Reversing Type 2 Diabetes: A Narrative Review of the Evidence
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
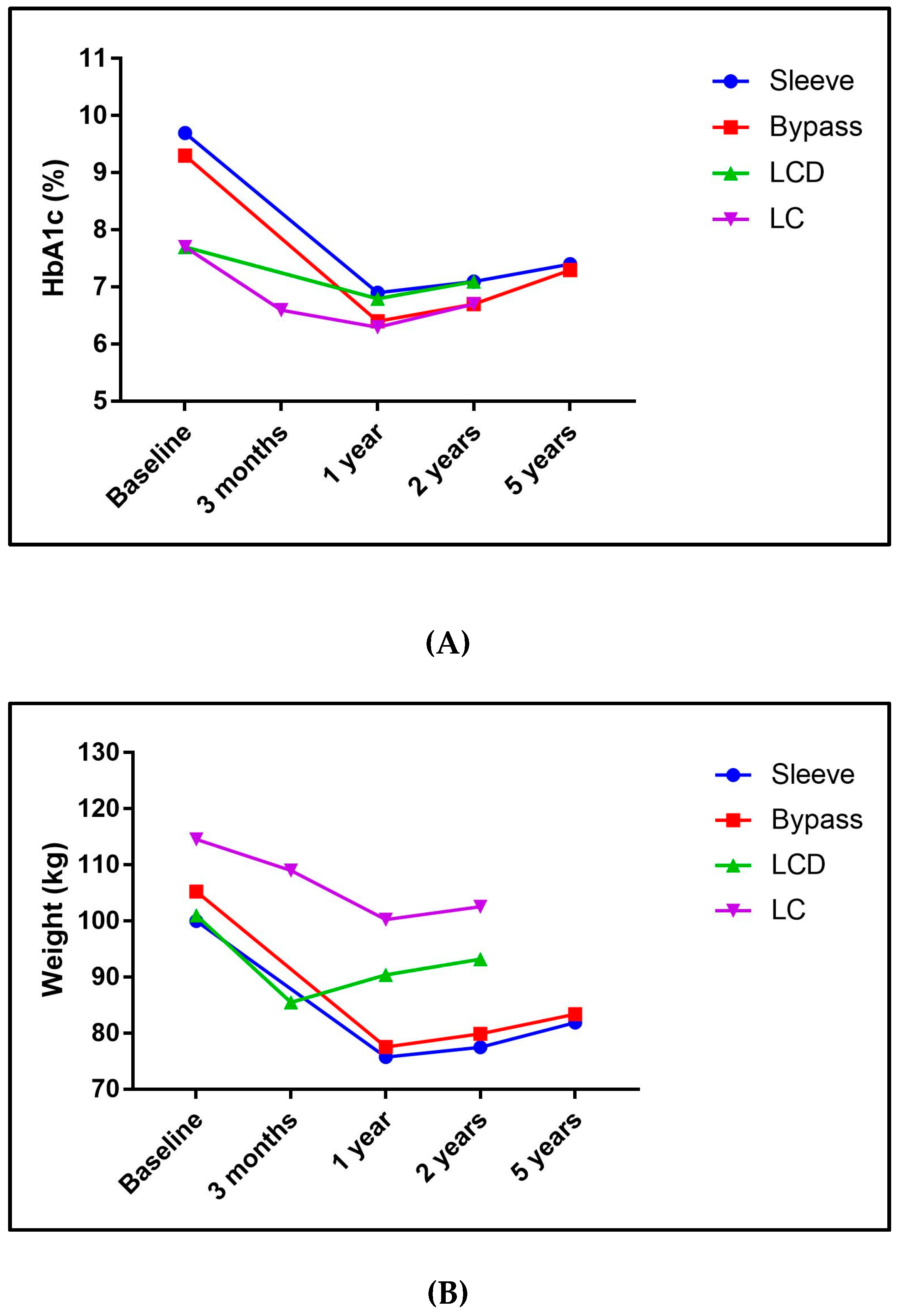
3.1. Bariatric Surgery
3.2. Low-Calorie Diets (LCD)
3.3. Carbohydrate-Restricted Diets (LC)
4. Summary
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations:
| C | carbohydrate restriction |
| DSS-II | Diabetes Surgery Summit |
| RYGB | Roux-en-Y Gastric Bypass |
| SG | Sleeve Gastrectomy |
| CCK | cholecystokinin |
| PYY | peptide-tyrosine-tyrosine |
| GLP-1 | glucagon-like peptide 1 |
| LABS-2 | Longitudinal Assessment of Bariatric Surgery 2 |
| LCD | low-calorie diet |
| IMT | intensive medical intervention |
| DiRECT | Diabetes Remission Clinical Trial |
| ASD | European Association for the Study of Diabetes |
| VA/DOD | Veterans Affairs/Department of Defense |
References
- International Diabetes Federation. In IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Available online: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pd (accessed on 1 February 2019).
