High-Prevalence Vitamin D Deficiency among Korean Emergency Department Homeless, with a Comparison to a Healthy Korean Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Subjects
2.3. Measurement
2.4. Healthy Control
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Homeless
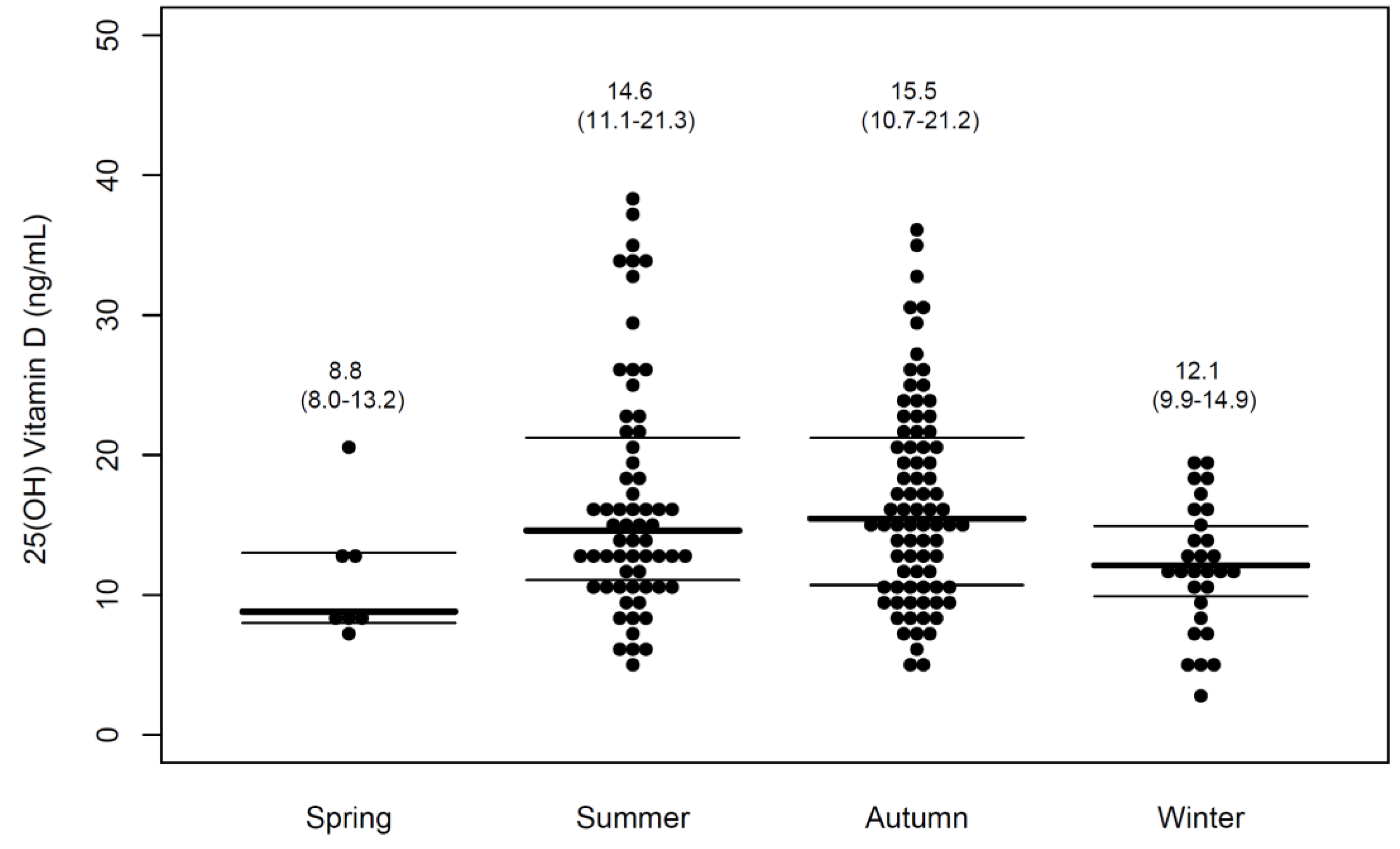
3.2. Level of 25 (OH)D
3.3. Factors Associated with Vitamin D Deficiency
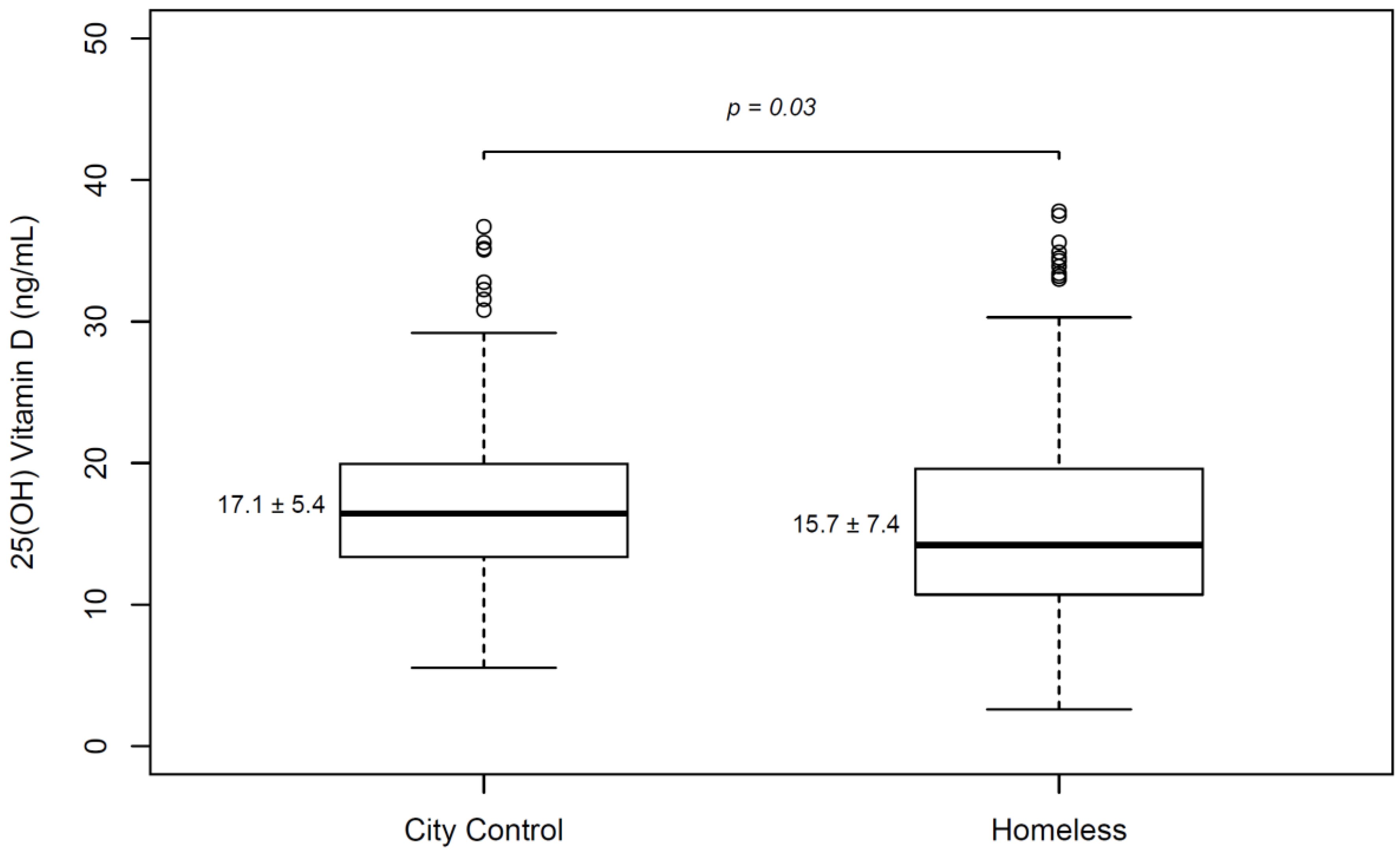
3.4. Comparison of Homeless Population with Healthy Controls
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Malmauret, L.; Leblanc, J.; Cuvelier, I.; Verger, P. Dietary intakes and vitamin status of a sample of homeless people in Paris. Eur. J. Clin. Nutr. 2002, 56, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Seale, J.V.; Fallaize, R.; Lovegrove, J.A. Nutrition and the homeless: The underestimated challenge. Nutr. Res. Rev. 2016, 29, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Jackson, J.; Thorley, H.; Porter, K.; Fleming, C.; Richards, A.; Bonner, A.; Savovic, J. Nutritional deficiencies in homeless persons with problematic drinking: A systematic review. Int. J. Equity Health 2017, 16, 71. [Google Scholar] [CrossRef]
- Darmon, N. A Fortified Street Food to Prevent Nutritional Deficiencies in Homeless Men in France. J. Am. Coll. Nutr. 2009, 28, 196–202. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- De Haan, K.; Groeneveld, A.B.; de Geus, H.R.; Egal, M.; Struijs, A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: Systematic review and meta-analysis. Crit. Care 2014, 18, 660. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Scragg, R.; Schwartz, R.S.; Camargo, C.A., Jr. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J. Am. Geriatr. Soc. 2009, 57, 1595–1603. [Google Scholar] [CrossRef]
- Skaaby, T.; Husemoen, L.L.; Pisinger, C.; Jorgensen, T.; Thuesen, B.H.; Fenger, M.; Linneberg, A. Vitamin D status and incident cardiovascular disease and all-cause mortality: A general population study. Endocrine 2013, 43, 618–625. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Altieri, B.; Annweiler, C.; Balercia, G.; Pal, H.B.; Boucher, B.J.; Cannell, J.J.; Foresta, C.; Grubler, M.R.; Kotsa, K.; et al. Vitamin D and chronic diseases: The current state of the art. Arch. Toxicol. 2017, 91, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Gilboy, N.; Tanabe, P.; Travers, D.A. The Emergency Severity Index Version 4: Changes to ESI level 1 and pediatric fever criteria. J. Emerg. Nurs. 2005, 31, 357–362. [Google Scholar] [CrossRef]
