β-carotene in Obesity Research: Technical Considerations and Current Status of the Field
Abstract
1. Introduction
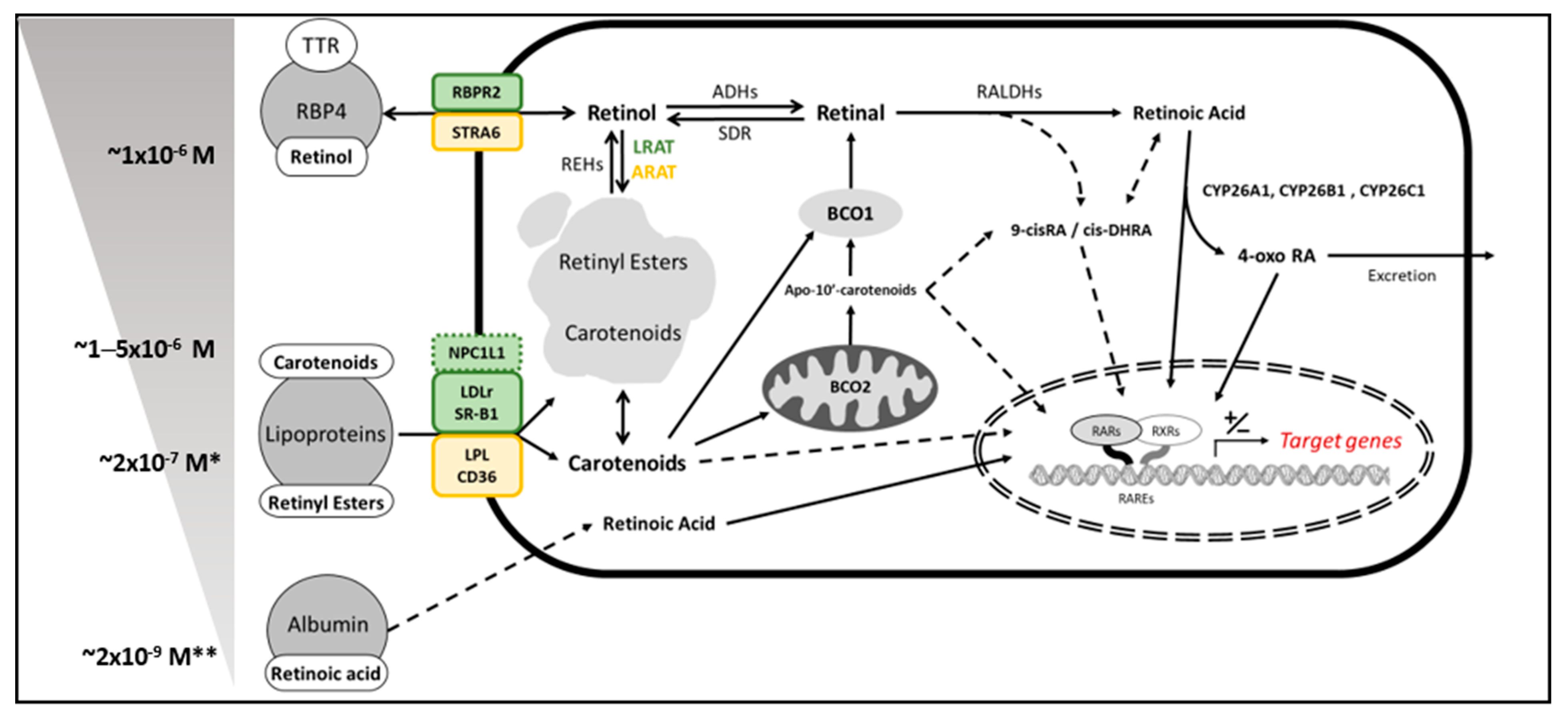
2. Vitamin A Sources in Mammals—Provitamin A Carotenoids
3. β–Carotene Oxygenase 1 as Solely Responsible for Vitamin A Production in Mammals
4. β-Carotene and Obesity; Key Findings and Technical Limitations
4.1. Cell Culture Studies—β-carotene and Adipogenesis
4.2. Cell Culture Studies—Retinoic Acid and Adipogenesis
4.3. Animal Models in Carotenoid Research
4.4. Animal Models—β-carotene and Obesity
4.5. Animal Models—Vitamin A and Obesity
4.6. Human Studies—β-Carotene and Obesity
4.7. Human Studies—Synthetic Retinoids and Obesity
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016; NCHS Data Brief No. 288; Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2017; pp. 1–8. [Google Scholar]
- Briggs, A.M.; Woolf, A.D.; Dreinhofer, K.; Homb, N.; Hoy, D.G.; Kopansky-Giles, D.; Akesson, K.; March, L. Reducing the global burden of musculoskeletal conditions. Bull. World Health Organ. 2018, 96, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- Kimmons, J.E.; Blanck, H.M.; Tohill, B.C.; Zhang, J.; Khan, L.K. Associations between body mass index and the prevalence of low micronutrient levels among US adults. MedGenMed 2006, 8, 59. [Google Scholar] [PubMed]
- Church, T.; Martin, C.K. The Obesity Epidemic: A Consequence of Reduced Energy Expenditure and the Uncoupling of Energy Intake? Obesity 2018, 26, 14–16. [Google Scholar] [CrossRef]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. The adipose organ at a glance. Dis. Model. Mech. 2012, 5, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Jirtle, R.L.; Skinner, M.K. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007, 8, 253–262. [Google Scholar] [CrossRef]
- Cohen, P.; Spiegelman, B.M. Cell biology of fat storage. Mol. Biol. Cell 2016, 27, 2523–2527. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Flier, J.S. Obesity and the regulation of energy balance. Cell 2001, 104, 531–543. [Google Scholar] [CrossRef]
- Klein, S.; Wadden, T.; Sugerman, H.J. AGA technical review on obesity. Gastroenterology 2002, 123, 882–932. [Google Scholar] [CrossRef]
- Hall, K.D.; Sacks, G.; Chandramohan, D.; Chow, C.C.; Wang, Y.C.; Gortmaker, S.L.; Swinburn, B.A. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011, 378, 826–837. [Google Scholar] [CrossRef]
- Makris, A.; Foster, G.D. Dietary approaches to the treatment of obesity. Psychiatr. Clin. N. Am. 2011, 34, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Physical activity, food intake, and body weight regulation: Insights from doubly labeled water studies. Nutr. Rev. 2010, 68, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Su, L.; Wang, J.; Duan, X.; Jiang, X. Fruit and vegetable consumption and risk of the metabolic syndrome: A meta-analysis. Public Health Nutr. 2018, 21, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Maria, A.G.; Graziano, R.; Nicolantonio, D.O. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Edwards, D.; Hamernig, I.; Jian, L.; James, A.P.; Johnson, S.K.; Tapsell, L.C. Vegetables containing phytochemicals with potential anti-obesity properties: A review. Food Res. Int. 2013, 52, 323–333. [Google Scholar] [CrossRef]
- Bonet, M.L.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids in Adipose Tissue Biology and Obesity. In Carotenoids in Nature; Springer: Cham, Switzerland, 2016; Volume 79, pp. 377–414. [Google Scholar]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef]
- Rowles, J.L., 3rd; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W., Jr. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Chen, X.; Jha, K.; Beydoun, H.A.; Zonderman, A.B.; Canas, J.A. Carotenoids, vitamin A, and their association with the metabolic syndrome: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Melendez-Martinez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Moise, A.R.; Al-Babili, S.; Wurtzel, E.T. Mechanistic aspects of carotenoid biosynthesis. Chem. Rev. 2014, 114, 164–193. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.E.; Mohn, E.S.; Hason, N.; Erdman, J.W., Jr.; Johnson, E.J. Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids. Adv. Nutr. 2018, 9, 465–492. [Google Scholar] [CrossRef] [PubMed]
- von Lintig, J.; Eggersdorfer, M.; Wyss, A. News and views about carotenoids: Red-hot and true. Arch. Biochem. Biophys. 2018, 657, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and β carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
