [CrossRef] [PubMed]
- Molyneaux, E.; Telesia, L.A.; Henshaw, C.; Boath, E.; Bradley, E.; Howard, L.M. Antidepressants for preventing postnatal depression. Cochrane Database Syst. Rev. 2018, 4, CD004363. [Google Scholar] [CrossRef]
- Molenaar, N.M.; Kamperman, A.M.; Boyce, P.; Bergink, V. Guidelines on treatment of perinatal depression with antidepressants: An international review. Aust. N. Zeal. J. Psychiatry 2018, 52, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef]
- Lauritzen, L.; Hansen, H.S.; Jørgensen, M.H.; Michaelsen, K.F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 2001, 40, 1–94. [Google Scholar] [CrossRef]
- Hibbeln, J.R. Seafood consumption, the DHA content of mothers’ milk and prevalence rates of postpartum depression: A cross-national, ecological analysis. J. Affect. Disord. 2002, 69, 15–29. [Google Scholar] [CrossRef]
- Dos Santos Vaz, J.; Kac, G.; Emmett, P.; Davis, J.M.; Golding, J.; Hibbeln, J.R. Dietary patterns, n-3 fatty acids intake from seafood and high levels of anxiety symptoms during pregnancy: Findings from the Avon Longitudinal Study of Parents and Children. PLoS ONE 2013, 8, e67671. [Google Scholar]
- Golding, J.; Steer, C.; Emmett, P.; Davis, J.M.; Hibbeln, J.R. High Levels of Depressive Symptoms in Pregnancy with Low Omega-3 Fatty Acid Intake from Fish. Epidemiology 2009, 20, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, C.M.M.; Kac, G. High dietary ratio of omega-6 to omega-3 polyunsaturated acids during pregnancy and prevalence of post-partum depression. Matern. Child Nutr. 2012, 8, 36–48. [Google Scholar] [CrossRef]
- Chong, M.F.F.; Ong, Y.-L.; Calder, P.C.; Colega, M.; Wong, J.X.Y.; Tan, C.S.; Lim, A.L.; Fisk, H.L.; Cai, S.; Pang, W.W.; et al. Long-Chain Polyunsaturated Fatty Acid Status During Pregnancy and Maternal Mental Health in Pregnancy and the Postpartum Period. J. Clin. Psychiatry 2015, 76, e848–e856. [Google Scholar] [CrossRef] [PubMed]
- Strøm, M.; Mortensen, E.L.; Halldorsson, T.I.; Thorsdottir, I.; Olsen, S.F. Fish and long-chain n-3 polyunsaturated fatty acid intakes during pregnancy and risk of postpartum depression: A prospective study based on a large national birth cohort. Am. J. Clin. Nutr. 2009, 90, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Sioen, I.; van Lieshout, L.; Eilander, A.; Fleith, M.; Lohner, S.; Szommer, A.; Petisca, C.; Eussen, S.; Forsyth, S.; Calder, P.C.; et al. Systematic Review on N-3 and N-6 Polyunsaturated Fatty Acid Intake in European Countries in Light of the Current Recommendations—Focus on Specific Population Groups. Ann. Nutr. Metab. 2017, 70, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.J.; Houwelingen, A.C.; Antal, M.; Manninen, A.; Godfrey, K.; López-Jaramillo, P.; Hornstra, G. Maternal and neonatal essential fatty acid status in phospholipids: An international comparative study. Eur. J. Clin. Nutr. 1997, 51, 232–242. [Google Scholar] [CrossRef]
- Al, M.D.; van Houwelingen, A.C.; Kester, A.D.; Hasaart, T.H.; de Jong, A.E.; Hornstra, G. Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. Br. J. Nutr. 1995, 74, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.J.; de Groot, R.H.M.; Hornstra, G. Increased risk of postpartum depressive symptoms is associated with slower normalization after pregnancy of the functional docosahexaenoic acid status. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 237–243. [Google Scholar] [CrossRef]
- De Vriese, S.R.; Christophe, A.B.; Maes, M. Lowered serum n-3 polyunsaturated fatty acid (PUFA) levels predict the occurrence of postpartum depression: Further evidence that lowered n-PUFAs are related to major depression. Life Sci. 2003, 73, 3181–3187. [Google Scholar] [CrossRef] [PubMed]
- Markhus, M.W.; Skotheim, S.; Graff, I.E.; Frøyland, L.; Braarud, H.C.; Stormark, K.M.; Malde, M.K. Low omega-3 index in pregnancy is a possible biological risk factor for postpartum depression. PLoS ONE 2013, 8, e67617. [Google Scholar] [CrossRef]