- Lee, H.J.; Shin, J.; Hong, K.; Jung, J.H. Vitamin C Deficiency of Korean Homeless Patients Visiting to Emergency Department with Acute Alcohol Intoxication. J. Korean Med. Sci. 2015, 30, 1874–1880. [Google Scholar] [CrossRef]
- Choi, H.S.; Oh, H.J.; Choi, H.; Choi, W.H.; Kim, J.G.; Kim, K.M.; Kim, K.J.; Rhee, Y.; Lim, S.K. Vitamin D insufficiency in Korea—A greater threat to younger generation: The Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J. Clin. Endocrinol. Metab. 2011, 96, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Darmon, N.; Coupel, J.; Deheeger, M.; Briend, A. Dietary inadequacies observed in homeless men visiting an emergency night shelter in Paris. Public Health Nutr. 2001, 4, 155–161. [Google Scholar] [CrossRef]
- Smith, C.; Richards, R. Dietary intake, overweight status, and perceptions of food insecurity among homeless Minnesotan youth. Am. J. Hum. Biol. 2008, 20, 550–563. [Google Scholar] [CrossRef]
- Hoogenboom, S.A.; Lekkerkerker, S.J.; Fockens, P.; Boermeester, M.A.; van Hooft, J.E. Systematic review and meta-analysis on the prevalence of vitamin D deficiency in patients with chronic pancreatitis. Pancreatology 2016, 16, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Ceglia, L. Vitamin D and its role in skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Arik, G.; Ulger, Z. Vitamin D in sarcopenia: Understanding its role in pathogenesis, prevention and treatment. Eur. Geriatr. Med. 2016, 7, 207–213. [Google Scholar] [CrossRef]
- Wijnia, J.W.; Wielders, J.P.; Lips, P.; van de Wiel, A.; Mulder, C.L.; Nieuwenhuis, K.G. Is vitamin D deficiency a confounder in alcoholic skeletal muscle myopathy? Alcohol. Clin. Exp. Res. 2013, 37 (Suppl. 1), E209–E215. [Google Scholar] [CrossRef]
- Smith, E.M.; Tangpricha, V. Vitamin D and Anemia: Insights into an Emerging Association. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 432–438. [Google Scholar] [CrossRef]