- Home, P.; Riddle, M.; Cefalu, W.T.; Bailey, C.J.; Del Prato, S.; Leroith, D.; Schemthaner, G.; van Gaal, L.; Raz, I. Insulin therapy in people with Type 2 diabetes: Opportunities and challenges. Diabetes Care 2014, 37, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Diabetes. 2016. Available online: https://www.who.int/diabetes/publications/grd-2016/en/ (accessed on 1 February 2019).
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycemia in Type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Levi, A.M.; Cabrerizo, L.; Matia, P.; Sanchez-Pernaute, A.; Torres, A.J.; Rubio, M.A. Which criteria should be used to define type 2 diabetes remission after bariatric surgery. BMC Surgery 2013, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.H.; Trigo, E.; Martinez, M.; Katkhouda, N.; Beale, E.; Wang, X.; Wu, J.; Chow, T.; Montgomery, C.; Nayak, K.S.; et al. Impact of gastric banding versus metformin on β-cell function in adults with impaired glucose tolerance or mild type 2 diabetes. Diabetes Care 2018, 41, 2544–2551. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Long-term effects of metformin on diabetes prevention: Identification of subgroups that benefited most in the diabetes prevention program and diabetes prevention outcomes study. Diabetes Care 2019, dc181970. [Google Scholar] [CrossRef]
- Buse, J.B.; Caprio, S.; Cefalu, W.T.; Ceriello, A.; Del Prato, S.; Inzucchi, S.E.; McLaughlin, S.; Phillips, G.L., II; Robertson, R.P.; Rubino, F.; et al. How do we define cure of diabetes? Diabetes Care 2009, 32, 2133–2135. [Google Scholar] [CrossRef]
- Karter, A.J.; Nundy, S.; Parker, M.M.; Moffet, H.H.; Huang, E.S. Incidence of remission in adults with Type 2 diabetes: The Diabetes & Aging Study. Diabetes Care 2014, 37, 3188–3195. [Google Scholar]
- Steven, S.; Carey, P.E.; Small, P.K.; Taylor, R. Reversal of Type 2 diabetes after bariatric surgery is determined by the degree of achieved weight loss in both short- and long-duration diabetes. Diabet Med. 2015, 32, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Nathan, D.; Eckel, R.H.; Schauer, P.R.; Alberti, K.G.; Zimmet, P.Z.; Del Prato, S.; Ji, L.; Sadikot, S.M.; Herman, W.H.; et al. Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: A joint statement by International Diabetes Organizations. Diabetes Care 2016, 39, 861–877. [Google Scholar] [CrossRef]
- Anhe, F.F.; Varin, T.V.; Schertzer, J.D.; Marette, A. The Gut Microbiota as a Mediator of Metabolic Benefits after Bariatric Surgery. Can. J. Diabetes 2017, 41, 439–447. [Google Scholar] [CrossRef]
- Medina, D.A.; Pedreros, J.P.; Turiel, D.; Quezada, N.; Pimentel, F.; Escalona, A.; Garrido, D. Distinct patterns in the gut microbiota after surgical or medical therapy in obese patients. PeerJ 2017, 5, e3443. [Google Scholar] [CrossRef]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Sioka, E.; Chatedaki, C.; Zacharoulis, D. Impact of Bariatric Surgery on Metabolic and Gut Microbiota Profile: A Systematic Review and Meta-analysis. Obes. Surg. 2017, 27, 1345–1357. [Google Scholar] [CrossRef]
- Murphy, R.; Tsai, P.; Jullig, M.; Liu, A.; Plank, L.; Booth, M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes. Surg. 2017, 27, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Kaska, L.; Sledzinski, T.; Chomiczewska, A.; Dettlaff-Pokora, A.; Swierczynski, J. Improved glucose metabolism following bariatric surgery is associated with increased circulating bile acid concentrations and remodeling of the gut microbiome. World J. Gastroenterol. 2016, 22, 8698–8719. [Google Scholar] [CrossRef] [PubMed]
- Penney, N.C.; Kinross, J.; Newton, R.C.; Purkayastha, S. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int. J. Obes. (Lond). 2015, 39, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Morton, J.M. The human gut microbiome: A review of the effect of obesity and surgically induced weight loss. JAMA Surg. 2013, 148, 563–569. [Google Scholar] [CrossRef]
- Rubino, F.; Gagner, M. Potential of surgery for curing type 2 diabetes mellitus. Ann. Surg. 2002, 236, 554–559. [Google Scholar] [CrossRef]
