Associations between Weight Loss, Food Likes, Dietary Behaviors, and Chemosensory Function in Bariatric Surgery: A Case-Control Analysis in Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Procedure and Measures
2.3. Statistical Analysis
2.4. Power Analysis
3. Results
3.1. Demographics of the Participants
3.2. Self-Reported Chemosensory Function
3.3. Sweet Taste, Retronasal Probe, and Sweet Liking
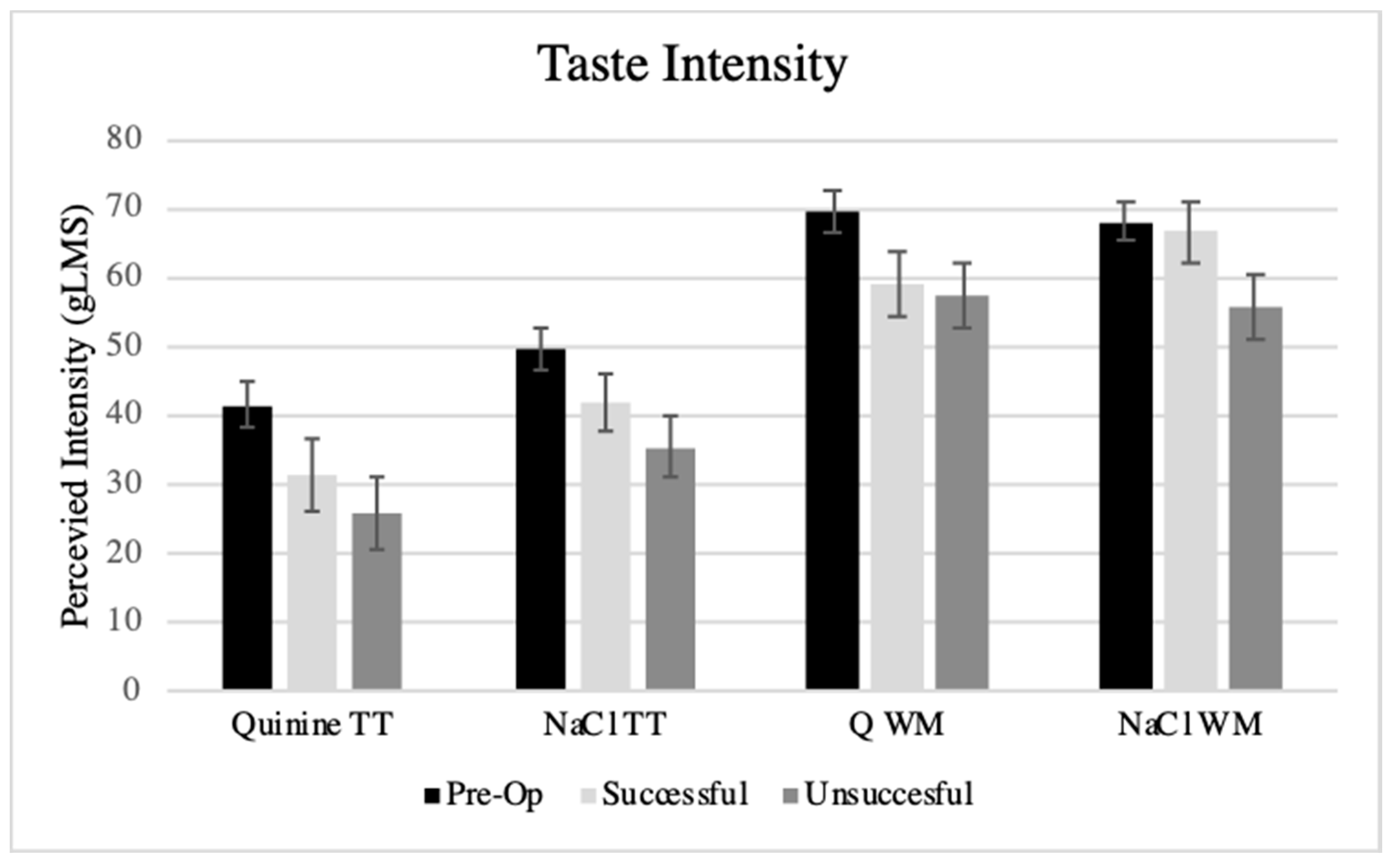
3.4. Taste Function and PROP Tasting
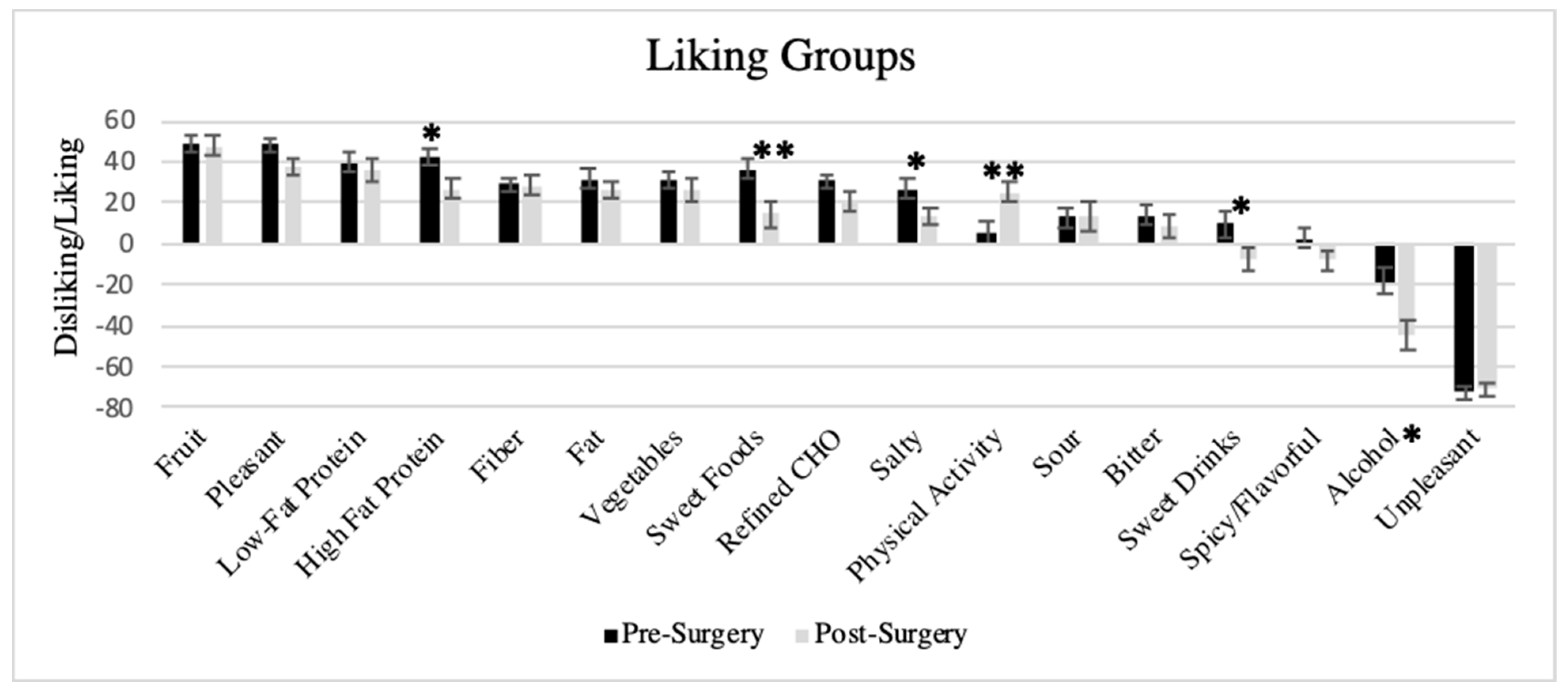
3.5. Liking Survey
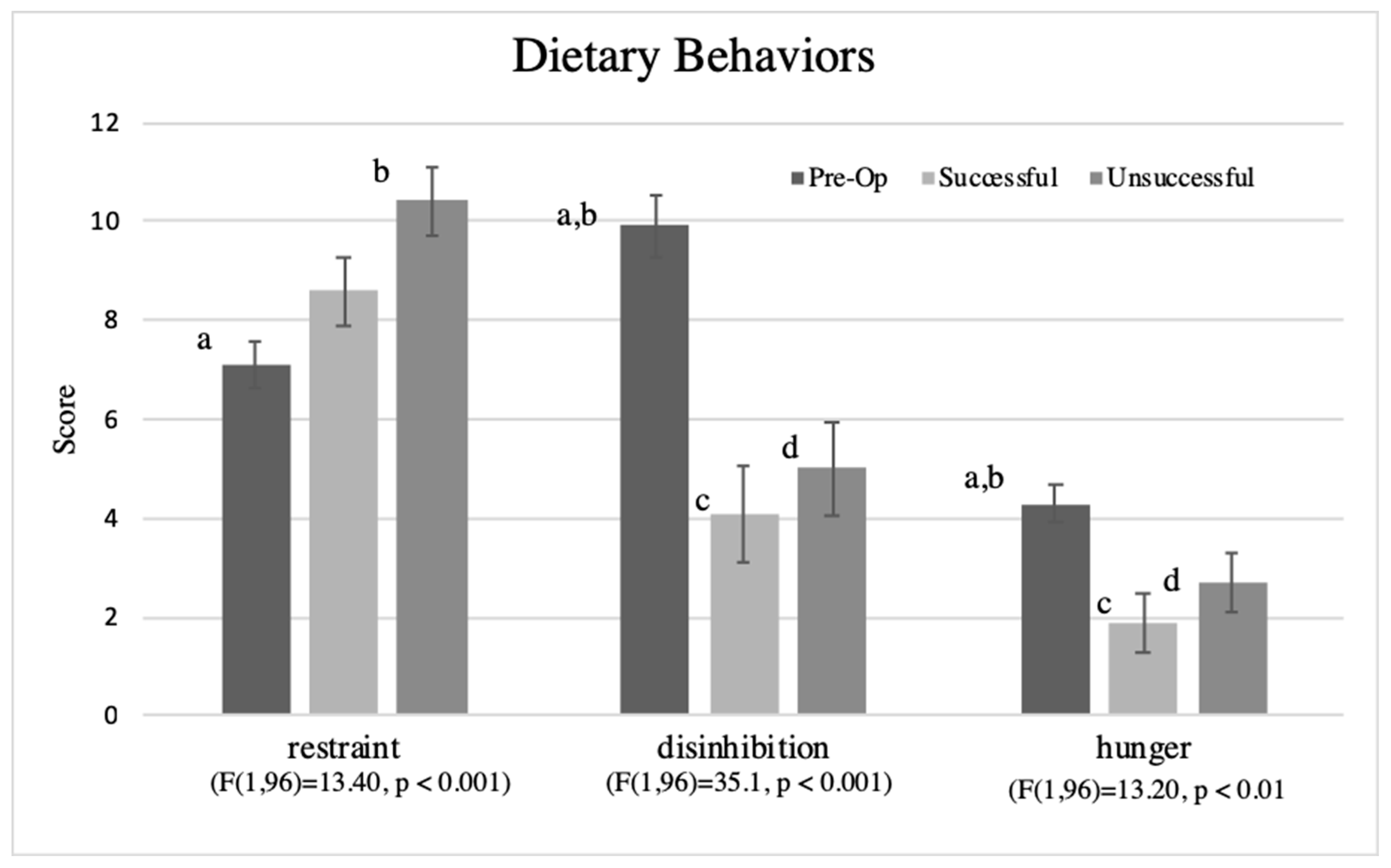
3.6. Dietary Behaviors
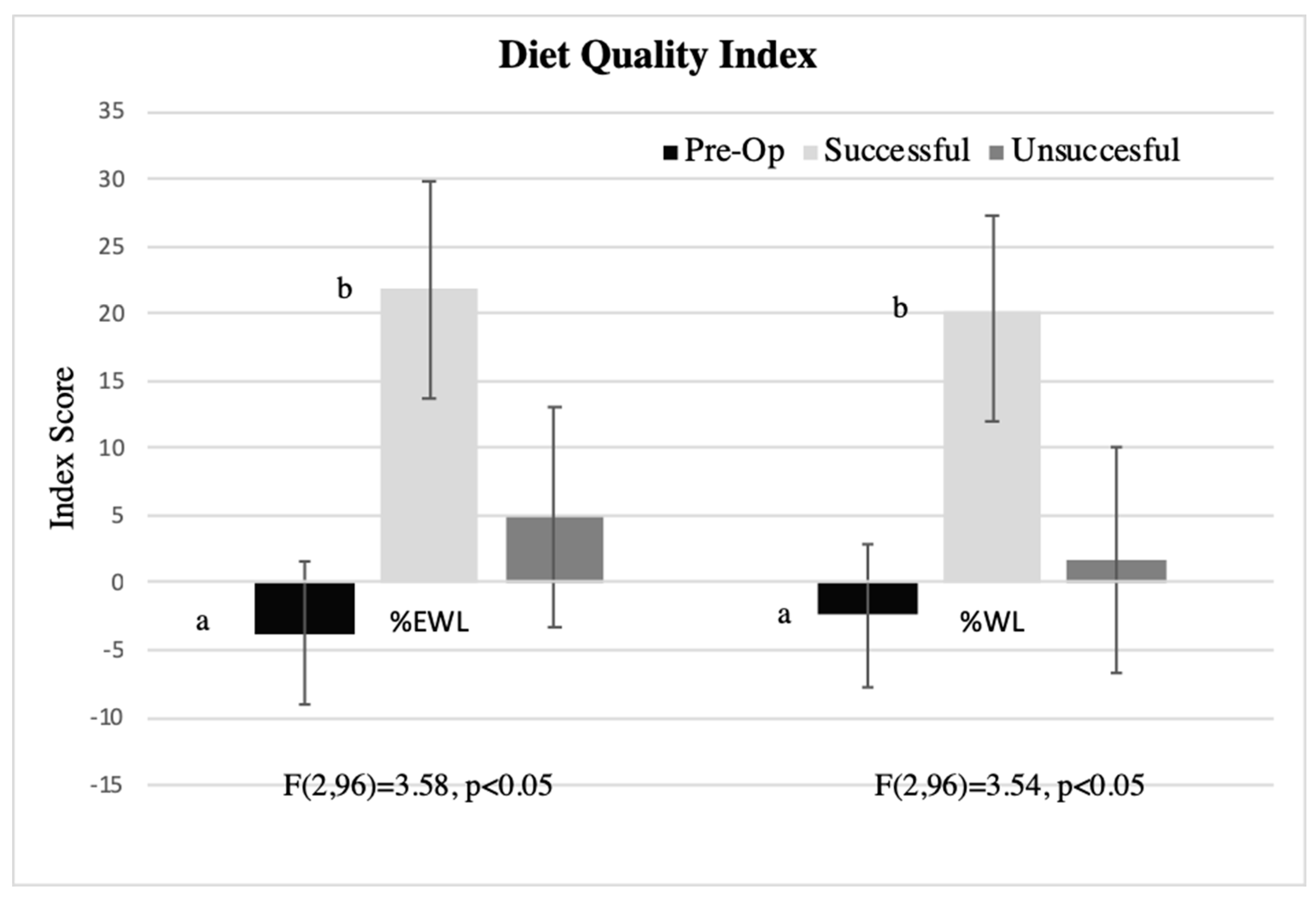
3.7. Associations between Liking-Based Indexes, Dietary Behaviors, and Percent Weight Loss in Post-Surgical Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- National Center for Health Statistics. Health, United States, 2017: With Special Feature on Mortality. Available online: https://www.cdc.gov/nchs/data/hus/hus17.pdf (accessed on 18 February 2019).
- Buchwald, H. Consensus conference statement bariatric surgery for morbid obesity: Health implications for patients, health professionals, and third-party payers. Surg. Obes. Relat. Dis. 2005, 1, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Panteliou, E.; Miras, A.D. What is the role of bariatric surgery in the management of obesity? Climacteric 2017, 20, 97–102. [Google Scholar] [CrossRef] [PubMed]
- American Society for Metabolic and Bariatric Society. Estimate of Bariatric Surgery Numbers, 2011–2017. Available online: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers (accessed on 9 February 2019).
- Young, M.T.; Phelan, M.J.; Nguyen, N.T. A decade analysis of trends and outcomes of male vs female patients who underwent bariatric surgery. J. Am. Coll. Surg. 2016, 222, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern Med. 2013, 273, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Bonouvrie, D.S.; Uittenbogaart, M.; Luijten, A.; van Dielen, F.M.H.; Leclercq, W.K.G. Lack of standard definitions of primary and secondary (non)responders after primary gastric bypass and gastric sleeve: A systematic review. Obes. Surg. 2019, 29, 691–697. [Google Scholar] [CrossRef]
- Brethauer, S.A.; Kim, J.; el Chaar, M.; Papasavas, P.; Eisenberg, D.; Rogers, A.; Ballem, N.; Kligman, M.; Kothari, S.; Committee, A.C.I. Standardized outcomes reporting in metabolic and bariatric surgery. Surg. Obes. Relat. Dis. 2015, 11, 489–506. [Google Scholar] [CrossRef]
- Reinhold, R.B. Critical analysis of long term weight loss following gastric bypass. Surg. Gynecol. Obstet. 1982, 155, 385–394. [Google Scholar]
- Hatoum, I.J.; Kaplan, L.M. Advantages of percent weight loss as a method of reporting weight loss after Roux-en-Y gastric bypass. Obesity 2013, 21, 1519–1525. [Google Scholar] [CrossRef]
- Montero, P.N.; Stefanidis, D.; Norton, H.J.; Gersin, K.; Kuwada, T. Reported excess weight loss after bariatric surgery could vary significantly depending on calculation method: A plea for standardization. Surg. Obes. Relat. Dis. 2011, 7, 531–534. [Google Scholar] [CrossRef]