- Chalon, S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 259–269. [Google Scholar] [CrossRef]
- Gerbi, A.; Zérouga, M.; Debray, M.; Durand, G.; Chanez, C.; Bourre, J.M. Effect of fish oil diet on fatty acid composition of phospholipids of brain membranes and on kinetic properties of Na+,K(+)-ATPase isoenzymes of weaned and adult rats. J. Neurochem. 1994, 62, 1560–1569. [Google Scholar] [CrossRef]
- Lichtstein, D.; Ilani, A.; Rosen, H.; Horesh, N.; Singh, S.; Buzaglo, N.; Hodes, A. Na+, K+-ATPase Signaling and Bipolar Disorder. Int. J. Mol. Sci. 2018, 19, 2314. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E. Inflammation and depression: A causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018, 30, 1–16. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Chang, C.-H.; Chong, M.F.-F.; Chen, H.; Su, K.-P. Polyunsaturated Fatty Acids in Perinatal Depression: A Systematic Review and Meta-analysis. Biol. Psychiatry 2017, 82, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.; Vieira, M.C.; Lepsch, J.; Rebelo, F.; Poston, L.; Pasupathy, D.; Kac, G. A systematic review of the associations between maternal nutritional biomarkers and depression and/or anxiety during pregnancy and postpartum. J. Affect. Disord. 2018, 232, 185–203. [Google Scholar] [CrossRef]
- Sallis, H.; Steer, C.; Paternoster, L.; Davey Smith, G.; Evans, J. Perinatal depression and omega-3 fatty acids: A Mendelian randomisation study. J. Affect. Disord. 2014, 166, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Wisner, K.L.; Luther, J.F.; Powers, R.W.; Evans, R.W.; Gallaher, M.J.; Newby, P.K. An exploratory factor analysis of nutritional biomarkers associated with major depression in pregnancy. Public Health Nutr. 2012, 15, 1078–1086. [Google Scholar] [CrossRef]
- Mattes, E.; McCarthy, S.; Gong, G.; van Eekelen, J.A.M.; Dunstan, J.; Foster, J.; Prescott, S.L. Maternal mood scores in mid-pregnancy are related to aspects of neonatal immune function. Brain. Behav. Immun. 2009, 23, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.; Hegarty, B.; Granville-Smith, I.; Ho, J.; Paterson, A.; Gokiert, A.; Hadzi-Pavlovic, D. Is essential fatty acid status in late pregnancy predictive of post-natal depression? Acta Psychiatr. Scand. 2015, 131, 148–156. [Google Scholar] [CrossRef]
- Harris, W.S.; Thomas, R.M. Biological variability of blood omega-3 biomarkers. Clin. Biochem. 2010, 43, 338–340. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hoge, A.; Bernardy, F.; Donneau, A.-F.; Dardenne, N.; Degée, S.; Timmermans, M.; Nisolle, M.; Guillaume, M.; Castronovo, V. Low omega-3 index values and monounsaturated fatty acid levels in early pregnancy: An analysis of maternal erythrocytes fatty acids. Lipids Health Dis. 2018, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.; Van den Akker, O. The retrospective diagnosis of postnatal depression by questionnaire. J. Psychosom. Res. 1992, 36, 67–75. [Google Scholar] [CrossRef]
- World Health Organization WHO. Process of Translation and Adaptation of Instruments; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Amaru, D.; Le Bon, O. La dépression du post-partum: Corrélats et facteurs prédictifs. Rev. Med. Brux. 2014, 35, 10–16. [Google Scholar] [PubMed]
- Lands, B.; Bibus, D.; Stark, K.D. Dynamic interactions of n-3 and n-6 fatty acid nutrients. Prostaglandins Leukot. Essent. Fatty Acids 2018, 136, 15–21. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Georgin-Lavialle, S.; Moura, D.S.; Salvador, A.; Chauvet-Gelinier, J.-C.; Launay, J.-M.; Damaj, G.; Côté, F.; Soucié, E.; Chandesris, M.-O.; Barète, S.; et al. Mast cells’ involvement in inflammation pathways linked to depression: Evidence in mastocytosis. Mol. Psychiatry 2016, 21, 1511–1516. [Google Scholar] [CrossRef]
- Maes, M.; Smith, R.; Christophe, A.; Cosyns, P.; Desnyder, R.; Meltzer, H. Fatty acid composition in major depression: Decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J. Affect. Disord. 1996, 38, 35–46. [Google Scholar] [CrossRef]
