Unhealthy yet Avoidable—How Cognitive Bias Modification Alters Behavioral and Brain Responses to Food Cues in Individuals with Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Behavioral Assessment
2.3. Questionnaire Measures
2.4. fMRI Task
2.5. Selection of Stimuli
2.6. Neuroimaging
2.7. Data Analyses
2.7.1. Behavioral Analysis
2.7.2. fMRI Data Analysis
Data Preprocessing
AAT fMRI Data Analysis
GLM1: Pre and Post Data Analysis
GLM2: Psychophysiological Interactions Analysis
Resting-State fMRI Data Analysis
Seed-Based Connectivity Analysis
Seed Definition
Degree Centrality
3. Results
3.1. Behavioral Results
AAT
3.2. Neuroimaging Results
3.2.1. GLM1
Baseline Food Approach and Avoidance
Pre- to Post- Changes
3.2.2. GLM 2: PPI Analysis
3.2.3. Resting-State Data
Seed-Based Connectivity Analysis
Degree Centrality
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castellanos, E.H.; Charboneau, E.; Dietrich, M.S.; Park, S.; Bradley, B.P.; Mogg, K.; Cowan, R.L. Obese adults have visual attention bias for food cue images: Evidence for altered reward system function. Int. J. Obes. 2009, 33, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Werthmann, J.; Roefs, A.; Nederkoorn, C.; Mogg, K.; Bradley, B.P.; Jansen, A. Can (not) take my eyes off it: Attention bias for food in overweight participants. Health Psychol. 2011, 30, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Kemps, E.; Tiggemann, M. Approach bias for food cues in obese individuals. Psychol. Health 2015, 30, 370–380. [Google Scholar] [CrossRef]
- Mehl, N.; Mueller-Wieland, L.; Mathar, D.; Horstmann, A. Retraining automatic action tendencies in obesity. Physiol. Behav. 2018, 192, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Lake, A.; Townshend, T. Obesogenic environments: Exploring the built and food environments. J. R. Soc. Promot. Health 2006, 126, 262–267. [Google Scholar] [CrossRef]
- Cohen, D.; Farley, T.A. Eating as an Automatic Behavior. Prev. Chronic Dis. 2007, 5, A23. [Google Scholar]
- Strack, F.; Deutsch, R. Reflective and Impulsive Determinants of Social Behavior. Personal. Soc. Psychol. Rev. 2004, 8, 220–247. [Google Scholar] [CrossRef]
- Wiers, R.W.; Rinck, M.; Kordts, R.; Houben, K.; Strack, F. Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction 2010, 105, 279–287. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The Neural Basis of Drug Craving—An Incentive-Sensitization Theory of Addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef]