- Makaronidis, J.M.; Batterham, R.L. Potential mechanisms mediating sustained weight loss following Roux-en-Y Gastric Bypass and Sleeve Gastrectomy. Endocrinol Metab. Clin. North. Am. 2016, 45, 539–552. [Google Scholar] [CrossRef]
- Mulla, C.M.; Middelbeek, R.J.W.; Patti, M.E. Mechanisms of weight loss and improved metabolism following bariatric surgery. Ann. N. Y. Acad. Sci. 2018, 1411, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C.; Robinson, T.E.; Aldridge, J.W. Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Curr. Opin. Pharmacol. 2009, 9, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Duffy, V.B.; Hayes, J.E.; Moskowitz, H.R.; Snyder, D.J. Psychophysics of sweet and fat perception in obesity: Problems, solutions and new perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci 2006, 361, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Schultes, B.; Ernst, B.; Wilms, B.; Thurnheer, M.; Hallschmid, M. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am. J. Clin. Nutr. 2010, 92, 277–283. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Bueter, M. The physiology of altered eating behaviour after Roux-en-Y gastric bypass. Exp. Physiol. 2014, 99, 1128–1132. [Google Scholar] [CrossRef]
- Ullrich, J.; Ernst, B.; Wilms, B.; Thurnheer, M.; Schultes, B. Roux-en Y Gastric Bypass Surgery reduces hedonic hunger and improves dietary habits in severely obese subjects. Obes. Surg. 2013, 23, 50–55. [Google Scholar] [CrossRef]
- Kapoor, N.; Al-Najim, W.; le Roux, C.W.; Docherty, N.G. Shifts in food preferences after bariatric surgery: Observational reports and proposed mechanisms. Curr. Obes. Rep. 2017, 6, 246–252. [Google Scholar] [CrossRef]
- Li, G.; Ji, G.; Hu, Y.; Liu, L.; Jin, Q.; Zhang, W.; Liu, L.; Wang, Y.; Zhao, J.; von Deneen, K.M.; et al. Reduced plasma ghrelin concentrations are associated with decreased brain reactivity to food cues after laparoscopic sleeve gastrectomy. Psychoneuroendocrinology 2019, 100, 229–236. [Google Scholar] [CrossRef]
- Gero, D.; Steinert, R.E.; le Roux, C.W.; Bueter, M. Do food preferences change after bariatric surgery? Curr. Atheroscler. Rep. 2017, 19, 38. [Google Scholar] [CrossRef]
- Primeaux, S.D.; de Silva, T.; Tzeng, T.H.; Chiang, M.C.; Hsia, D.S. Recent advances in the modification of taste and food preferences following bariatric surgery. Rev. Endocr. Metab. Dis. 2016, 17, 195–207. [Google Scholar] [CrossRef]
- Trijsburg, L.; Geelen, A.; Hollman, P.C.; Hulshof, P.J.; Feskens, E.J.; Van’t Veer, P.; Boshuizen, H.C.; de Vries, J.H. BMI was found to be a consistent determinant related to misreporting of energy, protein and potassium intake using self-report and duplicate portion methods. Pub. Health Nutr. 2016, 20, 598–607. [Google Scholar] [CrossRef]