- Adams, P.B.; Lawson, S.; Sanigorski, A.; Sinclair, A.J. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids 1996, 31, S157–S161. [Google Scholar] [CrossRef] [PubMed]
- Friesen, R.W.; Innis, S.M. Dietary Arachidonic Acid to EPA and DHA Balance Is Increased among Canadian Pregnant Women with Low Fish Intake. J. Nutr. 2009, 139, 2344–2350. [Google Scholar] [CrossRef]
- McCullough, L.E.; Miller, E.E.; Calderwood, L.E.; Shivappa, N.; Steck, S.E.; Forman, M.R.; Mendez, M.A.; Fuemmeler, B.F.; Kollins, S.H.; et al. Maternal inflammatory diet and adverse pregnancy outcomes: Circulating cytokines and genomic imprinting as potential regulators? Epigenetics 2017, 12, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary Aspects of Diet: The Omega-6/Omega-3 Ratio and the Brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Van der Wurff, I.S.M.; von Schacky, C.; Bergeland, T.; Leontjevas, R.; Zeegers, M.P.; Kirschner, P.A.; de Groot, R.H.M. Exploring the association between whole blood Omega-3 Index, DHA, EPA, DHA, AA and n-6 DPA, and depression and self-esteem in adolescents of lower general secondary education. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Milte, C.M.; Sinn, N.; Howe, P.R. Polyunsaturated fatty acid status in attention deficit hyperactivity disorder, depression, and Alzheimer’s disease: Towards an omega-3 index for mental health? Nutr. Rev. 2009, 67, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Bigornia, S.J.; Harris, W.S.; Falcón, L.M.; Ordovás, J.M.; Lai, C.-Q.; Tucker, K.L. The Omega-3 Index Is Inversely Associated with Depressive Symptoms among Individuals with Elevated Oxidative Stress Biomarkers. J. Nutr. 2015, 146, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Baghai, T.C.; Varallo-Bedarida, G.; Born, C.; Häfner, S.; Schüle, C.; Eser, D.; Rupprecht, R.; Bondy, B.; von Schacky, C. Major Depressive Disorder Is Associated With Cardiovascular Risk Factors and Low Omega-3 Index. J. Clin. Psychiatry 2011, 72, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Sparling, T.M.; Nesbitt, R.C.; Henschke, N.; Gabrysch, S. Nutrients and perinatal depression: A systematic review. J. Nutr. Sci. 2017, 6, e61. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-C.; Tung, C.-Y.; Chen, H.-E. Omega-3 polyunsaturated fatty acid supplementation in prevention and treatment of maternal depression: Putative mechanism and recommendation. J. Affect. Disord. 2018, 238, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.; Dos, S.; Farias, D.R.; Adegboye, A.R.A.; Nardi, A.E.; Kac, G. Omega-3 supplementation from pregnancy to postpartum to prevent depressive symptoms: A randomized placebo-controlled trial. BMC Pregnancy Childbirth 2017, 17, 180. [Google Scholar] [CrossRef]
- Xie, L.; Innis, S.M. Genetic Variants of the FADS1 FADS2 Gene Cluster Are Associated with Altered (n-6) and (n-3) Essential Fatty Acids in Plasma and Erythrocyte Phospholipids in Women during Pregnancy and in Breast Milk during Lactation. J. Nutr. 2008, 138, 2222–2228. [Google Scholar] [CrossRef]
- Meldrum, S.J.; Li, Y.; Zhang, G.; Heaton, A.E.M.; D’Vaz, N.; Manz, J.; Reischl, E.; Koletzko, B.V.; Prescott, S.L.; Simmer, K. Can polymorphisms in the fatty acid desaturase (FADS) gene cluster alter the effects of fish oil supplementation on plasma and erythrocyte fatty acid profiles? An exploratory study. Eur. J. Nutr. 2018, 57, 2583–2594. [Google Scholar] [CrossRef]
- Bergmann, R.L.; Haschke-Becher, E.; Klassen-Wigger, P.; Bergmann, K.E.; Richter, R.; Dudenhausen, J.W.; Grathwohl, D.; Haschke, F. Supplementation with 200 mg/day docosahexaenoic acid from mid-pregnancy through lactation improves the docosahexaenoic acid status of mothers with a habitually low fish intake and of their infants. Ann. Nutr. Metab. 2008, 52, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Imhoff-Kunsch, B. WHO|Marine Oil Supplementation in Pregnancy and Maternal and Neonatal Health Outcomes. Available online: http://www.who.int/elena/titles/commentary/fish_oil_pregnancy/en/ (accessed on 30 January 2018).