- Nijs, I.M.; Franken, I.H. Attentional Processing of Food Cues in Overweight and Obese Individuals. Curr. Obes. Rep. 2012, 1, 106–113. [Google Scholar] [CrossRef]
- Wiers, R.W.; Rinck, M.; Kordts, R.; Houben, K.; Strack, F. Implicit and explicit alcohol-related cognitions in heavy and light drinkers. J. Abnorm. Psychol. 2002, 111, 648–658. [Google Scholar] [CrossRef] [PubMed]
- De Houwer, J.; Crombez, G.; Koster, E.H.W.; Beul, N.D. Implicit alcohol-related cognitions in a clinical sample of heavy drinkers. J. Behav. Ther. Exp. Psychiatry 2004, 35, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Palfai, T.P.; Ostafin, B.D. Alcohol-related motivational tendencies in hazardous drinkers: Assessing implicit response tendencies using the modified-IAT. Behav. Res. Ther. 2003, 41, 1149–1162. [Google Scholar] [CrossRef]
- Peeters, M.; Wiers, R.W.; Monshouwer, K.; van de Schoot, R.; Janssen, T.; Vollebergh, W.A. Automatic processes in at-risk adolescents: The role of alcohol-approach tendencies and response inhibition in drinking behavior. Addiction 2012, 107, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.B.; Sharpe, L. Cognitive bias modification: A review of meta-analyses. J. Affect. Disord. 2017, 223, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Eberl, C.; Wiers, R.W.; Pawelczack, S.; Rinck, M.; Becker, E.S.; Lindenmeyer, J. Implementation of approach bias re-training in alcoholism-how many sessions are needed? Alcohol. Clin. Exp. Res. 2014, 38, 587–594. [Google Scholar] [CrossRef]
- Machulska, A.; Zlomuzica, A.; Rinck, M.; Assion, H.-J.; Margraf, J. Approach bias modification in inpatient psychiatric smokers. J. Psychiatr. Res. 2016, 76, 44–51. [Google Scholar] [CrossRef]
- Kakoschke, N.; Kemps, E.; Tiggemann, M. Approach bias modification training and consumption: A review of the literature. Addict. Behav. 2017, 64, 21–28. [Google Scholar] [CrossRef]
- Schumacher, S.E.; Kemps, E.; Tiggemann, M. Bias modification training can alter approach bias and chocolate consumption. Appetite 2016, 96, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Jostmann, N.B.; Wiers, R.W.; Holland, R.W. Approach avoidance training in the eating domain: Testing the effectiveness across three single session studies. Appetite 2015, 85, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wiers, C.E.; Ludwig, V.U.; Gladwin, T.E.; Park, S.Q.; Heinz, A.; Wiers, R.W.; Rinck, M.; Lindenmeyer, J.; Walter, H.; Bermpohl, F. Effects of cognitive bias modification training on neural signatures of alcohol approach tendencies in male alcohol-dependent patients. Addict. Biol. 2015, 20, 990–999. [Google Scholar] [CrossRef]
- Cousijn, J.; Goudriaan, A.E.; Ridderinkhof, K.R.; Brink, W.; Veltman, D.J.; Wiers, R.W. Approach-Bias Predicts Development of Cannabis Problem Severity in Heavy Cannabis Users: Results from a Prospective FMRI Study. PLoS ONE 2012, 7, e42394. [Google Scholar] [CrossRef] [PubMed]
- Wiers, C.E.; Stelzel, C.; Park, S.Q.; Gawron, C.K.; Ludwig, V.U.; Gutwinski, S.; Heinz, A.; Lindenmeyer, J.; Wiers, R.W.; Walter, H.; et al. Neural Correlates of Alcohol-Approach Bias in Alcohol Addiction: The Spirit is Willing but the Flesh is Weak for Spirits. Neuropsychopharmacology 2014, 39, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Stunkard, A.J.; Messick, S. The 3-Factor Eating Questionnaire to Measure Dietary Restraint, Disinhibition and Hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Carver, C.S.; White, T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J. Personal. Soc. Psychol. 1994, 67, 319–333. [Google Scholar] [CrossRef]
- Blechert, J.; Meule, A.; Busch, N.A.; Ohla, K. Food-pics: An. image database for experimental research on eating and appetite. Front. Psychol. 2014, 5, 617. [Google Scholar] [CrossRef]
- Wahrburg, U.; Egert, S. Die große Wahrburg/Egert Kalorien- und Nährwerttabelle. 3. Auflage; TRIAS: Stuttgart, Germany, 2014; p. 180. ISBN 978-3-8304-8220-8. [Google Scholar]
- Guenther, P.M.; Casavale, K.O.; Reedy, J.; Kirkpatrick, S.I.; Hiza, H.A.; Kuczynski, K.J.; Kahle, L.L.; Krebs-Smith, S.M. Update of the healthy eating index: HEI-2010. J. Acad. Nutr. Diet. 2013, 113, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Fogli-Cawley, J.J.; Dwyer, J.T.; Saltzman, E.; McCullough, M.L.; Troy, L.M.; Jacques, P.F. The 2005 dietary guidelines for Americans adherence index: Development and application. J. Nutr. 2006, 136, 2908–2915. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef]
- Cox, R.W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef]