- Kittrell, H.; Graber, W.; Mariani, E.; Czaja, K.; Hajnal, A.; Di Lorenzo, P.M. Taste and odor preferences following Roux-en-Y surgery in humans. PLoS ONE 2018, 13, e0199508. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Bradley, D.; Eagon, J.C.; Sullivan, S.; Abumrad, N.A.; Klein, S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity 2014, 22, E13–E20. [Google Scholar] [CrossRef]
- Nance, K.; Eagon, J.C.; Klein, S.; Pepino, M.Y. Effects of Sleeve Gastrectomy vs. Roux-en-Y Gastric Bypass on eating behavior and sweet taste perception in subjects with obesity. Nutrients 2017, 10, 18. [Google Scholar] [CrossRef]
- Miras, A.D.; Jackson, R.N.; Jackson, S.N.; Goldstone, A.P.; Olbers, T.; Hackenberg, T.; Spector, A.C.; le Roux, C.W. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am. J. Clin. Nutr. 2012, 96, 467–473. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Christensen, B.J.; Ritz, C.; Rasmussen, S.; Hansen, T.T.; Bredie, W.L.P.; le Roux, C.W.; Sjodin, A.; Schmidt, J.B. Roux-En-Y Gastric Bypass and Sleeve Gastrectomy does not affect food preferences when assessed by an ad libitum buffet meal. Obes. Surg. 2017, 27, 2599–2605. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard Nielsen, M.; Rasmussen, S.; Just Christensen, B.; Ritz, C.; le Roux, C.W.; Berg Schmidt, J.; Sjodin, A. Bariatric surgery does not affect food preferences, but individual changes in food preferences may predict weight loss. Obesity 2018, 26, 1879–1887. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; Agriculture, U.S.D.o. 2015–2020 Dietary Guidelines For Americans. Available online: https://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 18 February 2019).
- Molin Netto, B.D.; Earthman, C.P.; Farias, G.; Landi Masquio, D.C.; Grotti Clemente, A.P.; Peixoto, P.; Bettini, S.C.; von Der Heyde, M.E.; Damaso, A.R. Eating patterns and food choice as determinant of weight loss and improvement of metabolic profile after RYGB. Nutrition 2017, 33, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Keskitalo, K.; Knaapila, A.; Kallela, M.; Palotie, A.; Wessman, M.; Sammalisto, S.; Peltonen, L.; Tuorila, H.; Perola, M. Sweet taste preferences are partly genetically determined: Identification of a trait locus on chromosome 16. Am. J. Clin. Nutr. 2007, 86, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.; Peterson, J.; Dinehart, M.; Bartoshuk, L. Genetic and environmental variation in taste: Associations with sweet intensity, preference, and intake. Top. Clin. Nutr. 2003, 18, 209–220. [Google Scholar] [CrossRef]
- Hayes, J.E.; Sullivan, B.S.; Duffy, V.B. Explaining variability in sodium intake through oral sensory phenotype, salt sensation and liking. Physiol. Behav. 2010, 100, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, M.; Hayes, J.E.; Duffy, V.B. Masking vegetable bitterness to improve palatability depends on vegetable type and taste phenotype. Chemosens. Percept. 2013, 6, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Tuorila, H.; Huotilainen, A.; Lähteenmäki, L.; Ollila, S.; Tuomi-Nurmi, S.; Urala, N. Comparison of affective rating scales and their relationship to variables reflecting food consumption. Food Qual. Pref. 2008, 19, 51–61. [Google Scholar] [CrossRef]