- Cohen, R.; Caravatto, P.P.; Correa, J.L.; Noujaim, P.; Petry, T.Z.; Salles, J.E.; Schiavon, C.A. Glycemic control after stomach-sparing duodenal-jejunal bypass surgery in diabetic patients with low body mass index. Surg. Obes. Relat. Dis. 2012, 8, 375–380. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Tolone, S.; Gravina, A.G.; Patrone, V.; Romano, M.; Tuccillo, C.; Mozzillo, A.L.; Amoroso, V.; Misso, G.; et al. Gastrointestinal Hormones, Intestinal Microbiota and Metabolic Homeostasis in Obese Patients: Effect of Bariatric Surgery. In Vivo 2016, 30, 321–330. [Google Scholar]
- Peat, C.M.; Kleiman, S.C.; Bulik, C.M.; Carroll, I.M. The Intestinal Microbiome in Bariatric Surgery Patients. Eur. Eat. Disord. Rev. 2015, 23, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Morton, J.M. Metabolic surgery: Action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 727–740. [Google Scholar] [CrossRef]
- Ma, I.T.; Madura, J.A. Gastrointestinal Complications after Bariatric Surgery. Gastroenterol. Hepatol. (N Y). 2015, 11, 526–535. [Google Scholar]
- Rubino, F.; Schauer, P.R.; Kaplan, L.M.; Cummings, D.E. Metabolic surgery to treat type 2 diabetes: Clinical outcome and mechanisms of action. Annu. Rev. Med. 2010, 61, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Ikramuddin, S.; Jahansouz, C.; Arafat, F.; Hevelone, N.; Leslie, D. Trends in bariatric surgery: Procedure selection, revisional surgeries, and readmissions. Obes. Surg. 2016, 26, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Deloose, E. Complications of bariatric surgery: Dumping syndrome, reflux and vitamin deficiencies. Best Prac. Res. Clin. Gastroenterol. 2014, 28, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarg, D.; Azagury, D.E.; Ghiassi, S.; Grover, B.T.; Ki, J.J. ASMBS position statement on postprandial hyperinsulinemic hypoglycemia after bariatric surgery. Surg. Obes. Relat. Dis. 2017, 13, 371–378. [Google Scholar] [CrossRef]
- Pories, W.J.; Mehaffey, J.H.; Staton, K.M. The surgical treatment of type two diabetes mellitus. Surg Clin. N. Am. 2011, 91, 821–836. [Google Scholar] [CrossRef]
- Purnell, J.Q.; Selzer, F.; Wahed, A.S.; Pender, J.; Pories, W.; Pomp, A.; Dakin, G.; Mitchell, J.; Garcia, L.; Staten, M.A.; et al. Type 2 Diabetes Remission Rates After Laparoscopic Gastric Bypass and Gastric Banding: Results of the Longitudinal Assessment of Bariatric Surgery Study. Diabetes Care 2016, 39, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Salminen, P.; Helmio, M.; Ovaska, J.; Juuti, A.; Leivonen, M.; Peromaa-Haavista, P.; Hurme, S.; Soinio, M.; Nuutila, P.; Victorzon, M. Effect of laparoscopic sleeve gastrectomy vs. laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA 2018, 319, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Kashyap, S.R.; Wolski, K.; Brethauer, S.A.; Kirwan, J.P.; Pothier, C.E.; Thomas, S.; Abood, B.; Nissen, S.E.; Bhatt, D.L. Bariatric surgery versus medical therapy in obese patients with diabetes. N. Engl. J. Med. 2012, 366, 1567–1576. [Google Scholar] [CrossRef]
- Kashyap, S.R.; Bhatt, D.L.; Wolski, K.; Watanabe, R.M.; Abdul-Ghani, M.; Abood, B.; Pothier, C.E.; Brethauer, S.; Nissen, S.; Gupta, M.; et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: Analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care 2013, 36, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric surgery versus intensive medical therapy for diabetes: 5-year outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Doble, B.; Wordsworth, S.; Rogers, C.A.; Welbourn, R.; Byrne, J.; Blazeby, J.M.; By-Band-Sleeve Trial Management Group. What are the real procedural costs of bariatric surgery? A systematic literature review of published cost analyses. Obes. Surg. 2017, 27, 2179–2192. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.A.; Ewing, J.A.; Hale, A.L.; Blackhurst, D.W.; Bour, E.S.; Scott, J.D. Cost-effectiveness of bariatric surgery: Increasing the economic viability of the most effective treatment for type II diabetes mellitus. Am. Surgeon. 2015, 81, 807–811. [Google Scholar]