- Christen, W.; Glynn, R.; Sperduto, R.; Chew, E.; Buring, J. Age-related cataract in a randomized trial of β-carotene in women. Ophthalmic Epidemiol. 2004, 11, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Hennekens, C.H. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: The Women’s Health Study. J. Natl. Cancer Inst. 1999, 91, 2102–2106. [Google Scholar] [CrossRef] [PubMed]
- van Poppel, G. Epidemiological evidence for β-carotene in prevention of cancer and cardiovascular disease. Eur. J. Clin. Nutr. 1996, 50 (Suppl. 3), S57–S61. [Google Scholar]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.A.; Biesalski, H.K. Beta-carotene is an important vitamin A source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy of Medicine: Washington, DC, USA, 2001. [Google Scholar]
- Burri, B.J. Beta-cryptoxanthin as a source of vitamin A. J. Sci. Food Agric. 2015, 95, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Burri, B.J.; La Frano, M.R.; Zhu, C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutr. Rev. 2016, 74, 69–82. [Google Scholar] [CrossRef]
- Amengual, J.; Widjaja-Adhi, M.A.; Rodriguez-Santiago, S.; Hessel, S.; Golczak, M.; Palczewski, K.; von Lintig, J. Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J. Biol. Chem. 2013, 288, 34081–34096. [Google Scholar] [CrossRef] [PubMed]
- Farook, V.S.; Reddivari, L.; Mummidi, S.; Puppala, S.; Arya, R.; Lopez-Alvarenga, J.C.; Fowler, S.P.; Chittoor, G.; Resendez, R.G.; Kumar, B.M.; et al. Genetics of serum carotenoid concentrations and their correlation with obesity-related traits in Mexican American children. Am. J. Clin. Nutr. 2017, 106, 52–58. [Google Scholar] [CrossRef]
- Meyers, K.J.; Johnson, E.J.; Bernstein, P.S.; Iyengar, S.K.; Engelman, C.D.; Karki, C.K.; Liu, Z.; Igo, R.P., Jr.; Truitt, B.; Klein, M.L.; et al. Genetic determinants of macular pigments in women of the Carotenoids in Age-Related Eye Disease Study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2333–2345. [Google Scholar] [CrossRef] [PubMed]
- Widjaja-Adhi, M.A.; Lobo, G.P.; Golczak, M.; Von Lintig, J. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum. Mol. Genet. 2015, 24, 3206–3219. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, S.; Urata, M.; Wai Kun, R.Y.; Masaki, M.; Shidoji, Y. Common SNP rs6564851 in the BCO1 Gene Affects the Circulating Levels of β-Carotene and the Daily Intake of Carotenoids in Healthy Japanese Women. PLoS ONE 2016, 11, e0168857. [Google Scholar] [CrossRef]
- Haskell, M.J.; Brown, K.H. Maternal vitamin A nutriture and the vitamin A content of human milk. J. Mammary Gland Biol. Neoplasia 1999, 4, 243–257. [Google Scholar] [CrossRef]
- Dary, O.; Mora, J.O.; International Vitamin A Consultative Group. Food fortification to reduce vitamin A deficiency: International Vitamin A Consultative Group recommendations. J. Nutr. 2002, 132, 2927S–2933S. [Google Scholar] [CrossRef]
- Ye, X.; Al-Babili, S.; Kloti, A.; Zhang, J.; Lucca, P.; Beyer, P.; Potrykus, I. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 2000, 287, 303–305. [Google Scholar] [CrossRef]
- La Frano, M.R.; Woodhouse, L.R.; Burnett, D.J.; Burri, B.J. Biofortified cassava increases β-carotene and vitamin A concentrations in the TAG-rich plasma layer of American women. Br. J. Nutr. 2013, 110, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Welsch, R.; Arango, J.; Bar, C.; Salazar, B.; Al-Babili, S.; Beltran, J.; Chavarriaga, P.; Ceballos, H.; Tohme, J.; Beyer, P. Provitamin A accumulation in cassava (Manihot esculenta) roots driven by a single nucleotide polymorphism in a phytoene synthase gene. Plant Cell 2010, 22, 3348–3356. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.Y.; Romer, A.I.; Hu, M.; Lepourcelet, M.; Mechoor, A.; Yesilaltay, A.; Krieger, M.; Gray, P.A.; Shivdasani, R.A. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development 2006, 133, 4119–4129. [Google Scholar] [CrossRef] [PubMed]
- Lobo, G.P.; Amengual, J.; Baus, D.; Shivdasani, R.A.; Taylor, D.; von Lintig, J. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J. Biol. Chem. 2013, 288, 9017–9027. [Google Scholar] [CrossRef]
- Lobo, G.P.; Amengual, J.; Palczewski, G.; Babino, D.; von Lintig, J. Mammalian carotenoid-oxygenases: Key players for carotenoid function and homeostasis. Biochim. Biophys. Acta 2012, 1821, 78–87. [Google Scholar] [CrossRef]