- Arranz, L.; de Vicente, A.; Munoz, M.; De la Fuente, M. Impaired immune function in a homeless population with stress-related disorders. Neuroimmunomodulation 2009, 16, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Nurbazlin, M.; Chee, W.S.; Rokiah, P.; Tan, A.T.; Chew, Y.Y.; Nusaibah, A.R.; Chan, S.P. Effects of sun exposure on 25(OH) vitamin D concentration in urban and rural women in Malaysia. Asia Pac. J. Clin. Nutr. 2013, 22, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B.A.; Manning, T.; Peiris, A.N. The impact of living in rural and urban areas: Vitamin D and medical costs in veterans. J. Rural Health 2012, 28, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Christopher, K.L.; Wiggins, A.T.; Van Meter, E.M.; Means, R.T., Jr.; Hayslip, J.W.; Roach, J.P. Differences in vitamin D nutritional status between newly diagnosed cancer patients from rural or urban settings in Kentucky. Nutr. Cancer 2013, 65, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hong, I.Y.; Chung, J.W.; Choi, H.S. Vitamin D status in South Korean population: Seven-year trend from the KNHANES. Medicine 2018, 97, e11032. [Google Scholar] [CrossRef]
- Ritu, G.; Gupta, A. Fortification of foods with vitamin D in India. Nutrients 2014, 6, 3601–3623. [Google Scholar] [CrossRef]
- Farrar, M.D.; Kift, R.; Felton, S.J.; Berry, J.L.; Durkin, M.T.; Allan, D.; Vail, A.; Webb, A.R.; Rhodes, L.E. Recommended summer sunlight exposure amounts fail to produce sufficient vitamin D status in UK adults of South Asian origin. Am. J. Clin. Nutr. 2011, 94, 1219–1224. [Google Scholar] [CrossRef]
- Calvo, M.S.; Whiting, S.J.; Barton, C.N. Vitamin D intake: A global perspective of current status. J. Nutr. 2005, 135, 310–316. [Google Scholar] [CrossRef]
| Deficient | Not Deficient | p-Value * | Total | ||
|---|---|---|---|---|---|
| (25(OH)D < 20 ng/mL) | (25(OH)D ≥ 20 ng/mL) | ||||
| n = 132 | n = 47 | n = 179 | |||
| Age | median (IQR) | 53 (46–59) | 52 (46–57) | 52 (47–58) | |
| Male | n (%) | 124 (94%) | 46 (98%) | 0.45 | 170 (95%) |
| Alcohol-intoxicated state | n (%) | 112 (85%) | 41 (87%) | 0.69 | 153 (86%) |
| Past Medical History | |||||
| Alcohol dependency | n (%) | 121 (92%) | 44 (94%) | 0.67 | 165 (92%) |
| Diabetes | n (%) | 19 (14%) | 5 (11%) | 0.62 | 24 (13%) |
| Hypertension | n (%) | 14 (11%) | 7 (15%) | 0.43 | 21 (12%) |
| Liver cirrhosis | n (%) | 12 (9%) | 4 (9%) | 1.00 | 16 (9%) |