- Pallister, T.; Sharafi, M.; Lachance, G.; Pirastu, N.; Mohney, R.P.; MacGregor, A.; Feskens, E.J.; Duffy, V.; Spector, T.D.; Menni, C. Food preference patterns in a UK twin cohort. Twin Res. Hum. Genet. 2015, 18, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, M.; Rawal, S.; Fernandez, M.L.; Huedo-Medina, T.B.; Duffy, V.B. Taste phenotype associates with cardiovascular disease risk factors via diet quality in multivariate modeling. Physiol. Behav. 2018, 194, 103–112. [Google Scholar] [CrossRef]
- Guenther, P.M.; Kirkpatrick, S.I.; Reedy, J.; Krebs-Smith, S.M.; Buckman, D.W.; Dodd, K.W.; Casavale, K.O.; Carroll, R.J. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J. Nutr. 2014, 144, 399–407. [Google Scholar] [CrossRef]
- Sharafi, M.; Duffy, V.B.; Miller, R.J.; Winchester, S.B.; Sullivan, M.C. Dietary behaviors of adults born prematurely may explain future risk for cardiovascular disease. Appetite 2016, 99, 157–167. [Google Scholar] [CrossRef]
- Zoghbi, M.; Stone, A.; Papsavas, P.; Swede, H.; Hubert, P.; Tisher, D.; Duffy, V.B. Evaluating taste preferences and dietary quality with a simple liking survey: Application in bariatric treatment settings. Bariatr. Surg. Pract. Patient Care 2017, 11. [Google Scholar] [CrossRef]
- Iatridi, V.; Hayes, J.E.; Yeomans, M.R. Quantifying sweet taste liker phenotypes: Time for some consistency in the classification criteria. Nutrients 2019, 11, 129. [Google Scholar] [CrossRef]
- Shoar, S.; Naderan, M.; Shoar, N.; Modukuru, V.R.; Mahmoodzadeh, H. Alteration pattern of taste perception after bariatric surgery: A systematic review of four taste domains. Obes. Surg. 2019. [Google Scholar] [CrossRef]
- Tichansky, D.S.; Boughter, J.D., Jr.; Madan, A.K. Taste change after laparoscopic Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. Surg. Obes. Relat. Dis. 2006, 2, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.; Murty, G.; Bowrey, D.J. Taste, smell and appetite change after Roux-en-Y gastric bypass surgery. Obes. Surg. 2014, 24, 1463–1468. [Google Scholar] [CrossRef]
- Makaronidis, J.M.; Neilson, S.; Cheung, W.H.; Tymoszuk, U.; Pucci, A.; Finer, N.; Doyle, J.; Hashemi, M.; Elkalaawy, M.; Adamo, M.; et al. Reported appetite, taste and smell changes following Roux-en-Y gastric bypass and sleeve gastrectomy: Effect of gender, type 2 diabetes and relationship to post-operative weight loss. Appetite 2016, 107, 93–105. [Google Scholar] [CrossRef]
- Holinski, F.; Menenakos, C.; Haber, G.; Olze, H.; Ordemann, J. Olfactory and gustatory function after bariatric surgery. Obes. Surg. 2015, 25, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.J.; Rawal, S.; Li, C.M.; Duffy, V.B. New chemosensory component to the U.S. National Health and Nutrition Examination Survey (NHANES), first-year results for measured olfactory dysfunction. Rev. Endocr. Metab. Disord. 2016, 17, 221–240. [Google Scholar] [CrossRef]