- Beste, L.A.; Moseley, R.H.; Saint, S.; Cornia, P.B. CLINICAL PROBLEM-SOLVING. Too Much of a Good Thing. N. Engl. J. Med. 2016, 374, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Geubel, A.P.; De Galocsy, C.; Alves, N.; Rahier, J.; Dive, C. Liver damage caused by therapeutic vitamin A administration: Estimate of dose-related toxicity in 41 cases. Gastroenterology 1991, 100, 1701–1709. [Google Scholar] [CrossRef]
- Moore, T. Vitamin A and carotene: The absence of the liver oil vitamin A from carotene. VI. The conversion of carotene to vitamin A in vivo. Biochem. J. 1930, 24, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Fidge, N.H.; Smith, F.R.; Goodman, D.S. Vitamin A and carotenoids. The enzymic conversion of β-carotene into retinal in hog intestinal mucosa. Biochem. J. 1969, 114, 689–694. [Google Scholar] [CrossRef]
- Olson, J.A.; Hayaishi, O. The enzymatic cleavage of β-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc. Natl. Acad. Sci. USA 1965, 54, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- dela Sena, C.; Narayanasamy, S.; Riedl, K.M.; Curley, R.W., Jr.; Schwartz, S.J.; Harrison, E.H. Substrate specificity of purified recombinant human β-carotene 15,15′-oxygenase (BCO1). J. Biol. Chem. 2013, 288, 37094–37103. [Google Scholar] [CrossRef] [PubMed]
- Babino, D.; Golczak, M.; Kiser, P.D.; Wyss, A.; Palczewski, K.; von Lintig, J. The Biochemical Basis of Vitamin A3 Production in Arthropod Vision. ACS Chem. Biol. 2016, 11, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- von Lintig, J.; Wyss, A. Molecular analysis of vitamin A formation: Cloning and characterization of β-carotene 15,15′-dioxygenases. Arch. Biochem. Biophys. 2001, 385, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Gouranton, E.; van Helden, Y.G.; Hessel, S.; Ribot, J.; Kramer, E.; Kiec-Wilk, B.; Razny, U.; Lietz, G.; Wyss, A.; et al. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS ONE 2011, 6, e20644. [Google Scholar] [CrossRef]
- Kiefer, C.; Hessel, S.; Lampert, J.M.; Vogt, K.; Lederer, M.O.; Breithaupt, D.E.; von Lintig, J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 2001, 276, 14110–14116. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Tang, G.W.; Fox, J.G.; Krinsky, N.I.; Russell, R.M. Enzymatic conversion of β-carotene into β-apo-carotenals and retinoids by human, monkey, ferret, and rat tissues. Arch. Biochem. Biophys. 1991, 285, 8–16. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Ryan, L.; O’Brien, N. Comparison of the uptake and secretion of carotene and xanthophyll carotenoids by Caco-2 intestinal cells. Br. J. Nutr. 2007, 98, 38–44. [Google Scholar] [CrossRef]
- O’Sullivan, S.M.; Woods, J.A.; O’Brien, N.M. Use of Tween 40 and Tween 80 to deliver a mixture of phytochemicals to human colonic adenocarcinoma cell (CaCo-2) monolayers. Br. J. Nutr. 2004, 91, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Zolberg Relevy, N.; Bechor, S.; Harari, A.; Ben-Amotz, A.; Kamari, Y.; Harats, D.; Shaish, A. The inhibition of macrophage foam cell formation by 9-cis β-carotene is driven by BCMO1 activity. PLoS ONE 2015, 10, e0115272. [Google Scholar] [CrossRef] [PubMed]
- Gouranton, E.; Yazidi, C.E.; Cardinault, N.; Amiot, M.J.; Borel, P.; Landrier, J.F. Purified low-density lipoprotein and bovine serum albumin efficiency to internalise lycopene into adipocytes. Food Chem. Toxicol. 2008, 46, 3832–3836. [Google Scholar] [CrossRef]
- Scita, G. Stability of β-carotene under different laboratory conditions. Methods Enzym. 1992, 213, 175–185. [Google Scholar]
- Gouranton, E.; Thabuis, C.; Riollet, C.; Malezet-Desmoulins, C.; El Yazidi, C.; Amiot, M.J.; Borel, P.; Landrier, J.F. Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue. J. Nutr. Biochem. 2011, 22, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Gouranton, E.; Aydemir, G.; Reynaud, E.; Marcotorchino, J.; Malezet, C.; Caris-Veyrat, C.; Blomhoff, R.; Landrier, J.F.; Ruhl, R. Apo-10′-lycopenoic acid impacts adipose tissue biology via the retinoic acid receptors. Biochim. Biophys. Acta 2011, 1811, 1105–1114. [Google Scholar] [CrossRef]
- Marsh, R.S.; Yan, Y.; Reed, V.M.; Hruszkewycz, D.; Curley, R.W.; Harrison, E.H. {beta}-Apocarotenoids do not significantly activate retinoic acid receptors {alpha} or {beta}. Exp. Biol. Med. 2010, 235, 342–348. [Google Scholar] [CrossRef]