- Freeman, M.P.; Sinha, P. Tolerability of omega-3 fatty acid supplements in perinatal women. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Hoge, A.; Bernardy, F.; Donneau, A.-F.; Dardenne, N.; Degée, S.; Nisolle, M.; Guillaume, M.; Castronovo, V. Importance of n-3 PUFA consumption during pregnancy: Perception discrepancies between pregnant women and gynaecologists-obstetricians in Belgium. Public Health Nutr. 2019. [Google Scholar] [CrossRef]
- De Figueiredo, F.P.; Parada, A.P.; Cardoso, V.C.; Batista, R.F.L.; da Silva, A.A.M.; Barbieri, M.A.; de Carvalho Cavalli, R.; Bettiol, H.; Del-Ben, C.M. Postpartum depression screening by telephone: A good alternative for public health and research. Arch. Women’s Ment. Health 2015, 18, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Metabolic programming of long-term outcomes due to fatty acid nutrition in early life. Matern. Child Nutr. 2011, 7 (Suppl. 2), 112–123. [Google Scholar] [CrossRef]
| Variables | All | Depressive (n = 17) | Control (n = 54) | p-Value 1 |
|---|---|---|---|---|
| Age (years) | 29.1 ± 5.0 | 29.5 ± 4.6 | 28.9 ± 5.1 | 0.70 |
| Pre-pregnancy body mass index (BMI) class (kg/m²) | 0.29 | |||
| <25 | 47 (66.2) | 9 (52.9) | 38 (70.4) | |
| 25–30 | 20 (28.2) | 6 (35.3) | 14 (25.9) | |
| ≥30 | 4 (5.63) | 2 (11.8) | 2 (3.70) | |
| Gestational age at inclusion (weeks) | 10.6 ± 2.4 | 10.5 ± 2.6 | 10.6 ± 2.4 | 0.97 |
| Gestational age at delivery (weeks) | 39.1 ± 1.7 | 39.2 ± 1.7 | 39.0 ± 1.8 | 0.66 |
| Parity | 0.037 | |||
| Nulliparous | 31 (43.7) | 5 (29.4) | 26 (48.2) | |
| Primiparous | 26 (36.6) | 5 (29.4) | 21 (38.9) | |
| Multiparous | 14 (19.7) | 7 (41.2) | 7 (13.0) | |
| Nationality | 0.31 | |||
| Belgian | 47 (66.2) | 13 (76.5) | 34 (63.0) | |
| Other | 24 (33.8) | 4 (23.5) | 20 (37.0) | |
| Level of education | 0.63 | |||
| Low | 34 (47.9) | 9 (52.9) | 25 (46.3) | |
| High | 37 (52.1) | 8 (47.1) | 29 (53.7) | |
| Socio-professional occupation | 0.045 | |||
| Yes | 40 (56.3) | 6 (35.3) | 34 (63.0) | |
| No | 31 (43.6) | 11 (64.7) | 20 (37.0) | |
| In a relationship | 0.21 | |||
| Yes | 60 (84.5) | 16 (94.1) | 44 (81.5) | |
| No | 11 (15.5) | 1 (5.88) | 10 (18.5) |
| Variables | PPD | OR | 95% CI | p-Value 1 | |
|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | ||||
| Planned pregnancy | 0.71 | ||||
| Yes | 13 (81.3) | 46 (85.2) | - | - | |
| No | 3 (18.8) | 8 (14.8) | 1.33 | 0.31–5.73 | |
| Adverse life event(s) during pregnancy | 0.49 | ||||
| No | 7 (43.8) | 29 (53.7) | - | - | |
| Yes | 9 (56.3) | 25 (46.3) | 1.49 | 0.49–4.59 | |
| Adverse life event(s) after childbirth | 0.029 | ||||
| No | 4 (25.0) | 31 (57.4) | - | - | |
| Yes | 12 (75.0) | 23 (42.6) | 4.04 | 1.15–14.2 | |
| Satisfaction in social support from entourage during pregnancy | 0.52 2 | ||||