- Rawal, S.; Huedo-Medina, T.B.; Hoffman, H.J.; Swede, H.; Duffy, V.B. Structural equation modeling of associations among taste-related risk factors, taste functioning, and adiposity. Obesity 2017, 25, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Banni, S.; Melis, M.; Crnjar, R.; Tomassini Barbarossa, I. Genetic sensitivity to the bitter taste of 6-n-propylthiouracil (PROP) and its association with physiological mechanisms controlling body mass index (BMI). Nutrients 2014, 6, 3363–3381. [Google Scholar] [CrossRef]
- Brockmeyer, T.; Hamze Sinno, M.; Skunde, M.; Wu, M.; Woehning, A.; Rudofsky, G.; Friederich, H.C. Inhibitory control and hedonic response towards food interactively predict success in a weight loss programme for adults with obesity. Obes. Facts 2016, 9, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Hays, N.P.; Roberts, S.B. Aspects of eating behaviors “disinhibition” and “restraint” are related to weight gain and BMI in women. Obesity 2008, 16, 52–58. [Google Scholar] [CrossRef]
- Thomas, J.G.; Bond, D.S.; Phelan, S.; Hill, J.O.; Wing, R.R. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am. J. Prev. Med. 2014, 46, 17–23. [Google Scholar] [CrossRef]
- Neumann, M.; Holzapfel, C.; Muller, A.; Hilbert, A.; Crosby, R.D.; de Zwaan, M. Features and trajectories of eating behavior in weight-loss maintenance: Results from the German Weight Control Registry. Obesity 2018, 26, 1501–1508. [Google Scholar] [CrossRef]
- Figura, A.; Rose, M.; Ordemann, J.; Klapp, B.F.; Ahnis, A. Changes in self-reported eating patterns after laparoscopic sleeve gastrectomy: A pre-post analysis and comparison with conservatively treated patients with obesity. Surg. Obes. Relat. Dis. 2017, 13, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Rieber, N.; Giel Ke Fau-Meile, T.; Meile T Fau-Enck, P.; Enck P Fau-Zipfel, S.; Zipfel S Fau-Teufel, M.; Teufel, M. Psychological dimensions after laparoscopic sleeve gastrectomy: Reduced mental burden, improved eating behavior, and ongoing need for cognitive eating control. Surg. Obes. Relat. Dis. 2013, 9, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Kalarchian, M.A.; Wilson, G.T.; Brolin, R.E.; Bradley, L. Effects of bariatric surgery on binge eating and related psychopathology. Eat Weight Disord. 1999, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Burgmer, R.; Grigutsch, K.; Zipfel, S.; Wolf, A.M.; de Zwaan, M.; Husemann, B.; Albus, C.; Senf, W.; Herpertz, S. The influence of eating behavior and eating pathology on weight loss after gastric restriction operations. Obes. Surg. 2005, 15, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Duffy, V.B.; Green, B.G.; Hoffman, H.J.; Ko, C.W.; Lucchina, L.A.; Marks, L.E.; Snyder, D.J.; Weiffenbach, J.M. Valid across-group comparisons with labeled scales: The gLMS versus magnitude matching. Physiol. Behav. 2004, 82, 109–114. [Google Scholar] [CrossRef] [PubMed]
- CDC. National Health and Nutrition Examination Survey (NHANES) Taste and Smell Examination Component Manual [Internet]. Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/Taste_Smell.pdf. (accessed on 18 February 2019).