- Klein, S.; Ghosh, A.; Cremieux, P.Y.; Eapen, S.; McGavock, T.J. Economic impact of the clinical benefits of bariatric surgery in diabetes patients with BMI ≥ 35kg/m2. Obesity 2011, 19, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Picot, J.; Jones, J.; Colquitt, J.L.; Gospodarevskaya, E.; Loveman, E.; Baxter, L.; Clegg, A.J. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: A systematic review and economic evaluation. Health Technol. Assess. 2009, 13, 1–190. [Google Scholar] [CrossRef]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Barbarisi, A.; Cesaretti, M.; Iannelli, A. A 4-Week Preoperative Ketogenic Micronutrient-Enriched Diet Is Effective in Reducing Body Weight, Left Hepatic Lobe Volume, and Micronutrient Deficiencies in Patients Undergoing Bariatric Surgery: A Prospective Pilot Study. Obes. Surg. 2018, 28, 2215–2224. [Google Scholar] [CrossRef]
- Leonetti, F.; Campanile, F.C.; Coccia, F.; Capoccia, D.; Alessandroni, L.; Puzziello, A.; Coluzzi, I.; Silecchia, G. Very low-carbohydrate ketogenic diet before bariatric surgery: Prospective evaluation of a sequential diet. Obes. Surg. 2015, 25, 64–71. [Google Scholar] [CrossRef]
- Gumbs, A.A.; Pomp, A.; Gagner, M. Revisional bariatric surgery for inadequate weight loss. Obes. Surg. 2007, 17, 1137–1145. [Google Scholar] [CrossRef]
- Velapati, S.R.; Shah, M.; Kuchkuntla, A.R.; Abu-Dayyeh, B.; Grothe, K.; Hurt, R.T.; Mundi, M.S. Weight Regain After Bariatric Surgery: Prevalence, Etiology, and Treatment. Curr. Nutr. Rep. 2018, 7, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Shoar, S.; Nguyen, T.; Ona, M.A.; Reddy, M.; Anand, S.; Alkuwari, M.J.; Saber, A.A. Roux-end-Y gastric bypass reversal: A systematic review. Surg. Obes. Relat. Dis. 2016, 12, 1366–1372. [Google Scholar] [CrossRef]
- Bojsen-Moller, K.N. Mechanisms of improved glycemic control after Roux-en-Y gastric bypass. Dan. Med. J. 2015, 62, B5057. [Google Scholar] [PubMed]
- Pories, W.J.; Swanson, M.S.; MacDonald, K.G.; Long, S.B.; Morris, P.G.; Brown, B.M.; Barakat, H.A.; de Ramon, R.A.; Israel, G.; Dolezal, J.M. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann. Surg. 1995, 222, 339–350. [Google Scholar] [CrossRef]
- Sjostrom, L.; Lindroos, A.K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjostrom, D.; et al. Lifestyle, diabetes and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Bistrian, B.R.; Blackburn, G.L.; Flatt, J.P.; Sizer, J.; Scrimshaw, N.S.; Sherman, M. Nitrogen metabolism and insulin requirements in obese diabetic adults on a protein-sparing modified fast. Diabetes 1976, 25, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Bauman, W.A.; Schwartz, E.; Rose, H.G.; Eisenstein, H.N.; Johnson, D.W. Early and long term effects of acute caloric deprivation in obese diabetic patients. Am. J. Med. 1988, 85, 38–46. [Google Scholar] [CrossRef]
- Hughes, T.A.; Gwynne, J.T.; Switzer, B.R.; Herbst, C.; White, G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am. J. Med. 1984, 77, 7–17. [Google Scholar] [CrossRef]
- Hammer, S.; Snel, M.; Lamb, H.J.; Jazet, I.M.; van der Meer, R.W.; Pijl, H.; Meinders, E.A.; Romijn, J.A.; de Roos, A.; Smit, J.W. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J. Am. Coll. Cardiol. 2008, 52, 1006–1012. [Google Scholar] [CrossRef]
- Snel, M.; Jonker, J.T.; Hammer, S.; Kerpershoek, G.; Lamb, H.J.; Meinders, A.E.; Pijl, H.; de Roos, A.; Romijn, J.A.; Smit, J.W.A. Long-term beneficial effect of a 16-week very low calorie diet on pericardial fat in obese type 2 diabetes mellitus patients. Obesity 2012, 20, 1572–1576. [Google Scholar] [CrossRef]
- Paisey, R.B.; Harvey, P.; Rice, S.; Belka, I.; Bower, L.; Dunn, M.; Taylor, P.; Paisey, R.M. An intensive weight loss programme in established type 2 diabetes and controls: Effect on weight and atherosclerosis risk factors at 1 year. Diabet. Med. 1998, 15, 73–79. [Google Scholar] [CrossRef]
- Wing, R.R.; Blair, E.; Marcus, M.; Epstein, L.H.; Harvey, J. Year-long weight loss treatment for obese patients with type II diabetes: Does including an intermittent very-low-calorie diet improve outcome? Am. J. Med. 1994, 97, 354–362. [Google Scholar] [CrossRef]