- Avants, B.B.; Tustison, N.J.; Song, G.; Cook, P.A.; Klein, A.; Gee, J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage 2011, 54, 2033–2044. [Google Scholar] [CrossRef]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Greve, D.N.; Fischl, B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage 2009, 48, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Alakörkkö, T.; Saarimäki, H.; Glerean, E.; Saramäki, J.; Korhonen, O. Effects of spatial smoothing on functional brain networks. Eur. J. Neurosci. 2017, 46, 2471–2480. [Google Scholar] [CrossRef]
- Pruim, R.H.R.; Mennes, M.; van Rooij, D.; Llera, A.; Buitelaar, J.K.; Beckmann, C.F. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage 2015, 112, 267–277. [Google Scholar] [CrossRef]
- Grinband, J.; Wager, T.D.; Lindquist, M.; Ferrera, V.P.; Hirsch, J. Detection of time-varying signals in event-related fMRI designs. NeuroImage 2008, 43, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Shirer, W.R.; Ryali, S.; Rykhlevskaia, E.; Menon, V.; Greicius, M.D. Decoding Subject-Driven Cognitive States with Whole-Brain Connectivity Patterns. Cereb. Cortex 2012, 22, 158–165. [Google Scholar] [CrossRef]
- Maldjian, J.A.; Laurienti, P.J.; Kraft, R.A.; Burdette, J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 2003, 19, 1233–1239. [Google Scholar] [CrossRef]
- Van der Laan, L.N.; de Ridder, D.T.D.; Viergever, M.A.; Smeets, P.A.M. The first taste is always with the eyes: A meta-analysis on the neural correlates of processing visual food cues. NeuroImage 2011, 55, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Kable, J.W.; Glimcher, P.W. The neural correlates of subjective value during intertemporal choice. Nat. Neurosci. 2007, 10, 1625–1633. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.-J. The addicted human brain viewed in the light of imaging studies: Brain circuits and treatment strategies. Neuropharmacology 2004, 47 (Suppl. 1), 3–13. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of Addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S.E.; Malenka, S.C.; Nestler, E.J. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu. Rev. Neurosci. 2006, 29, 565–598. [Google Scholar] [CrossRef]
- Heinz, A.; Beck, A.; Grüsser, S.M.; Grace, A.A.; Wrase, J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict. Biol. 2009, 14, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Hare, T.A.; Malmaud, J.; Rangel, A. Focusing Attention on the Health Aspects of Foods Changes Value Signals in vmPFC and Improves Dietary Choice. J. Neurosci. 2011, 31, 11077–11087. [Google Scholar] [CrossRef]