- Coldwell, S.E.; Mennella, J.A.; Duffy, V.B.; Pelchat, M.L.; Griffith, J.W.; Smutzer, G.; Cowart, B.J.; Breslin, P.A.; Bartoshuk, L.M.; Hastings, L.; et al. Gustation assessment using the NIH Toolbox. Neurology 2013, 80, S20–S24. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Duffy, V.B.; Miller, I.J., Jr. PTC/PROP Tasting: Anatomy, psychophysics, and sex effects. Physiol. Behav. 1994, 56, 1165–1171. [Google Scholar] [CrossRef]
- Hayes, J.E.; Bartoshuk, L.M.; Kidd, J.R.; Duffy, V.B. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem. Senses 2008, 33, 255–265. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Westenhoefer, J.; Stunkard Albert, J.; Pudel, V. Validation of the flexible and rigid control dimensions of dietary restraint. Int. J. Eat Disord. 1999, 26, 53–64. [Google Scholar] [CrossRef]
- Robinson, A.H.; Adler, S.; Stevens, H.B.; Darcy, A.M.; Morton, J.M.; Safer, D.L. What variables are associated with successful weight loss outcomes for bariatric surgery after 1 year? Surg. Obes. Relat. Dis. 2014, 10, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Sillen, L.; Andersson, E. Patient factors predicting weight loss after Roux-en-Y Gastric Bypass. J. Obes. 2017, 2017, 3278751. [Google Scholar] [CrossRef] [PubMed]
- Courcoulas, A.P.; Christian Nj Fau-Belle, S.H.; Belle Sh Fau-Berk, P.D.; Berk Pd Fau-Flum, D.R.; Flum Dr Fau-Garcia, L.; Garcia L Fau-Horlick, M.; Horlick M Fau-Kalarchian, M.A.; Kalarchian Ma Fau-King, W.C.; King Wc Fau-Mitchell, J.E.; Mitchell Je Fau-Patterson, E.J.; et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013, 310, 2416–2425. [Google Scholar] [CrossRef] [PubMed]
- Taboada, D.; Navio M Fau-Jurado, R.; Jurado R Fau-Fernandez, V.; Fernandez V Fau-Bayon, C.; Bayon C Fau-Alvarez, M.J.; Alvarez Mj Fau-Morales, I.; Morales I Fau-Ponce, G.; Ponce G Fau-Rubio, G.; Rubio G Fau-Mingote, J.C.; Mingote Jc Fau-Cruz, F.; et al. Factor structure and psychometric properties of the TFEQ in morbid obese patients, candidates to bariatric surgery. Psicothema 2015, 27, 141–150. [Google Scholar] [PubMed]
- Tepper, B.J. Nutritional implications of genetic taste variation: The role of PROP sensitivity and other taste phenotypes. Ann. Rev. Nutr. 2008, 28, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Coutts, D.; Wang, T.; Cakmak, Y.O. Systematic review of olfactory shifts related to obesity. Obes. Rev. 2019, 20, 325–338. [Google Scholar] [CrossRef]
- Snyder, D.J.; Bartoshuk, L.M. Oral sensory nerve damage: Causes and consequences. Rev. Endocr. Metab. Disord. 2016, 17, 149–158. [Google Scholar] [CrossRef]
- Oor, J.E.; Roks, D.J.; Unlu, C.; Hazebroek, E.J. Laparoscopic sleeve gastrectomy and gastroesophageal reflux disease: A systematic review and meta-analysis. Am. J. Surg. 2016, 211, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Althuwaini, S.; Bamehriz, F.; Aldohayan, A.; Alshammari, W.; Alhaidar, S.; Alotaibi, M.; Alanazi, A.; Alsahabi, H.; Almadi, M.A. Prevalence and predictors of gastroesophageal reflux disease after Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2018, 28, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Laboure, H.; Repoux, M.; Courcoux, P.; Feron, G.; Guichard, E. Inter-individual retronasal aroma release variability during cheese consumption: Role of food oral processing. Food Res. Int. 2014, 64, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Canterini, C.C.; Gaubil-Kaladjian, I.; Vatin, S.; Viard, A.; Wolak-Thierry, A.; Bertin, E. Rapid eating is linked to emotional eating in obese women relieving from bariatric surgery. Obes. Surg. 2018, 28, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Peterson, J.; Bartoshuk, L.M. Associations between taste genetics, oral sensations and alcohol intake. Physiol. Behav. 2004, 82, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Dinehart, M.E.; Hayes, J.E.; Bartoshuk, L.M.; Lanier, S.L.; Duffy, V.B. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol. Behav. 2006, 87, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.E.; Cruickshanks, K.J.; Schubert, C.R.; Pinto, A.; Huang, G.H.; Klein, B.E.; Klein, R.; Pankow, J.S. The association of taste with change in adiposity-related health measures. J. Acad. Nutr. Diet 2014, 114, 1195–1202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ortega, F.J.; Aguera, Z.; Sabater, M.; Moreno-Navarrete, J.M.; Alonso-Ledesma, I.; Xifra, G.; Botas, P.; Delgado, E.; Jimenez-Murcia, S.; Fernandez-Garcia, J.C.; et al. Genetic variations of the bitter taste receptor TAS2R38 are associated with obesity and impact on single immune traits. Mol. Nutr. Food Res. 2016, 60, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, I.; Raparelli, L.; Guarnacci, L.; Paone, E.; Del Genio, G.; le Roux, C.W.; Silecchia, G. Food intake and changes in eating behavior after Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2016, 26, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Zoon, H.F.A.; de Bruijn, S.E.M.; Smeets, P.A.M.; de Graaf, C.; Janssen, I.M.C.; Schijns, W.; Aarts, E.O.; Jager, G.; Boesveldt, S. Altered neural responsivity to food cues in relation to food preferences, but not appetite-related hormone concentrations after RYGB-surgery. Behav. Brain Res. 2018, 353, 194–202. [Google Scholar] [CrossRef]