- Damms-Machado, A.; Weser, G.; Bischoff, SC. Micronutrient deficiency in obese subjects undergoing low calorie diet. Nutr. J. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Kim, S.; Bersamin, A.; Dopler-Nelson, M.; Otten, J.; Oelrich, B.; Cherin, R. Micronutrient quality of weight-loss diets that focus on macronutrients: Results from the A to Z study. Am. J. Clin. Nutr. 2010, 92, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.H.; Espeland, M.A.; Foster, G.D.; Haffner, S.M.; Hubbard, V.S.; Johnson, K.C.; Kahn, S.E.; Knowler, W.C.; Yanovski, S.Z.; Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003, 24, 610–628. [Google Scholar] [PubMed]
- Gregg, E.W.; Chen, H.; Wagenknecht, L.E.; Clark, J.M.; Delahanty, L.M.; Bantle, J.; Pownall, H.J.; Johnson, K.C.; Safford, M.M.; Kitabchi, A.E.; et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012, 308, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Mottalib, A.; Sakr, M.; Shehabeldin, M.; Hamdy, O. Diabetes remission after nonsurgical intensive lifestyle intervention in obese patients with Type 2 diabetes. J. Diabetes Res. 2015, 468704. [Google Scholar] [CrossRef]
- Ades, P.A.; Savage, P.D.; Marney, A.M.; Harvey, J.; Evans, K.A. Remission of recently diagnosed type 2 diabetes mellitus with weight loss and exercise. J. Cardiopulm. Rehabil. Prev. 2015, 35, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.A.; Choudhari, P.K.; Mahajan, R.R.; Sayyad, M.G.; Pratyush, D.D.; Hasan, I.; Javherani, R.S.; Bothale, M.M.; Purandare, V.B.; Unnikrishnan, A.G. Effect of a low-calorie diet on restoration of normoglycemia in obese subjects with Type 2 diabetes. Indian J. Endocrinol. Metab. 2017, 21, 776–780. [Google Scholar] [PubMed]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetol 2011, 54, 2506–2514. [Google Scholar] [CrossRef]
- Steven, S.; Hollingsworth, K.G.; Al-Mrabeh, A.; Avery, L.; Aribisala, B.; Caslake, M.; Taylor, R. Very low-calorie diet and 6 months of weight stability in type 2 diabetes: Pathophysiological changes in responders and nonresponders. Diabetes Care 2016, 39, 808–815. [Google Scholar] [CrossRef]
- Lean, M.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet. 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019. [CrossRef]
- McInnes, N.; Smith, A.; Otto, R.; Vandermey, J.; Punthakee, Z.; Sherifali, D.; Balasubramaniam, K.; Hall, S.; Gerstein, HC. Piloting a remission strategy in type 2 diabetes: Results of a randomized controlled trial. J. Clin. Endocrinol. Metab. 2017, 102, 1596–1605. [Google Scholar] [CrossRef]
- Fothergill, E.; Guo, J.; Howard, L.; Kerns, J.C.; Knuth, N.D.; Brychta, R.; Chen, K.Y.; Skarulis, M.C.; Walter, M.; Walter, P.J.; et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity 2016, 24, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L. Physiological adaptations to weight loss and factors favouring weight regain. Int. J. Obes. 2015, 39, 1188–1196. [Google Scholar] [CrossRef]
- Campbell, W.R. Dietetic treatment in diabetes mellitus. Can. Med. Assoc. J. 1923, 13, 487–492. [Google Scholar] [PubMed]
- Westman, E.C.; Yancy, W.S.; Humphreys, M. Dietary treatment of diabetes mellitus in the pre-insulin era (1914–1922). Perspect. Biol. Med. 2006, 49, 77–83. [Google Scholar] [CrossRef]
- Arky, R.; Wylie-Rosett, J.; El-Beheri, B. Examination of current dietary recommendations for individuals with diabetes mellitus. Diabetes Care 1982, 5, 59–63. [Google Scholar] [CrossRef]
- Anderson, J.W.; Geil, P.B. New perspectives in nutrition management of diabetes mellitus. Am. J. Med. 1988, 85, 159–165. [Google Scholar] [CrossRef]
- American Diabetes Association. Summary of Revisions: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S4–S6. [Google Scholar]
- Department of Veteran Affairs and Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care. Version 5.0. Available online: https://www.healthquality.va.gov/guidelines/cd/diabetes/ (accessed on 20 January 2019).