- Dietrich, A.; Hollmann, M.; Mathar, D.; Villringer, A.; Horstmann, A. Brain regulation of food craving: Relationships with weight status and eating behavior. Int. J. Obes. 2016, 40, 982–989. [Google Scholar] [CrossRef]
- Zuo, X.-N.; Ehmke, R.; Mennes, M.; Imperati, D.; Castellanos, F.X.; Sporns, O.; Milham, M.P. Network Centrality in the Human Functional Connectome. Cereb. Cortex 2012, 22, 1862–1875. [Google Scholar] [CrossRef]
- Bzdok, D.; Langner, R.; Schilbach, L.; Jakobs, O.; Roski, C.; Caspers, S.; Laird, A.R.; Fox, P.T.; Zilles, K.; Eickhoff, S.B. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. NeuroImage 2013, 81, 381–392. [Google Scholar] [CrossRef]
- Krall, S.C.; Rottschy, C.; Oberwelland, E.; Bzdok, D.; Fox, P.T.; Eickhoff, S.B.; Fink, G.R.; Konrad, K. The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Struct. Funct. 2015, 220, 587–604. [Google Scholar] [CrossRef]
- Garavan, H.; Ross, T.J.; Stein, E.A. Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proc. Natl. Acad. Sci. USA 1999, 96, 8301–8306. [Google Scholar] [CrossRef]
- Simmonds, D.J.; Pekar, J.J.; Mostofsky, S.H. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 2008, 46, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Boehler, C.N.; Appelbaum, L.G.; Krebs, R.M.; Hopf, J.M.; Woldorff, M.G. Pinning down response inhibition in the brain—Conjunction analyses of the Stop-signal task. NeuroImage 2010, 52, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Ryali, S.; Chen, T.; Li, C.-S.R.; Menon, V. Dissociable Roles of Right Inferior Frontal Cortex and Anterior Insula in Inhibitory Control: Evidence from Intrinsic and Task-Related Functional Parcellation, Connectivity, and Response Profile Analyses across Multiple Datasets. J. Neurosci. 2014, 34, 14652–14667. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, M.F.S.; Ellison, A.; Walsh, V. Complementary localization and lateralization of orienting and motor attention. Nat. Neurosci. 2001, 4, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Schiff, S.; Bardi, L.; Basso, D.; Mapelli, D. Timing Spatial Conflict within the Parietal Cortex: A TMS Study. J. Cogn. Neurosci. 2011, 23, 3998–4007. [Google Scholar] [CrossRef]
- Seghier, M.L. The Angular Gyrus: Multiple Functions and Multiple Subdivisions. Neuroscientist 2013, 19, 43–61. [Google Scholar] [CrossRef]