- Eroglu, A.; Hruszkewycz, D.P.; dela Sena, C.; Narayanasamy, S.; Riedl, K.M.; Kopec, R.E.; Schwartz, S.J.; Curley, R.W., Jr.; Harrison, E.H. Naturally occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J. Biol. Chem. 2012, 287, 15886–15895. [Google Scholar] [CrossRef] [PubMed]
- Narayanasamy, S.; Sun, J.; Pavlovicz, R.E.; Eroglu, A.; Rush, C.E.; Sunkel, B.D.; Li, C.; Harrison, E.H.; Curley, R.W., Jr. Synthesis of apo-13- and apo-15-lycopenoids, cleavage products of lycopene that are retinoic acid antagonists. J. Lipid Res. 2017, 58, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Shete, V.; Quadro, L. Mammalian metabolism of β-carotene: Gaps in knowledge. Nutrients 2013, 5, 4849–4868. [Google Scholar] [CrossRef]
- Ross, A.C. Vitamin A and retinoic acid in T cell-related immunity. Am. J. Clin. Nutr. 2012, 96, 1166S–1172S. [Google Scholar] [CrossRef] [PubMed]
- von Lintig, J. Provitamin A metabolism and functions in mammalian biology. Am. J. Clin. Nutr. 2012, 96, 1234S–1244S. [Google Scholar] [CrossRef]
- Shete, V.; Costabile, B.K.; Kim, Y.K.; Quadro, L. Low-Density Lipoprotein Receptor Contributes to β-Carotene Uptake in the Maternal Liver. Nutrients 2016, 8, 765. [Google Scholar] [CrossRef]
- Benbrook, D.M.; Chambon, P.; Rochette-Egly, C.; Asson-Batres, M.A. History of retinoic acid receptors. Subcell. Biochem. 2014, 70, 1–20. [Google Scholar] [PubMed]
- Shaw, N.; Elholm, M.; Noy, N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor β/delta. J. Biol. Chem. 2003, 278, 41589–41592. [Google Scholar] [CrossRef]
- Ruhl, R.; Krzyzosiak, A.; Niewiadomska-Cimicka, A.; Rochel, N.; Szeles, L.; Vaz, B.; Wietrzych-Schindler, M.; Alvarez, S.; Szklenar, M.; Nagy, L.; et al. 9-cis-13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice. PLoS Genet. 2015, 11, e1005213. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, R.; Krezel, W.; de Lera, A.R. 9-Cis-13,14-dihydroretinoic acid, a new endogenous mammalian ligand of retinoid X receptor and the active ligand of a potential new vitamin A category: Vitamin A5. Nutr. Rev. 2018, 76, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.J.; Reginato, M.J.; Shao, D.; Krakow, S.L.; Lazar, M.A. Retinoic acid blocks adipogenesis by inhibiting C/EBPβ-mediated transcription. Mol. Cell. Biol. 1997, 17, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Mercader, J.; Ribot, J.; Murano, I.; Felipe, F.; Cinti, S.; Bonet, M.L.; Palou, A. Remodeling of white adipose tissue after retinoic acid administration in mice. Endocrinology 2006, 147, 5325–5332. [Google Scholar] [CrossRef]
- Wang, B.; Fu, X.; Liang, X.; Deavila, J.M.; Wang, Z.; Zhao, L.; Tian, Q.; Zhao, J.; Gomez, N.A.; Trombetta, S.C.; et al. Retinoic acid induces white adipose tissue browning by increasing adipose vascularity and inducing beige adipogenesis of PDGFRalpha(+) adipose progenitors. Cell Discov. 2017, 3, 17036. [Google Scholar] [CrossRef]
- Serra, F.; Bonet, M.L.; Puigserver, P.; Oliver, J.; Palou, A. Stimulation of uncoupling protein 1 expression in brown adipocytes by naturally occurring carotenoids. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Lobo, G.P.; Amengual, J.; Li, H.N.; Golczak, M.; Bonet, M.L.; Palczewski, K.; von Lintig, J. β,β-carotene decreases peroxisome proliferator receptor gamma activity and reduces lipid storage capacity of adipocytes in a β,β-carotene oxygenase 1-dependent manner. J. Biol. Chem. 2010, 285, 27891–27899. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y. Animal models in carotenoids research and lung cancer prevention. Transl. Oncol. 2011, 4, 271–281. [Google Scholar] [CrossRef]
- Lee, C.M.; Boileau, A.C.; Boileau, T.W.; Williams, A.W.; Swanson, K.S.; Heintz, K.A.; Erdman, J.W., Jr. Review of animal models in carotenoid research. J. Nutr. 1999, 129, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Babino, D.; Palczewski, G.; Widjaja-Adhi, M.A.; Kiser, P.D.; Golczak, M.; von Lintig, J. Characterization of the Role of β-Carotene 9,10-Dioxygenase in Macular Pigment Metabolism. J. Biol. Chem. 2015, 290, 24844–24857. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Vachali, P.P.; Gorusupudi, A.; Shen, Z.; Sharifzadeh, H.; Besch, B.M.; Nelson, K.; Horvath, M.M.; Frederick, J.M.; Baehr, W.; et al. Inactivity of human β,β-carotene-9′,10′-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proc. Natl. Acad. Sci. USA 2014, 111, 10173–10178. [Google Scholar] [CrossRef]
- Hessel, S.; Eichinger, A.; Isken, A.; Amengual, J.; Hunzelmann, S.; Hoeller, U.; Elste, V.; Hunziker, W.; Goralczyk, R.; Oberhauser, V.; et al. CMO1 deficiency abolishes vitamin A production from β-carotene and alters lipid metabolism in mice. J. Biol. Chem. 2007, 282, 33553–33561. [Google Scholar] [CrossRef]