- Mack, I.; Olschlager, S.; Sauer, H.; von Feilitzsch, M.; Weimer, K.; Junne, F.; Peeraully, R.; Enck, P.; Zipfel, S.; Teufel, M. Does Laparoscopic Sleeve Gastrectomy improve depression, stress and eating behaviour? A 4-Year Follow-up Study. Obes. Surg. 2016, 26, 2967–2973. [Google Scholar] [CrossRef]
- Karlsson, J.; Persson, L.O.; Sjostrom, L.; Sullivan, M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1715–1725. [Google Scholar] [CrossRef]
- Lang, T.; Hauser, R.; Buddeberg, C.; Klaghofer, R. Impact of gastric banding on eating behavior and weight. Obes. Surg. 2002, 12, 100–107. [Google Scholar] [CrossRef]
- Roehrig, M.; Masheb Rm Fau-White, M.A.; White Ma Fau-Rothschild, B.S.; Rothschild Bs Fau-Burke-Martindale, C.H.; Burke-Martindale Ch Fau-Grilo, C.M.; Grilo, C.M. Chronic dieting among extremely obese bariatric surgery candidates. Obes. Surg. 2009, 19, 1116–1123. [Google Scholar] [CrossRef]
- Lowe, M.R.; Doshi Sd Fau-Katterman, S.N.; Katterman Sn Fau-Feig, E.H.; Feig, E.H. Dieting and restrained eating as prospective predictors of weight gain. Front. Psychol. 2013, 4, 577. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, K.; Anderson, D.A.; Anderson, L.M.; Reilly, E.E.; Gorrell, S. Dietary restraint: What’s the harm? A review of the relationship between dietary restraint, weight trajectory and the development of eating pathology. Clin. Obes. 2016, 6, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Polivy, J.; Herman, C.P. Dieting and binging. A causal analysis. Am. Psychol. 1985, 40, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Van de Laar, A.W.; van Rijswijk, A.S.; Kakar, H.; Bruin, S.C. Sensitivity and specificity of 50% excess weight loss (50%EWL) and twelve other bariatric criteria for weight loss success. Obes. Surg. 2018, 28, 2297–2304. [Google Scholar] [CrossRef]
- Andromalos, L.; Crowley, N.; Brown, J.; Craggs-Dino, L.; Handu, D.; Isom, K.; Lynch, A.; DellaValle, D. Nutrition Care in Bariatric Surgery: An Academy Evidence Analysis Center Systematic Review. J. Acad. Nutr. Diet. 2018, 119, 678–686. [Google Scholar] [CrossRef] [PubMed]
| Variables | Pre-Op n = 49 | Post-Surg n = 48 | Success %EWL n = 23 | Unsuccess %EWL n = 25 | Success %WL n = 26 | Unsuccess %WL n = 22 |
|---|---|---|---|---|---|---|
| Age † | 45.7 ± 1.6 | 48.44 ± 1.6 | 47.8 ± 1.8 | 49.0 ± 2.5 | 46.4 ± 2.2 | 50.8 ± 2.2 |
| Race ¥ | ||||||
| Black | 25 | 21 | 13 | 28 | 15 | 27 |
| Other | 4 | 10 | 17 | 4 | 15 | 5 |
| White | 71 | 69 | 70 | 68 | 70 | 68 |
| Ethnicity ¥ | ||||||
| Hispanic | 16 | 13 | 22 | 4 | 19 | 5 |
| Not-Hispanic | 84 | 87 | 78 | 96 | 82 | 95 |
| Surgery Type ¥ | ||||||
| RYGB | 51 | 50 | 48 | 52 | 59 | 38 |
| SG | 49 | 50 | 52 | 48 | 41 | 62 |
| Pre-Op † | ||||||
| Weight (lbs) | 266.5 ± 6.7 | 273.4 ± 8.8 | 238.4 ± 6.4 b | 305.6 ± 12.7 a | 277.8 ± 14.9 | 268.1 ± 7.5 |
| BMI †† | 45.7 ± 1.2 | 47.0 ± 1.6 | 41.0 ± 1.3 b | 52.6 ± 2.4 a | 48.0 ± 2.8 | 45.9 ± 1.4 |
| Post-Surgery † (1 year) | ||||||
| Weight (lbs) | 198.3 ± 7.9 | 156.1 ± 4.3 b | 240.6 ± 8.0 a | 183.7 ± 11.7 b | 219.5 ± 6.8 a | |
| BMI †† | 33.8 ± 1.4 | 26.4 ± 0.7 b | 41.2 ± 1.7 a | 31.4 ± 2.1 b | 37.3 ± 1.4 a |
| %WL | ||||
|---|---|---|---|---|
| Successful | Unsuccessful | Total | ||
| %EWL | Successful | 19 | 4 | 23 |
| Unsuccessful | 7 | 18 | 25 | |
| Total | 26 | 22 | 48 | |
| Smell † | Post-Op - %EWL | Post-Op - %WL | Pre-Op | NHANES | ||
|---|---|---|---|---|---|---|
| Successful | Unsuccessful | Successful | Unsuccessful | |||
| Problem since surgery | ||||||
| Yes | 9 | 8 | 10 | 5 | ||
| No | 87 | 92 | 85 | 95 | ||
| Don’t Know | 4 | 0 | 5 | 0 | ||
| Problem in past year | ||||||
| Yes | 9 | 8 | ||||
| No | 91 | 92 | ||||
| Description since surgery | ||||||
| Excellent | 35 | 42 | 41 | 35 | ||
| Good | 35 | 46 | 37 | 45 | ||
| Little trouble | 17 | 8 | 11 | 15 | ||
| Moderate trouble | 4 | 0 | 4 | 0 | ||
| A lot of trouble | 0 | 0 | 0 | 0 | ||
| Loss smell | 0 | 0 | 0 | 0 | ||
| Don’t Know | 9 | 4 | 7 | 5 | ||
| Change since 25 years | ||||||
| Better | 22 | 6 | ||||
| Worse | 7 | 14 | ||||
| No Change | 65 | 79 | ||||
| Don’t Know | 7 | 0 | ||||
| Change since surgery | ||||||
| Better | 22 | 24 | 30 | 14 | ||
| Worse | 4 | 4 | 4 | 5 | ||
| No Change | 74 | 68 | 67 | 76 | ||
| Don’t Know | 0 | 4 | 0 | 5 | ||
| Specific problem since surgery | ||||||
| None | 65 | 68 | 59 | 76 | ||
| less able | 0 | 8 | 0 | 9 | ||
| parosmia or phantom | 26 | 8 | 33 | 5 | ||
| smell stronger/make sick or anxious | 4 | 12 | 4 | 5 | ||
| Don’t Know | 4 | 4 | 4 | 5 | ||
| Specific problem in past year | ||||||
| None | 72 | |||||
| less able | 4 | |||||
| parosmia or phantom | 11 | 7 | ||||
| smell stronger/make sick or anxious | 13 | |||||
| Don’t Know | 0 | |||||
| Taste † | Post-Op - EWL | Post-Op - % Wt Loss | Pre-Op | NHANES | ||
|---|---|---|---|---|---|---|
| Successful | Unsuccessful | Successful | Unsuccessful | |||
| Problem since surgery | ||||||
| Yes | 26 | 28 | 30 | 24 | ||