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Low carbohydrate nutrition and metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Stern, L.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, M.; Gracely, E.J.; Samaha, F.F. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: One-year follow-up of a randomized trial. Ann. Intern. Med. 2004, 140, 778–785. [Google Scholar] [CrossRef]
- Miyashita, Y.; Koide, N.; Ohtsuka, M.; Ozaki, H.; Itoh, Y.; Oyama, T.; Uetake, T.; Ariga, K.; Shirai, K. Beneficial effect of low carbohydrate in low calorie diets on visceral fat reduction in type 2 diabetic patients with obesity. Diabetes Res. Clin. Pract. 2004, 65, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, T.; Granfeldt, Y.; Ahren, B.; Branell, U.C.; Pålsson, G.; Hansson, A.; Söderström, M.; Lindeberg, S. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: A randomized cross-over pilot study. Cardiovasc. Diabetol. 2009, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.J.; Tomuta, N.; Schechter, C.; Isasi, C.R.; Segal-Isaacson, C.J.; Stein, D.; Zonszein, J.; Wylie-Rosett, J. Comparative study of the effects of a 1-year dietary intervention of a low- carbohydrate diet versus a low-fat diet on weight and glycemic control in type 2 diabetes. Diabetes Care 2009, 32, 1147–1152. [Google Scholar] [CrossRef]
- Daly, M.E.; Paisey, R.; Paisey, R.; Millward, B.A.; Eccles, C.; Williams, K.; Hammersley, S.; MacLeod, K.M.; Gale, T.J. Short-term effects of severe dietary carbohydrate-restriction advice in type 2 diabetes: A randomized controlled trial. Diabet. Med. 2006, 23, 15–20. [Google Scholar] [CrossRef]
- Dyson, P.A.; Beatty, S.; Matthews, D.R. A low-carbohydrate diet is more effective in reducing body weight than healthy eating in both diabetic and non-diabetic subjects. Diabet. Med. 2007, 24, 1430–1435. [Google Scholar] [CrossRef]
- Wolever, T.M.; Gibbs, A.L.; Mehling, C.; Chiasson, J.L.; Connelly, P.W.; Josse, R.G.; Leiter, L.A.; Maheux, P.; Rabasa-Lhoret, R.; Rodger, N.W.; et al. The Canadian trial of carbohydrates in diabetes (CCD), a 1-yr controlled of low-glycemic index dietary carbohydrate in type 2 diabetes: No effect on glycated hemoglobin but reduction in C-reactive protein. Am. J. Clin. Nutr. 2008, 87, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Vetter, M.L.; Moore, R.H.; Chittams, J.L.; Dalton-Bakes, C.V.; Dowd, M.; Williams-Smith, C.; Cardillo, S.; Wadden, T.A. Effects of a low-intensity intervention that prescribed a low-carbohydrate vs. a low-fat diet in obese, diabetic participants. Obesity (Silver Spring) 2010, 18, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Goday, A.; Bellido, D.; Sajoux, I.; Crujeiras, A.B.; Burguera, B.; García-Luna, P.P.; Casanueva, F.F. Short-term safety, tolerability and efficacy of a very low-calorie ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus. Nutr. Diabetes. 2016, 6, e230. [Google Scholar] [CrossRef]
- Saslow, L.R.; Mason, A.E.; Kim, S.; Goldman, V.; Ploutz-Snyder, R.; Bayandorian, H.; Daubenmier, J.; Hecht, F.M.; Moskowitz, J.T. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: A randomized controlled trial. J. Med. Int. Res. 2017, 19, e36. [Google Scholar] [CrossRef] [PubMed]
- Saslow, L.R.; Daubenmier, J.J.; Moskowitz, J.T.; Kim, S.; Murphy, E.J.; Phinney, S.D.; Ploutz-Snyder, R.; Goldman, V.; Cox, R.M.; Mason, A.E.; et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr. Diabetes. 2017, 7, 304. [Google Scholar] [CrossRef]
- Yamada, Y.; Uchida, J.; Izumi, H.; Tsukamoto, Y.; Inoue, G.; Watanabe, Y.; Irie, J.; Yamada, S. A non-calorie-restricted low-carbohydrate diet is effective as an alternative therapy for patients with type 2 diabetes. Int. Med. 2014, 53, 13–19. [Google Scholar] [CrossRef]