- Cieslik, E.C.; Mueller, V.I.; Eickhoff, C.R.; Langner, R.; Eickhoff, S.B. Three key regions for supervisory attentional control: Evidence from neuroimaging meta-analyses. Neurosci. Biobehav. Rev. 2015, 48, 22–34. [Google Scholar] [CrossRef]
- Kolodny, T.; Mevorach, C.; Shalev, L. Isolating response inhibition in the brain: Parietal versus frontal contribution. Cortex 2017, 88, 173–185. [Google Scholar] [CrossRef]
- Ritschel, F.; Geisler, D.; King, J.A.; Bernardoni, F.; Seidel, M.; Boehm, I.; Vettermann, R.; Biemann, R.; Roessner, V.; Smolka, M.N.; et al. Neural correlates of altered feedback learning in women recovered from anorexia nervosa. Sci. Rep. 2017, 7, 5421. [Google Scholar] [CrossRef]
- Ferentzi, H.; Scheibner, H.; Wiers, R.; Becker, E.S.; Lindenmeyer, J.; Beisel, S.; Rinck, M. Retraining of automatic action tendencies in individuals with obesity: A randomized controlled trial. Appetite 2018, 126, 66–72. [Google Scholar] [CrossRef]
- Jankowski, J.; Scheef, L.; Hüppe, C.; Boecker, H. Distinct striatal regions for planning and executing novel and automated movement sequences. NeuroImage 2009, 44, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Mestres-Missé, A.; Turner, R.; Friederici, A.D. An anterior-posterior gradient of cognitive control within the dorsomedial striatum. NeuroImage 2012, 62, 41–47. [Google Scholar] [CrossRef]
- Swick, D.; Ashley, V.; Turken, A.U. Dorsal striatum mediates cognitive control, not cognitive effort per se, in decision-making: An event-related fMRI study. NeuroImage 2015, 114, 170–184. [Google Scholar]
- Liljeholm, M.; O’Doherty, J.P. Contributions of the striatum to learning, motivation, and performance: An associative account. Trends Cogn. Sci. 2012, 16, 467–475. [Google Scholar] [CrossRef]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef]
- Zwosta, K.; Ruge, H.; Wolfensteller, U. Neural mechanisms of goal-directed behavior: Outcome-based response selection is associated with increased functional coupling of the angular gyrus. Front. Hum. Neurosci. 2015, 9, 180. [Google Scholar] [CrossRef]
- Swick, D.; Ashley, V.; Turken, A.U. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008, 9, 102. [Google Scholar] [CrossRef]