| Yes | 16 (100.0) | 50 (92.6) | - | - | |
| No | 0 (0.00) | 4 (7.41) | 0.34 | 0.01–9.35 | |
| Satisfaction in social support from entourage after childbirth | 0.43 2 | ||||
| Yes | 16 (100.0) | 49 (90.7) | - | - | |
| No | 0 (0.00) | 5 (9.3) | 0.27 | 0.01–6.83 | |
| Emotional distress during pregnancy | 0.26 | ||||
| No | 6 (37.5) | 29 (53.7) | - | - | |
| Yes | 10 (62.5) | 25 (46.3) | 1.93 | 0.62–6.07 | |
| Fatty Acids (%) | PPD | Unadjusted Model | Adjusted Model 1 | |||
|---|---|---|---|---|---|---|
| Yes, Mean ± SD | No, Mean ± SD | OR (95% CI) | p-Value 2 | OR (95% CI) | p-Value 2 | |
| ALA | 0.14 ± 0.07 | 0.13 ± 0.04 | 1.05 (0.25–4.49) | 0.95 3 | 2.31 (0.43–12.5) | 0.33 3 |
| EPA | 0.46 ± 0.26 | 0.58 ± 0.26 | 0.10 (0.00–1.99) | 0.13 | 0.11 (0.00–2.48) | 0.17 |
| DHA | 4.85 ± 1.36 | 5.72 ± 1.34 | 0.55 (0.33–0.93) | 0.026 | 0.53 (0.30–0.95) | 0.034 |
| Total n-3 PUFA | 5.45 ± 1.52 | 6.43 ± 1.52 | 0.58 (0.35–0.94) | 0.027 | 0.57 (0.34–0.98) | 0.043 |
| Omega-3 index | 5.31 ± 1.53 | 6.30 ± 1.54 | 0.58 (0.36–0.97) | 0.028 | 0.57 (0.34–0.98) | 0.040 |
| Total n-6 PUFA | 24.6 ± 1.63 | 23.8 ± 1.36 | 1.51 (1.00–2.26) | 0.05 | 1.55 (0.96–2.51) | 0.07 |
| n-6/n-3 ratio | 4.92 ± 1.60 | 3.91 ± 0.94 | 2.09 (1.24–3.52) | 0.006 | 2.31 (1.20–4.45) | 0.013 |
| AA/EPA ratio | 39.3 ± 19.4 | 28.8 ± 11.3 | 1.05 (1.00–1.10) | 0.016 | 1.05 (1.00–1.11) | 0.043 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoge, A.; Tabar, V.; Donneau, A.-F.; Dardenne, N.; Degée, S.; Timmermans, M.; Nisolle, M.; Guillaume, M.; Castronovo, V. Imbalance between Omega-6 and Omega-3 Polyunsaturated Fatty Acids in Early Pregnancy Is Predictive of Postpartum Depression in a Belgian Cohort. Nutrients 2019, 11, 876. https://doi.org/10.3390/nu11040876
Hoge A, Tabar V, Donneau A-F, Dardenne N, Degée S, Timmermans M, Nisolle M, Guillaume M, Castronovo V. Imbalance between Omega-6 and Omega-3 Polyunsaturated Fatty Acids in Early Pregnancy Is Predictive of Postpartum Depression in a Belgian Cohort. Nutrients. 2019; 11(4):876. https://doi.org/10.3390/nu11040876
Chicago/Turabian StyleHoge, Axelle, Valentine Tabar, Anne-Françoise Donneau, Nadia Dardenne, Sylvie Degée, Marie Timmermans, Michelle Nisolle, Michèle Guillaume, and Vincenzo Castronovo. 2019. "Imbalance between Omega-6 and Omega-3 Polyunsaturated Fatty Acids in Early Pregnancy Is Predictive of Postpartum Depression in a Belgian Cohort" Nutrients 11, no. 4: 876. https://doi.org/10.3390/nu11040876
APA StyleHoge, A., Tabar, V., Donneau, A.-F., Dardenne, N., Degée, S., Timmermans, M., Nisolle, M., Guillaume, M., & Castronovo, V. (2019). Imbalance between Omega-6 and Omega-3 Polyunsaturated Fatty Acids in Early Pregnancy Is Predictive of Postpartum Depression in a Belgian Cohort. Nutrients, 11(4), 876. https://doi.org/10.3390/nu11040876