- Guldbrand, H.; Dizdar, B.; Bunjaku, B.; Lindström, T.; Bachrach-Lindström, M.; Fredrikson, M.; Östgren, C.J.; Nystrom, F.H. In type 2 diabetes, randomisation to advice to follow a low-carbohydrate diet transiently improves glycaemic control compared with advice to follow a low-fat diet producing a similar weight loss. Diabetologi. 2012, 55, 2118–2127. [Google Scholar] [CrossRef]
- Westman, E.C.; Yancy, W.S.; Mavropoulos, J.C.; Marquart, M.; McDuffie, J.R. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr. Metab. 2008, 19, 36. [Google Scholar] [CrossRef]
- Haimoto, H.; Iwata, M.; Wakai, K.; Umegaki, H. Long-term effects of a diet loosely restricting carbohydrates on HbA1c levels, BMI and tapering of sulfonylureas in type 2 diabetes: A 2-year follow-up study. Diabetes Res. Clin. Pract. 2008, 79, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Thompson, C.H.; Luscombe-Marsh, N.D.; Wycherley, T.P.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: A 2-year randomized clinical trial. Diabetes Obes. Metab. 2018, 20, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Wang, Q.; Hong, Y.; Ojo, O.; Jiang, Q.; Hou, Y.Y.; Huang, Y.-H.; Wang, X.H. The effect of low-carbohydrate diet on glycemic control in patients with type 2 diabetes mellitus. Nutrients 2018, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.N.; Mann, N.J.; Maclean, E.; Shaw, J.E. The effect of high-protein, low-carbohydrate diets in the treatment of type 2 diabetes: A 12 month randomised controlled trial. Diabetologia 2011, 54, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Kanazawa, A.; Makita, S.; Hatae, C.; Komiya, K.; Shimizu, T.; Ikeda, F.; Tamura, Y.; Ogihara, T.; Mita, T.; et al. A randomized controlled trial of 130g/day low-carbohydrate diet in type 2 diabetes with poor glycemic control. Clin. Nutr. 2017, 36, 992–1000. [Google Scholar] [CrossRef]
- Sanada, M.; Kabe, C.; Hata, H.; Uchida, J.; Inoue, G.; Tsukamoto, Y.; Yamada, Y.; Irie, J.; Tabata, S.; Tabata, M.; et al. Efficacy of a moderately low carbohydrate diet in a 36-month observational study of Japanese patients with Type 2 diabetes. Nutrients 2018, 10, 528. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Sargrad, K.; Homko, C.; Mozzoli, M.; Stein, T.P. Effect of a low carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann. Intern. Med. 2005, 142, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes 2004, 53, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.P.; Phinney, S.D.; et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: An open-label, non-randomized, controlled study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.D.; Bell, D.; Hall, R.; Parry-Strong, A.; Docherty, P.D.; Clarke, K.; Chase, J.G. Improvements in glucose metabolism and insulin sensitivity with a low-carbohydrate diet in obese patients with type 2 diabetes. J. Am. Coll. Nutr. 2013, 32, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.A.; Matthew, T.C.; Dashti, A.A.; Asfar, S.; Al-Zaid, N.; Dashti, H.M. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 2012, 28, 1016–1021. [Google Scholar] [CrossRef]
- Sasakabe, T.; Haimoto, H.; Umegaki, H.; Wakai, K. Effects of a moderate low-carbohydrate diet on preferential abdominal fat loss and cardiovascular risk factors in patients with type 2 diabetes. Diabetes Metab. Syndr. Obes. 2011, 4, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.V.; Joensson, E.A. Low carbohydrate diet in type 2 diabetes: Stable improvement of bodyweight and glycemic control during 44 months follow-up. Nutr. Metab. 2008, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.M.; Mathew, T.C.; Khadada, M.; Al-Mousawi, M.; Talib, H.; Asfar, S.K.; Behbahani, A.I.; Al-Zaid, N.S. Beneficial effects of ketogenic diet in obese diabetic subjects. Mol. Cell Biochem. 2007, 302, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Yancy, W.S.; Foy, M.; Chalecki, A.M.; Vernon, A.C.; Westman, E.C. A low carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. 