- Stice, E.; Yokum, S.; Veling, H.; Kemps, E.; Lawrence, N.S. Pilot test of a novel food response and attention training treatment for obesity: Brain imaging data suggest actions shape valuation. Behav. Res. Ther. 2017, 94, 60–70. [Google Scholar] [CrossRef]
- Meule, A.; Richard, A.; Dinic, R.; Blechert, J.; Meule, A. Effects of a smartphone-based approach–avoidance intervention on chocolate craving and consumption. JMIR mHealth and uHealth, in press. [CrossRef]
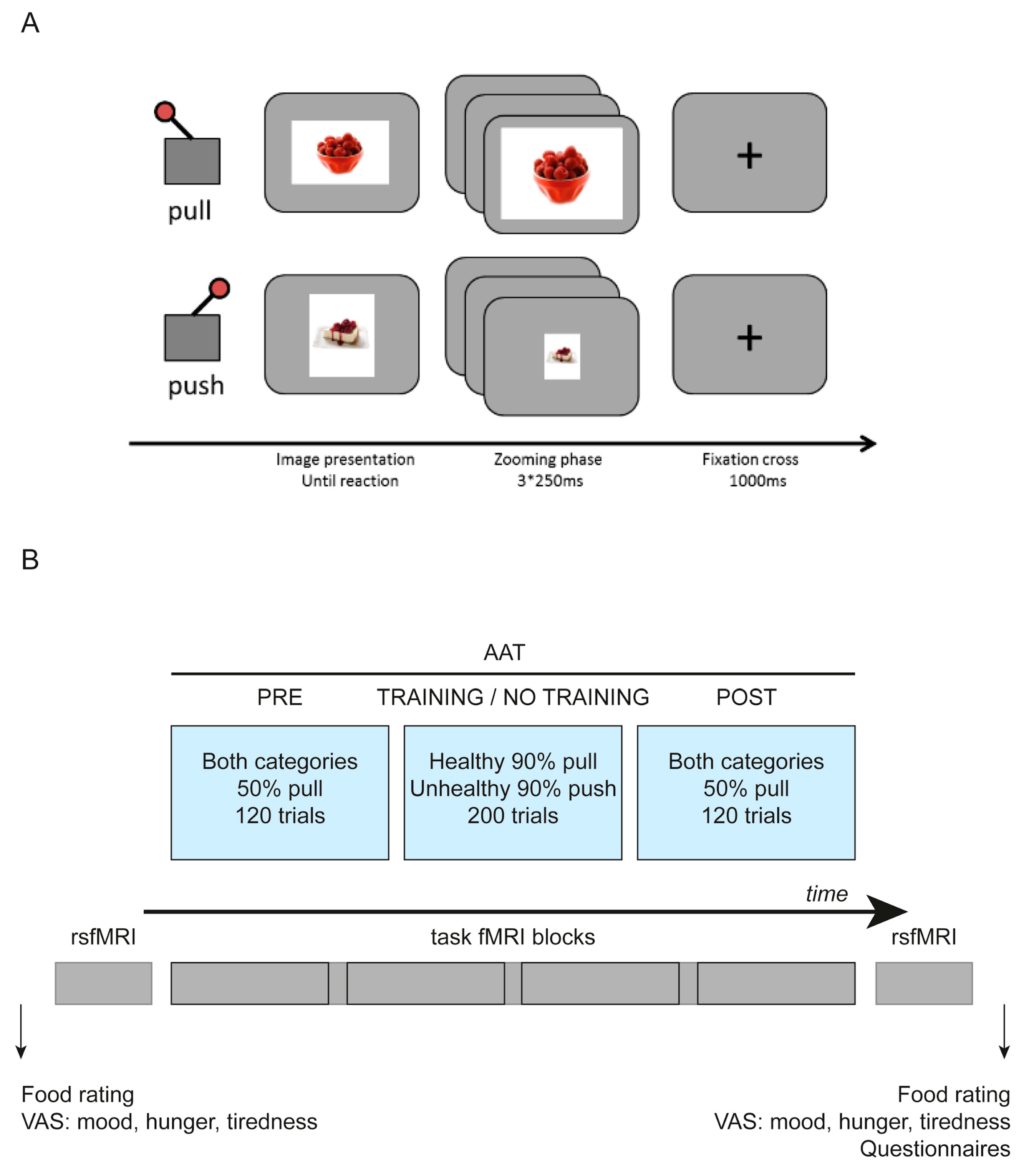
| Training Group | No-Training Group | p-Value/T(31) Value (Unless Otherwise Indicated) | Effect Size |D| (Unless Otherwise Indicated) | |
|---|---|---|---|---|
| Mean/SD (unless otherwise indicated) | ||||
| n | 17 | 16 | ||
| Sex | 11 ♀. 6 ♂ | 7 ♀. 9 ♂ | 0.227/χ2 = 1.460 | φ = 0.043 |
| Age (years) | 28/5 | 31/4 | 0.027/2.314 | 0.663 |
| BMI (kg/m2) | 35.57/4.63 | 36.95/7.63 | 0.530/0.635 | 0.219 |
| Hunger (VAS cm; not hungry–hungry) | 2.31/1.81 | 2.73/1.91 | 0.534/0.629 | 0.226 |
| Tiredness (VAS cm; not tired–tired) | 4.31/2.60 | 4.00/2.30 | 0.726/-0.354 | 0.126 |
| Mood (VAS cm; in a bad mood–in a good mood) | 7.69/1.58 | 8.20/1.26 | 0.711/ = 0.374 | 0.357 |
| Image Category | Training Group | Sham-Training Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | p-Value/ | Effect Size d | Pre | Post | p-Value/ | Effect Size d | |
| Mean/SD | t(16)-Value | Mean/SD | t(15)-Value | |||||
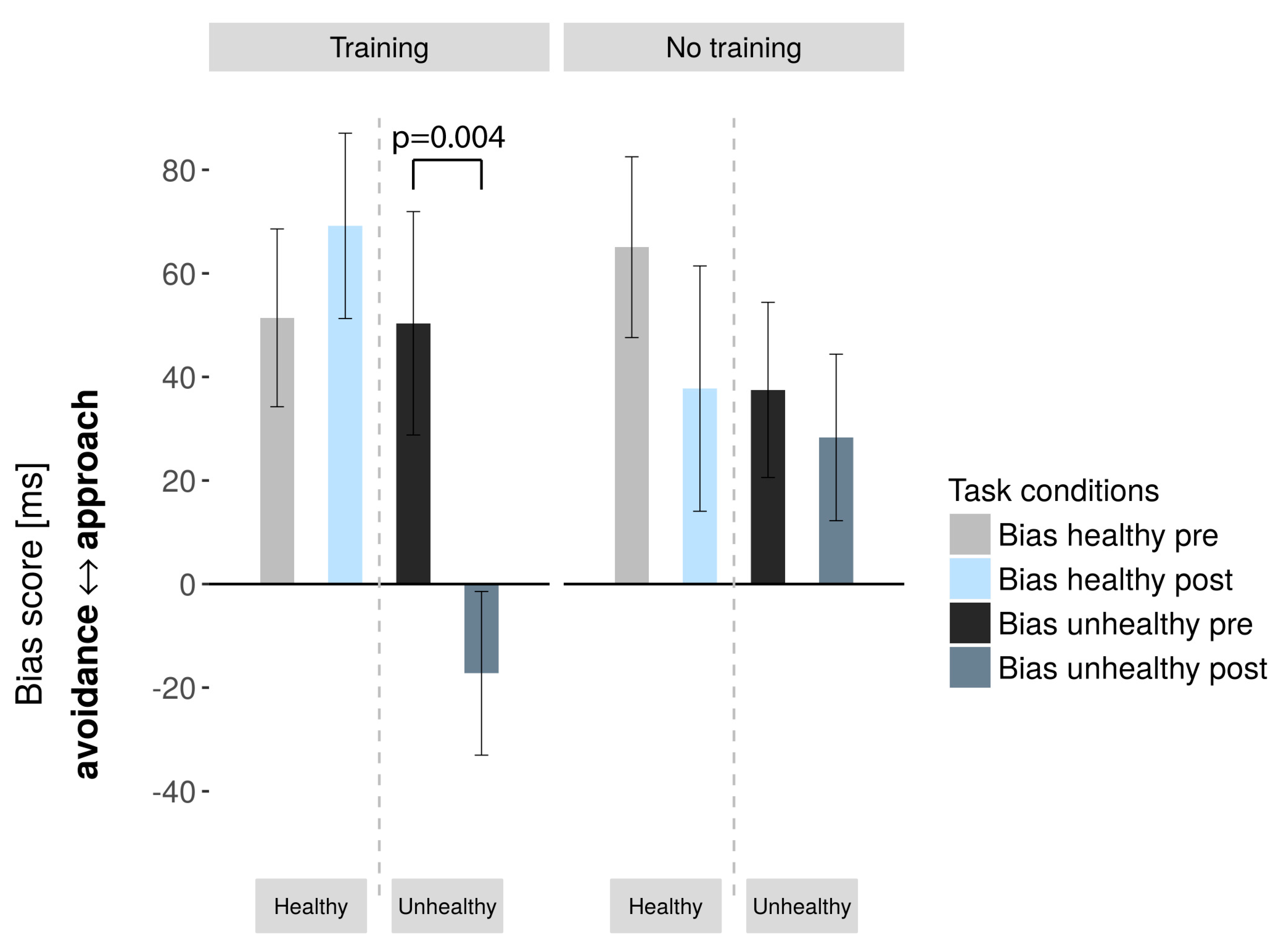
| Unhealthy | 50.35/ | −17.24/ | 0.004/ | 0, 81 | 37.50/ | 28.31/ | 0.585/ | 0, 14 |
| 88, 97 | 65, 18 | −3336 | 67, 63 | 64, 34 | 0, 559 | |||
| Healthy | 51.41/ | 69.18/ | 0.429/ | 0, 197 | 65.06/ | 37.75/ | 0.126/ | 0, 403 |
| 70, 8 | 73, 78 | −0, 812 | 69, 81 | 94, 76 | 1, 61 | |||
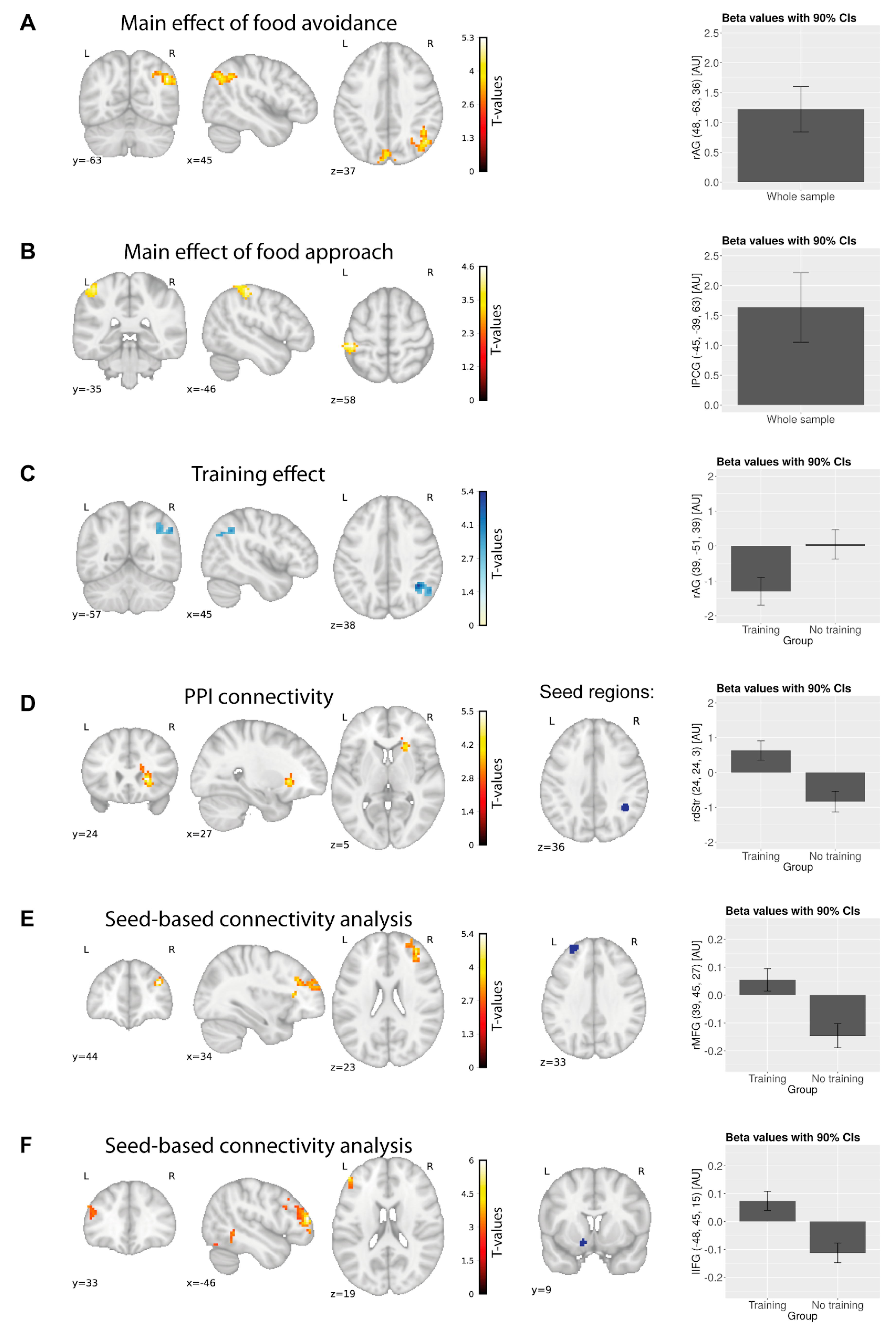
| Contrast (Pre > Post Phase) | Region of the Peak Voxel | Cluster Size (Voxels) | Coordinates (MNI) | Peak z Score | Peak t Score | |||
|---|---|---|---|---|---|---|---|---|
| Brain regions associated with baseline food approach/avoidance bias | ||||||||
| Food avoidance | Angular gyrus R | 178 | 48 | −63 | 36 | 4, 4 | 5, 28 | |
| Cuneus | 131 | 0 | −87 | 24 | 4, 21 | 4, 96 | ||
| Food approach | Postcentral gyrus L | 98 | −45 | −39 | 63 | 3, 98 | 4, 62 | |
| Unhealthy food avoidance | Angular gyrus R | 129 | 51 | −66 | 33 | 4, 51 | 5, 45 | |
| Cuneus | 212 | −3 | −87 | 24 | 4, 1 | 4, 8 | ||
| Brain regions showing training-related changes between- and within-groups | ||||||||