| Malignancy | n (%) | 1 (1%) | 1 (2%) | 0.43 | 2 (1%) |
| Medical conditions | |||||
| Active tuberculosis | n (%) | 4 (3%) | 0 (0%) † | 0.57 | 4 (2%) |
| Infection | n (%) | 28 (21%) | 8 (17%) | 0.54 | 36 (20%) |
| Trauma | n (%) | 35 (27%) | 12 (26%) | 0.90 | 47 (26%) |
| Fracture | n (%) | 7 (5%) | 4 (9%) † | 0.43 | 11 (6%) |
| Rhabdomyolysis | n (%) | 13 (10%) | 0 (0%) † | 0.03 | 13 (7%) |
| Laboratory test | |||||
| WBC (×103/uL) | median (IQR) | 7.2 (5.3–9.8) | 6.6 (5.6–9.0) | 0.55 | 7.1 (5.4–9.7) |
| Hb (g/dL) | median (IQR) | 13.4 (12.2–14.8) | 14.3 (12.9–15.7) | 0.02 | 13.8 (12.5–15.1) |
| Plt (x103/uL) | median (IQR) | 231 (167–278) | 219 (137–289) | 0.53 | 229 (155–279) |
| Sodium (mmol/L) | median (IQR) | 139.6 (135.8–142.3) | 140.2 (136.6–142.2) | 0.49 | 139.9 (136.3–142.2) |
| Potassium (mmol/L) | median (IQR) | 3.7 (3.4–4.0) | 3.7 (3.4–4.0) | 0.96 | 3.7 (3.4–4.0) |
| Chloride (mmol/L) | median (IQR) | 102.4 (96.1–105.5) | 102.4 (98.6–105.2) | 0.58 | 102.4 (96.9–105.4) |
| Calcium (mg/dL) | median (IQR) | 8.6 (8.4–9.1) | 8.8 (8.4–9.1) | 0.44 | 8.7 (8.4–9.1) |
| Phosphorus (mg/dL) | median (IQR) | 3.5 (2.9–4.3) | 3.7 (3.2–4.2) | 0.27 | 3.6 (3.0–4.2) |
| BUN (mg/dL) | median (IQR) | 11 (8–16) | 12 (9–16) | 0.62 | 12 (9–16) |
| Creatinine (mg/dL) | median (IQR) | 0.7 (0.6–0.9) | 0.8 (0.7–0.9) | 0.38 | 0.7 (0.6–0.9) |
| AST (IU/L) | median (IQR) | 52 (28–104) | 44 (27–82) | 0.27 | 50 (27–95) |
| ALT (IU/L) | median (IQR) | 23 (14–50) | 24 (16–47) | 0.76 | 23 (15–48) |
| T.bil (mg/dL) | median (IQR) | 1.0 (0.7–1.6) | 0.9 (0.7–1.3) | 0.51 | 1.0 (0.7–1.5) |
| CK (U/L) | median (IQR) | 209 (109–453) | 203 (122–329) | 0.79 | 208 (114–398) |
| CK-MB (U/L) | median (IQR) | 1.5 (0.8–4.7) | 2.5 (1.3–3.65) | 0.25 | 1.9 (0.9–4.3) |
| Ehtanol (mg/dL) | median (IQR) | 220 (2–315) | 212 (1–311) | 0.64 | 213 (2–311) |
| Total protein (g/dL) | median (IQR) | 6.7 (6.1–7.1) | 6.6 (6.2–7.1) | 0.27 | 6.7 (6.1–7.1) |
| Albumin (g/dL) | median (IQR) | 3.9 (3.6–4.2) | 4.1 (3.7–4.3) | 0.09 | 4.0 (3.7–4.2) |
| Cholesterol (mg/dL) | median (IQR) | 164 (134–203) | 172 (147–212) | 0.47 | 165 (136–207) |
| CRP (mg/L) | median (IQR) | 0.29 (0.09–0.91) | 0.08 (0.03–0.04) | 0.005 | 0.25 (0.05–0.88) |
| Osmolality (msom/kg) | median (IQR) | 343 (297–370) | 341 (295–368) | 0.75 | 342.5 (297–370) |