- Amengual, J.; Lobo, G.P.; Golczak, M.; Li, H.N.; Klimova, T.; Hoppel, C.L.; Wyss, A.; Palczewski, K.; von Lintig, J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011, 25, 948–959. [Google Scholar] [CrossRef]
- Ford, N.A.; Clinton, S.K.; von Lintig, J.; Wyss, A.; Erdman, J.W., Jr. Loss of carotene-9′,10′-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. J. Nutr. 2010, 140, 2134–2138. [Google Scholar] [CrossRef] [PubMed]
- Palczewski, G.; Amengual, J.; Hoppel, C.L.; von Lintig, J. Evidence for compartmentalization of mammalian carotenoid metabolism. FASEB J. 2014, 28, 4457–4469. [Google Scholar] [CrossRef]
- Wu, L.; Guo, X.; Lyu, Y.; Clarke, S.L.; Lucas, E.A.; Smith, B.J.; Hildebrand, D.; Wang, W.; Medeiros, D.M.; Shen, X.; et al. Targeted Metabolomics Reveals Abnormal Hepatic Energy Metabolism by Depletion of β-Carotene Oxygenase 2 in Mice. Sci. Rep. 2017, 7, 14624. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.E.; Ramkumar, S.; Sun, W.; Colon Ortiz, C.; Kiser, P.D.; Golczak, M.; von Lintig, J. The Biochemical Basis of Vitamin A Production from the Asymmetric Carotenoid β-Cryptoxanthin. ACS Chem. Biol. 2018, 13, 2121–2129. [Google Scholar] [CrossRef]
- Yonekura, L.; Kobayashi, M.; Terasaki, M.; Nagao, A. Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues. J. Nutr. 2010, 140, 1824–1831. [Google Scholar] [CrossRef]
- Wang, W.; Connor, S.L.; Johnson, E.J.; Klein, M.L.; Hughes, S.; Connor, W.E. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am. J. Clin. Nutr. 2007, 85, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, K.; Morimoto, S.; Shirakura, Y.; Mukai, K.; Sugiyama, T.; Tokuji, Y.; Ohnishi, M. Mechanism of visceral fat reduction in Tsumura Suzuki obese, diabetes (TSOD) mice orally administered β-cryptoxanthin from Satsuma mandarin oranges (Citrus unshiu Marc). J. Agric. Food Chem. 2011, 59, 12342–12351. [Google Scholar] [CrossRef] [PubMed]
- Fenni, S.; Hammou, H.; Astier, J.; Bonnet, L.; Karkeni, E.; Couturier, C.; Tourniaire, F.; Landrier, J.F. Lycopene and tomato powder supplementation similarly inhibit high-fat diet induced obesity, inflammatory response, and associated metabolic disorders. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of astaxanthin in obese mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2007, 71, 893–899. [Google Scholar] [CrossRef]
- Liu, M.; Liu, H.; Xie, J.; Xu, Q.; Pan, C.; Wang, J.; Wu, X.; Sanabil; Zheng, M.; Liu, J. Anti-obesity effects of zeaxanthin on 3T3-L1 preadipocyte and high fat induced obese mice. Food Funct. 2017, 8, 3327–3338. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D. Overview on the Antihypertensive and Anti-Obesity Effects of Secondary Metabolites from Seaweeds. Mar. Drugs 2018, 16, 237. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; DeSantis, D.; Soltanian, H.; Croniger, C.M.; Noy, N. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes 2012, 61, 1112–1121. [Google Scholar] [CrossRef]
- Mercader, J.; Madsen, L.; Felipe, F.; Palou, A.; Kristiansen, K.; Bonet, M.L. All-trans retinoic acid increases oxidative metabolism in mature adipocytes. Cell. Physiol. Biochem. 2007, 20, 1061–1072. [Google Scholar] [CrossRef]
- Bonet, M.L.; Ribot, J.; Felipe, F.; Palou, A. Vitamin A and the regulation of fat reserves. Cell. Mol. Life Sci. 2003, 60, 1311–1321. [Google Scholar] [CrossRef]
- Amengual, J.; Garcia-Carrizo, F.J.; Arreguin, A.; Musinovic, H.; Granados, N.; Palou, A.; Bonet, M.L.; Ribot, J. Retinoic Acid Increases Fatty Acid Oxidation and Irisin Expression in Skeletal Muscle Cells and Impacts Irisin In Vivo. Cell. Physiol. Biochem. 2018, 46, 187–202. [Google Scholar] [CrossRef]
- Amengual, J.; Petrov, P.; Bonet, M.L.; Ribot, J.; Palou, A. Induction of carnitine palmitoyl transferase 1 and fatty acid oxidation by retinoic acid in HepG2 cells. Int. J. Biochem. Cell Biol. 2012, 44, 2019–2027. [Google Scholar] [CrossRef]
- Amengual, J.; Ribot, J.; Bonet, M.L.; Palou, A. Retinoic acid treatment increases lipid oxidation capacity in skeletal muscle of mice. Obesity 2008, 16, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Ribot, J.; Bonet, M.L.; Palou, A. Retinoic acid treatment enhances lipid oxidation and inhibits lipid biosynthesis capacities in the liver of mice. Cell. Physiol. Biochem. 2010, 25, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Cryer, A.; Jones, H.M. The early development of white adipose tissue. Effects of litter size on the lipoprotein lipase activity of four adipose-tissue depots, serum immunoreactive insulin and tissue cellularity during the first four weeks of life in the rat. Biochem. J. 1979, 178, 711–724. [Google Scholar] [CrossRef]