| No | 70 | 68 | 66 | 71 | ||
| Don’t Know | 4 | 4 | 4 | 5 | ||
| Problem in past year | ||||||
| Yes | 6 | 5 | ||||
| No | 94 | 95 | ||||
| Description since surgery | ||||||
| Excellent | 43 | 42 | 48 | 35 | ||
| Good | 39 | 33 | 33 | 40 | ||
| Little trouble | 9 | 17 | 11 | 15 | ||
| Moderate trouble | 4 | 4 | 4 | 5 | ||
| A lot of trouble | 4 | 4 | 4 | 5 | ||
| Loss smell | 0 | 0 | 0 | 0 | ||
| Don’t Know | 0 | 0 | 0 | 0 | ||
| Change since 25 yrs across each taste quality | ||||||
| Better | 21.7 to 39.1 | 8.7 to 30.4 | 42.3 | 23.8 | 17–26 | 4–8 |
| Worse | 8.7 to 21.7 | 13.0 to 17.4 | 15.4 | 19.1 | 9–13 | 7–13 |
| No Change | 52.2 to 60.9 | 60.1 to 82.6 | 42.3 | 57.1 | 60–64 | 87–92 |
| Don’t Know | 0 to 8.7 | 0 to 17.4 | 0.0 | 0.0 | 6–9 | <1 |
| Ability to taste food flavor as good as when younger | ||||||
| Yes | 85 | 92 | ||||
| No | 6 | 7 | ||||
| Don’t Know | 8 | 1 | ||||
| Change since surgery across each taste quality | ||||||
| Better | 22–44 | 8–28 | 26–44 | 0–24 | ||
| Worse | 9–22 | 12–20 | 11–19 | 14–19 | ||
| No Change | 32–61 | 56–72 | 41–59 | 57–71 | ||
| Don’t Know | 0–9 | 0–12 | 0–7 | 0–14 | ||
| Ability to taste food flavor as good since surgery | ||||||
| Yes | 82 | 88 | 89 | 81 | ||
| No | 9 | 8 | 4 | 14 | ||
| Don’t Know | 9 | 4 | 7 | 5 | ||
| Specific problem since surgery | ||||||
| None | 39 | 52 | 37 | 55 | ||
| Can’t taste some things | 0 | 8 | 4 | 5 | ||
| Things don’t taste right | 48 | 20 | 45 | 27 | ||
| taste stronger | 13 | 20 | 15 | 14 | ||
| Specific problem in past year | ||||||
| None | 87 | |||||
| less able | 0 | |||||
| dysgeusia | 7 | 6 | ||||
| stronger | 2 | |||||
| Don’t Know | 4 | |||||
| %EWL | %WL | |||||
|---|---|---|---|---|---|---|
| Pre-Op | Post-Surg | Successful | Unsuccessful | Successful | Unsuccessful | |
| Nontaster | 29 | 26 | 30 | 21 | 27 | 24 |
| Medium taster | 51 | 45 | 43 | 46 | 46 | 43 |
| Supertaster | 20 | 30 | 26 | 33 | 27 | 33 |
| Group | Cronbach’s α | Mean ± SEM |
|---|---|---|
| Physical Activity—bicycling, working up a sweat, playing sports, exercising with others, exercising alone, going to the gym, taking the stairs | 0.84 | 15.12 ± 3.8 |
| Sweet foods—cookies, cake, or pie, jam or jelly, ice cream, icing | 0.81 | 25.46 ± 4.2 |
| Alcohol—vodka, gin, or scotch, white wine, red wine, beer | 0.79 | −31.54 ± 5.2 |
| High-Fat Protein—bacon, pizza, fried chicken, sausage, fried fish, pork chops, charred meat, cheddar cheese | 0.77 | 34.52 ± 3.1 |
| Sour—sour pickles, lemon, vinegar | 0.72 | 13.0 ± 4.3 |
| Vegetable—eggplant, spinach or greens, beets, sautéed mushrooms, asparagus, raw carrots, broccoli, tomatoes | 0.71 | 28.7 ± 3.3 |
| Refined Carbohydrate—crackers, white potato, cornflakes, white rice, pasta, bagel or rolls | 0.70 | 25.2 ± 3.1 |
| Fruit—strawberries, pineapple, cherries, pear, melon, banana | 0.70 | 48.2 ± 3.0 |
| Spicy/flavorful —Tabasco sauce, raw onion, chili pepper, garlic, soy sauce, blue cheese, dark chocolate | 0.67 | −2.8 ± 3.4 |
| Fat—olive oil, salad dressing, mayonnaise, butter | 0.66 | 28.9 ± 3.3 |
| Salt—salting foods, ham, pretzels, olives, tortilla or potato chips, French fries | 0.65 | 20.2 ± 3.3 |
| Sweet Drinks—orange juice, coffee drinks, sugar-sweetened coffee or tea, soda | 0.58 | 1.43 ± 4.3 |
| Bitter—tea, grapefruit juice, black coffee, unsweetened iced tea | 0.56 | 11.2 ± 3.6 |
| Pleasant—hearing favorite music, going to a coffee shop, going to a pub or bar, smell of cut grass, cooling off on a hot day, television | 0.55 | 43.2 ± 2.7 |
| Low-Fat Protein—tuna or salmon, baked chicken, plain yogurt, shrimp | 0.52 | 38.1 ± 3.4 |
| Fiber—fiber bar, oatmeal, lentils or beans, whole wheat bread | 0.40 | 28.9 ± 3.0 |
| Unpleasant—glare of headlights, car accident, seeing a mouse at home | 0.37 | −72.0 ± 2.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hubert, P.A.; Papasavas, P.; Stone, A.; Swede, H.; Huedo-Medina, T.B.; Tishler, D.; Duffy, V.B. Associations between Weight Loss, Food Likes, Dietary Behaviors, and Chemosensory Function in Bariatric Surgery: A Case-Control Analysis in Women. Nutrients 2019, 11, 804. https://doi.org/10.3390/nu11040804
Hubert PA, Papasavas P, Stone A, Swede H, Huedo-Medina TB, Tishler D, Duffy VB. Associations between Weight Loss, Food Likes, Dietary Behaviors, and Chemosensory Function in Bariatric Surgery: A Case-Control Analysis in Women. Nutrients. 2019; 11(4):804. https://doi.org/10.3390/nu11040804
Chicago/Turabian StyleHubert, Patrice A., Pavlos Papasavas, Andrea Stone, Helen Swede, Tania B. Huedo-Medina, Darren Tishler, and Valerie B. Duffy. 2019. "Associations between Weight Loss, Food Likes, Dietary Behaviors, and Chemosensory Function in Bariatric Surgery: A Case-Control Analysis in Women" Nutrients 11, no. 4: 804. https://doi.org/10.3390/nu11040804
APA StyleHubert, P. A., Papasavas, P., Stone, A., Swede, H., Huedo-Medina, T. B., Tishler, D., & Duffy, V. B. (2019). Associations between Weight Loss, Food Likes, Dietary Behaviors, and Chemosensory Function in Bariatric Surgery: A Case-Control Analysis in Women. Nutrients, 11(4), 804. https://doi.org/10.3390/nu11040804