| Vitamin B1 (mmol/L) | median (IQR) | 151 (114–200) | 154 (121–226) | 0.39 | 151 (116–205) |
| Vitamin B12 (pg/mL) | median (IQR) | 539 (389–766) | 627 (429–936) | 0.32 | 572 (392–804) |
| Vitamin B6 (mmol/L) | median (IQR) | 32.3 (22.1–51.7) | 39.2 (22.5–63.0) | 0.27 | 34.1 (22.1–53.3) |
| Vitamin C (umol/L) | median (IQR) | 3.9 (1.3–12.1) | 5.4 (1.6–14.7) | 0.23 | 4.1 (1.5–12.7) |
| Chief Complaints | N | % |
|---|---|---|
| Altered mental status | 68 | 38% |
| Extremity pain | 16 | 9% |
| General weakness | 14 | 8% |
| Abdominal pain | 12 | 7% |
| Multiple contusion | 12 | 7% |
| Chest pain | 9 | 5% |
| Fever, myalgia | 7 | 4% |
| Dyspnea | 7 | 4% |
| Dizziness | 5 | 3% |
| Laceration | 5 | 3% |
| Headache | 4 | 2% |
| Back pain | 4 | 2% |
| Facial pain | 4 | 2% |
| GI bleeding | 3 | 2% |
| Anxiety | 3 | 2% |
| Eye problem | 2 | 1% |
| Drug overdose | 1 | 1% |
| Seizure | 1 | 1% |
| Syncope | 1 | 1% |
| Hallucination | 1 | 1% |
| Total | 179 |
| Unadjusted Relative Risk * | 95% Confidence interval | Adjusted Relative Risk * | 95% Confidence Interval | |
|---|---|---|---|---|
| Winter/spring | 4.9 | 1.4–16.8 | 5.1 | 1.4–17.9 |
| Hemoglobin | 0.8 | 0.7–1.0 | 0.9 | 0.7–1.1 |
| Albumin | 0.6 | 0.2–1.2 | 1.0 | 0.3–2.7 |
| CRP | 1.1 | 1.0–1.2 | 1.1 | 0.9–1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.J.; Shin, J.; You, K.M. High-Prevalence Vitamin D Deficiency among Korean Emergency Department Homeless, with a Comparison to a Healthy Korean Population. Nutrients 2019, 11, 763. https://doi.org/10.3390/nu11040763
Lee HJ, Shin J, You KM. High-Prevalence Vitamin D Deficiency among Korean Emergency Department Homeless, with a Comparison to a Healthy Korean Population. Nutrients. 2019; 11(4):763. https://doi.org/10.3390/nu11040763
Chicago/Turabian StyleLee, Hui Jai, Jonghwan Shin, and Kyoung Min You. 2019. "High-Prevalence Vitamin D Deficiency among Korean Emergency Department Homeless, with a Comparison to a Healthy Korean Population" Nutrients 11, no. 4: 763. https://doi.org/10.3390/nu11040763
APA StyleLee, H. J., Shin, J., & You, K. M. (2019). High-Prevalence Vitamin D Deficiency among Korean Emergency Department Homeless, with a Comparison to a Healthy Korean Population. Nutrients, 11(4), 763. https://doi.org/10.3390/nu11040763