- Granados, N.; Amengual, J.; Ribot, J.; Musinovic, H.; Ceresi, E.; von Lintig, J.; Palou, A.; Bonet, M.L. Vitamin A supplementation in early life affects later response to an obesogenic diet in rats. Int. J. Obes. 2013, 37, 1169–1176. [Google Scholar] [CrossRef]
- Bonet, M.L.; Ribot, J.; Palou, A. Lipid metabolism in mammalian tissues and its control by retinoic acid. Biochim. Biophys. Acta 2012, 1821, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Prolonging healthy aging: Longevity vitamins and proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10836–10844. [Google Scholar] [CrossRef]
- Goncalves, A.; Amiot, M.J. Fat-soluble micronutrients and metabolic syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Osth, M.; Ost, A.; Kjolhede, P.; Stralfors, P. The concentration of β-carotene in human adipocytes, but not the whole-body adipocyte stores, is reduced in obesity. PLoS ONE 2014, 9, e85610. [Google Scholar] [CrossRef]
- Asemi, Z.; Alizadeh, S.A.; Ahmad, K.; Goli, M.; Esmaillzadeh, A. Effects of β-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 2016, 35, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Yao, Y.; Wu, X.; Han, D. Higher dose lutein and a longer supplementation period would be good for visual performance. Nutrition 2013, 29, 1072–1073. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.; Thornquist, M.; Grizzle, J.; Rosenstock, L.; Barnhart, S.; Balmes, J.; Cherniack, M.G.; Cullen, M.R.; Glass, A.; et al. The β-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high risk populations: Smokers and asbestos-exposed workers. Cancer Res. 1994, 54, 2038s–2043s. [Google Scholar] [PubMed]
- Greenwald, P. Beta-carotene and lung cancer: A lesson for future chemoprevention investigations? J. Natl. Cancer Inst. 2003, 95, E1. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyurek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015, 7, 308re8. [Google Scholar] [CrossRef]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014, 6, 221ra5. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, X.; Jiang, J.; Yuan, F.; Decker, E.A.; Gao, Y. Influence of pH, EDTA, α-tocopherol, and WPI oxidation on the degradation of β-carotene in WPI-stabilized oil-in-water emulsions. LWT-Food Sci. Technol. 2013, 54, 236–241. [Google Scholar] [CrossRef]
- Yi, J.; Fan, Y.; Yokoyama, W.; Zhang, Y.; Zhao, L. Thermal degradation and isomerization of β-carotene in oil-in-water nanoemulsions supplemented with natural antioxidants. J. Agric. Food Chem. 2016, 64, 1970–1976. [Google Scholar] [CrossRef]
- Yi, J.; Li, Y.; Zhong, F.; Yokoyama, W. The physicochemical stability and in vitro bioaccessibility of β-carotene in oil-in-water sodium caseinate emulsions. Food Hydrocoll. 2014, 35, 19–27. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Yu, K.; Mannisto, S.; Albanes, D. Serum Beta Carotene and Overall and Cause-Specific Mortality. Circ. Res. 2018, 123, 1339–1349. [Google Scholar] [CrossRef]
- Rimm, E.B. The Challenges of Deconstructing Fruits and Vegetables. Circ. Res. 2018, 123, 1267–1268. [Google Scholar] [CrossRef] [PubMed]
- Canas, J.A.; Lochrie, A.; McGowan, A.G.; Hossain, J.; Schettino, C.; Balagopal, P.B. Effects of Mixed Carotenoids on Adipokines and Abdominal Adiposity in Children: A Pilot Study. J. Clin. Endocrinol. Metab. 2017, 102, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Trasino, S.E.; Tang, X.H.; Jessurun, J.; Gudas, L.J. Obesity Leads to Tissue, but not Serum Vitamin A Deficiency. Sci. Rep. 2015, 5, 15893. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Mu, D.; Li, D.; Zhong, Y.; Jiang, N.; Zhang, Y.; Xia, M. Association of Serum Retinoic Acid With Risk of Mortality in Patients With Coronary Artery Disease. Circ. Res. 2016, 119, 557–563. [Google Scholar] [CrossRef]
- Birmingham, C.L. Hypercarotenemia. N. Engl. J. Med. 2002, 347, 222–223. Available online: https://www.ncbi.nlm.nih.gov/pubmed/12124418 (accessed on 24 March 2019). [PubMed]
- Pierach, C.A. Hypercarotenemia. N. Engl. J. Med. 2002, 347, 222–223. Available online: https://www.ncbi.nlm.nih.gov/pubmed/12132109/?ncbi_mmode=std (accessed on 24 March 2019).