2005, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.M.; Mathew, T.C.; Hussein, T.; Asfar, S.K.; Behbahani, A.; Khoursheed, M.A.; Al-Sayer, H.M.; Bo-Abbas, Y.Y.; Al-Zaid, N.S. Long-term effects of a ketogenic diet in obese patients. Exp. Clin. Cardiol. 2004, 9, 200–205. [Google Scholar]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Elhayany, A.; Lustman, A.; Abel, R.; Attal-Singer, J.; Vinker, S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: A 1-year prospective randomized intervention study. Diabetes Obes. Metab. 2010, 12, 204–209. [Google Scholar] [CrossRef]
- Athinarayanan, S.J.; Adams, R.N.; Hallberg, S.J.; McKenzie, A.L.; Bhanpuri, N.H.; Campbell, W.W.; Volek, J.S.; Phinney, S.D.; McCarter, J.P. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: A 2-year non-randomized clinical trial. bioRxiv 2018, 476275. [Google Scholar] [CrossRef]
- Snorgaard, O.; Poulsen, G.M.; Andersen, H.K.; Astrup, A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res. Care. 2017, 5, e000354. [Google Scholar] [CrossRef] [PubMed]
- Bhanpuri, N.H.; Hallberg, S.J.; Williams, P.T.; McKenzie, A.L.; Ballard, K.D.; Campbell, W.W.; McCarter, J.P.; Phinney, S.D.; Volek, J.S. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at one year: An open label, non-randomized, controlled study. Cardiovasc. Diabetol. 2018, 17, 56. [Google Scholar] [CrossRef]
- Wang, G.F.; Yan, Y.X.; Yin, D.; Hui, Y.; Zhang, J.P.; Han, G.J.; Ma, N.; Wu, Y.; Xu, J.Z.; Yang, T. Predictive factors of Type 2 diabetes mellitus remission following bariatric surgery: A Meta-analysis. Obes. Surg. 2015, 25, 199–208. [Google Scholar] [CrossRef]
- Yan, W.; Bai, R.; Li, Y.; Xu, J.; Zhong, Z.; Xing, Y.; Yan, M.; Lin, Y.; Song, M. Analysis of predictors of type 2 diabetes mellitus remission after roux-en-Y gastric bypass in 101 Chinese patients. Obes. Surg. 2019. [Google Scholar] [CrossRef]
- Brehm, B.J.; Seeley, R.J.; Daniels, S.R.; D’Alessio, D.A. A Randomized Trial Comparing a Very Low Carbohydrate Diet and a Calorie-Restricted Low Fat Diet on Body Weight and Cardiovascular Risk Factors in Healthy Women. J. Clin. Endocrinol. Metab. 2003, 88, 1617–1623. [Google Scholar] [CrossRef]
- Nordmann, A.J.; Nordmann, A.; Briel, M.; Keller, U.; Yancy, W.S.; Brehm, B.J.; Bucher, H.C. Effects of a low-carbohydrate vs. low-fat diets on weight loss and cardiovascular risk factors. Arch. Intern. Med. 2006, 166, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Yancy, W.S.; Edman, J.S.; Tomlin, K.F.; Perkins, CE. Effect of 6-month adherence to a very low carbohydrate diet program. Am. J. Med. 2002, 113, 30–36. [Google Scholar] [CrossRef]
- Nuttall, F.Q.; Gannon, M.C. The metabolic response to a high-protein, low-carbohydrate diet in men with type 2 diabetes mellitus. Metabolism 2006, 55, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Barry, J.D.; Stephens, J.W. Predicting the Resolution of Type 2 Diabetes after Bariatric Surgical Procedures: A Concise Review. J. Diabetes Metab. 2015, 6, 617. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallberg, S.J.; Gershuni, V.M.; Hazbun, T.L.; Athinarayanan, S.J. Reversing Type 2 Diabetes: A Narrative Review of the Evidence. Nutrients 2019, 11, 766. https://doi.org/10.3390/nu11040766
Hallberg SJ, Gershuni VM, Hazbun TL, Athinarayanan SJ. Reversing Type 2 Diabetes: A Narrative Review of the Evidence. Nutrients. 2019; 11(4):766. https://doi.org/10.3390/nu11040766
Chicago/Turabian StyleHallberg, Sarah J, Victoria M Gershuni, Tamara L Hazbun, and Shaminie J Athinarayanan. 2019. "Reversing Type 2 Diabetes: A Narrative Review of the Evidence" Nutrients 11, no. 4: 766. https://doi.org/10.3390/nu11040766
APA StyleHallberg, S. J., Gershuni, V. M., Hazbun, T. L., & Athinarayanan, S. J. (2019). Reversing Type 2 Diabetes: A Narrative Review of the Evidence. Nutrients, 11(4), 766. https://doi.org/10.3390/nu11040766