| Unhealthy food avoidance > healthy food avoidance | Training group | Angular gyrus R | 99 | 51 | −69 | 33 | 4, 06 | 4, 77 |
| Middle occipital gyrus L | 163 | −21 | −90 | −15 | −4, 26 | −5, 07 | ||
| Unhealthy food avoidance | Training group | Inferior parietal lobe R | 124 | 39 | −51 | 39 | 4, 46 | 5, 41 |
| Sham-training group | Lingual gyrus L | 202 | −3 | −75 | 9 | 3, 99 | 4, 65 | |
| Training>sham-training | Cuneus L | 163 | −15 | −75 | 9 | −3, 73 | −4, 27 | |
| Brain region showing increased activity for unhealthy food avoidance pre-training in the training group only | ||||||||
| Unhealthy food avoidance: pre-phase | Angular gyrus R | 97 | 51 | −66 | 30 | 4 | 4, 67 | |
| PPI connectivity differences in the training group from pre- to post-phase for unhealthy food avoidance | ||||||||
| PPI connectivity in the training group; seed: right angular gyrus | Putamen R | 170 | 24 | 24 | 3 | 4, 51 | 5, 53 | |
| Regions showing a group by time interaction in the resting-state measures of brain activity and connectivity; | ||||||||
| SCA, left middle frontal gyrus | Middle frontal gyrus R | 182 | 39 | 45 | 27 | 4, 443 | 5, 38 | |
| SCA, left nucleus accumbens | Inferior frontal gyrus L | 136 | −48 | 45 | 15 | 4, 8 | 6, 01 | |
| Inferior temporal gyrus L | 118 | −54 | −48 | −18 | 3, 73 | 4, 27 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehl, N.; Morys, F.; Villringer, A.; Horstmann, A. Unhealthy yet Avoidable—How Cognitive Bias Modification Alters Behavioral and Brain Responses to Food Cues in Individuals with Obesity. Nutrients 2019, 11, 874. https://doi.org/10.3390/nu11040874
Mehl N, Morys F, Villringer A, Horstmann A. Unhealthy yet Avoidable—How Cognitive Bias Modification Alters Behavioral and Brain Responses to Food Cues in Individuals with Obesity. Nutrients. 2019; 11(4):874. https://doi.org/10.3390/nu11040874
Chicago/Turabian StyleMehl, Nora, Filip Morys, Arno Villringer, and Annette Horstmann. 2019. "Unhealthy yet Avoidable—How Cognitive Bias Modification Alters Behavioral and Brain Responses to Food Cues in Individuals with Obesity" Nutrients 11, no. 4: 874. https://doi.org/10.3390/nu11040874
APA StyleMehl, N., Morys, F., Villringer, A., & Horstmann, A. (2019). Unhealthy yet Avoidable—How Cognitive Bias Modification Alters Behavioral and Brain Responses to Food Cues in Individuals with Obesity. Nutrients, 11(4), 874. https://doi.org/10.3390/nu11040874