- Tyler, I.; Wiseman, M.C.; Crawford, R.I.; Birmingham, C.L. Cutaneous manifestations of eating disorders. J. Cutan. Med. Surg. 2002, 6, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Curran-Celentano, J.; Erdman, J.W., Jr.; Nelson, R.A.; Grater, S.J. Alterations in vitamin A and thyroid hormone status in anorexia nervosa and associated disorders. Am. J. Clin. Nutr. 1985, 42, 1183–1191. [Google Scholar] [CrossRef]
- Priyadarshani, A.M.B. Insights of hypercarotenaemia: A brief review. Clin. Nutr. ESPEN 2018, 23, 19–24. [Google Scholar] [CrossRef]
- Penniston, K.L.; Tanumihardjo, S.A. The acute and chronic toxic effects of vitamin A. Am. J. Clin. Nutr. 2006, 83, 191–201. [Google Scholar] [CrossRef]
- Cooper, J.P.; Reynolds, C.P.; Cho, H.; Kang, M.H. Clinical development of fenretinide as an antineoplastic drug: Pharmacology perspectives. Exp. Biol. Med. 2017, 242, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.U.; Jun, H.S.; Choi, J.S.; Lim, J.H.; Kim, W.H.; Yoon, J.B.; Song, J. Fenretinide ameliorates insulin resistance and fatty liver in obese mice. Biol. Pharm. Bull. 2012, 35, 369–375. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, G.D.; Delibegovic, M.; Owen, C.; Stoney, P.N.; Shearer, K.D.; McCaffery, P.J.; Mody, N. Fenretinide treatment prevents diet-induced obesity in association with major alterations in retinoid homeostatic gene expression in adipose, liver, and hypothalamus. Diabetes 2013, 62, 825–836. [Google Scholar] [CrossRef]
- McIlroy, G.D.; Tammireddy, S.R.; Maskrey, B.H.; Grant, L.; Doherty, M.K.; Watson, D.G.; Delibegovic, M.; Whitfield, P.D.; Mody, N. Fenretinide mediated retinoic acid receptor signalling and inhibition of ceramide biosynthesis regulates adipogenesis, lipid accumulation, mitochondrial function and nutrient stress signalling in adipocytes and adipose tissue. Biochem. Pharm. 2016, 100, 86–97. [Google Scholar] [CrossRef]
- Morrice, N.; McIlroy, G.D.; Tammireddy, S.R.; Reekie, J.; Shearer, K.D.; Doherty, M.K.; Delibegovic, M.; Whitfield, P.D.; Mody, N. Elevated Fibroblast growth factor 21 (FGF21) in obese, insulin resistant states is normalised by the synthetic retinoid Fenretinide in mice. Sci. Rep. 2017, 7, 43782. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Golczak, M.; Palczewski, K.; von Lintig, J. Lecithin:retinol acyltransferase is critical for cellular uptake of vitamin A from serum retinol-binding protein. J. Biol. Chem. 2012, 287, 24216–24227. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Zhang, N.; Kemerer, M.; Maeda, T.; Palczewski, K.; Von Lintig, J. STRA6 is critical for cellular vitamin A uptake and homeostasis. Hum. Mol. Genet. 2014, 23, 5402–5417. [Google Scholar] [CrossRef]
- Poliakov, E.; Gubin, A.; Laird, J.; Gentleman, S.; Salomon, R.G.; Redmond, T.M. The mechanism of fenretinide (4-HPR) inhibition of β-carotene monooxygenase 1. New suspect for the visual side effects of fenretinide. Adv. Exp. Med. Biol. 2012, 723, 167–174. [Google Scholar]
- Poliakov, E.; Samuel, W.; Duncan, T.; Gutierrez, D.B.; Mata, N.L.; Redmond, T.M. Inhibitory effects of fenretinide metabolites N-[4-methoxyphenyl]retinamide (MPR) and 4-oxo-N-(4-hydroxyphenyl)retinamide (3-keto-HPR) on fenretinide molecular targets β-carotene oxygenase 1, stearoyl-CoA desaturase 1 and dihydroceramide Delta4-desaturase 1. PLoS ONE 2017, 12, e0176487. [Google Scholar]
- Beckenbach, L.; Baron, J.M.; Merk, H.F.; Loffler, H.; Amann, P.M. Retinoid treatment of skin diseases. Eur. J. Dermatol. 2015, 25, 384–391. [Google Scholar]
- Lestringant, G.G.; Frossard, P.M.; Agarwal, M.; Galadari, I.H. Variations in lipid and lipoprotein levels during isotretinoin treatment for acne vulgaris with special emphasis on HDL-cholesterol. Int. J. Dermatol. 1997, 36, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Roder, K.; Schweizer, M. Retinoic acid-mediated transcription and maturation of SREBP-1c regulates fatty acid synthase via cis-elements responsible for nutritional regulation. Biochem. Soc. Trans. 2007, 35, 1211–1214. [Google Scholar] [CrossRef] [PubMed]
- Roder, K.; Zhang, L.; Schweizer, M. SREBP-1c mediates the retinoid-dependent increase in fatty acid synthase promoter activity in HepG2. FEBS Lett. 2007, 581, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- Devereux, D.F.; Taylor, D.D.; Taylor, C.G.; Hollander, D.M. Effects of retinoic acid on lipolytic activity of tumor cells. Surgery 1987, 102, 277–282. [Google Scholar]
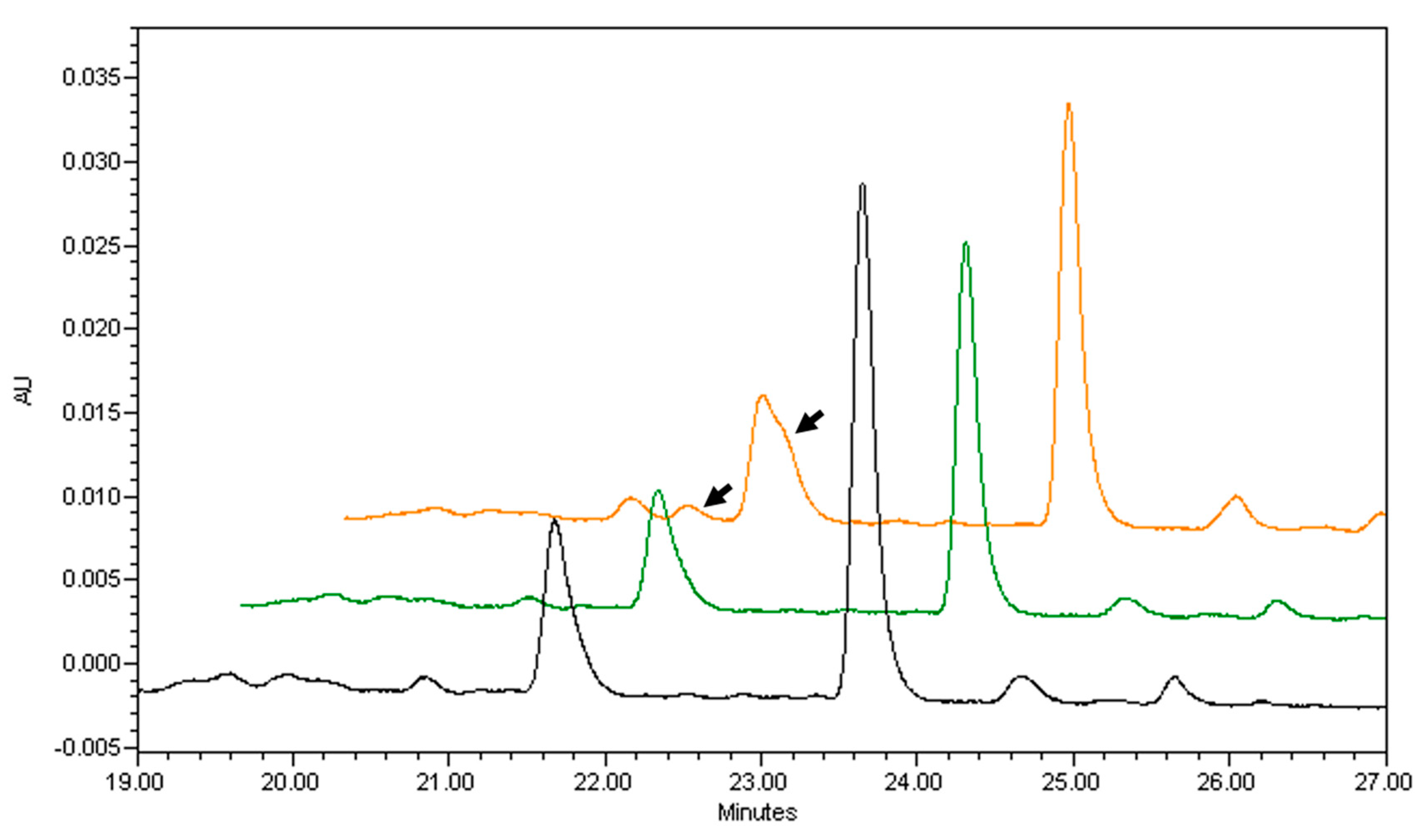
| Source | β-Carotene | α-Carotene | β-Cryptoxanthin | Vitamin A, RAE |
|---|---|---|---|---|
| Peppers | 42,891 | 6931 | - | 3863 |
| Carrots | 33,954 | 14,251 | - | 3423 |
| Paprika | 26,162 | 595 | 6186 | 2463 |
| Pepper (spices) | 21,840 | - | 6252 | 2081 |
| Grape leaves | 16,194 | 629 | 9 | 1376 |
| Chili powder | 15,000 | 2090 | 3490 | 1483 |
| Sweet potatoes | 12,498 | 47 | - | 1043 |
| Pumpkin | 6940 | 4795 | - | 778 |
| Lettuce | 5226 | - | - | 435 |
| Squash | 4226 | 834 | 3471 | 532 |
| Seaweed | 4872 | - | - | 406 |
| Fish oil, cod liver | - | - | - | 30,000 |
| Liver (beef, other meats) | - | - | - | 28,318 |
| Lamb, liver | - | - | - | 19,872 |
| Duck, liver | - | - | - | 11,984 |
| Turkey, liver | - | - | - | 10,751 |
| Chicken, liver | - | - | - | 4374 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coronel, J.; Pinos, I.; Amengual, J. β-carotene in Obesity Research: Technical Considerations and Current Status of the Field. Nutrients 2019, 11, 842. https://doi.org/10.3390/nu11040842
Coronel J, Pinos I, Amengual J. β-carotene in Obesity Research: Technical Considerations and Current Status of the Field. Nutrients. 2019; 11(4):842. https://doi.org/10.3390/nu11040842
Chicago/Turabian StyleCoronel, Johana, Ivan Pinos, and Jaume Amengual. 2019. "β-carotene in Obesity Research: Technical Considerations and Current Status of the Field" Nutrients 11, no. 4: 842. https://doi.org/10.3390/nu11040842
APA StyleCoronel, J., Pinos, I., & Amengual, J. (2019). β-carotene in Obesity Research: Technical Considerations and Current Status of the Field. Nutrients, 11(4), 842. https://doi.org/10.3390/nu11040842






