Lycopene Inhibits Reactive Oxygen Species-Mediated NF-κB Signaling and Induces Apoptosis in Pancreatic Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Culture Condition
2.2. Experimental Protocol
2.3. Determination of Cell Viability
2.4. Measurement of Intracellular Reactive Oxygen Species (ROS) Levels
2.5. Measurement of Mitochondrial ROS Levels
2.6. Measurement of Oxygen Consumption Rate (OCR)
2.7. Measurement of Mitochondrial Membrane Potential (MMP)
2.8. Real-Time Polymerase Chain Reaction (PCR) Analysis for cIAP1, cIAP2, and Survivin
2.9. Preparation of Cell Extracts
2.10. Western Blot Analysis for cIAP1, cIAP2, Survivin, IκBα, Bcl-2, and Bax
2.11. Electrophoretic Mobility Shift Assay (EMSA)
2.12. Statistical Analysis
3. Results
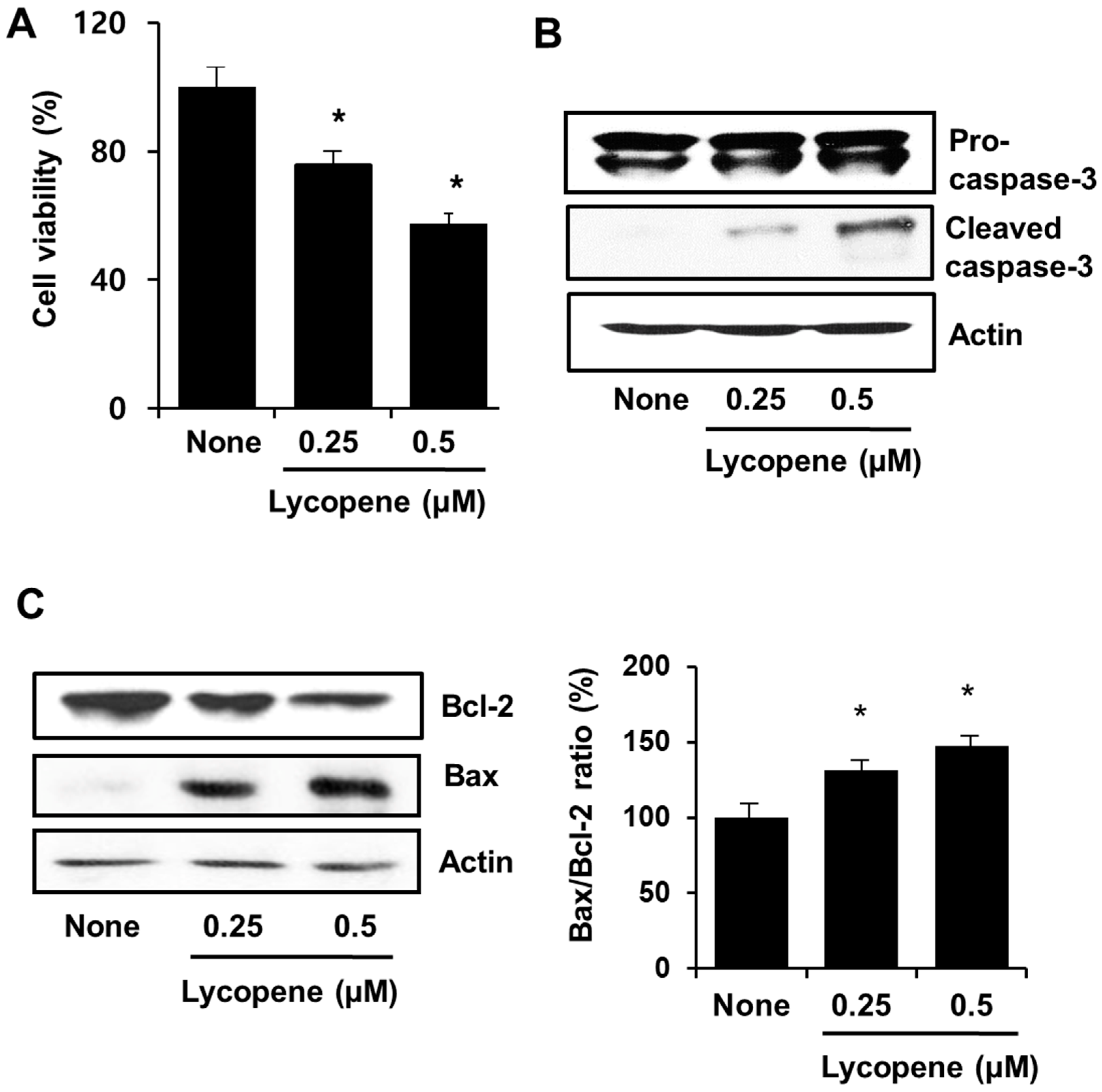
3.1. Lycopene Induces Apoptosis in PANC-1 Cells
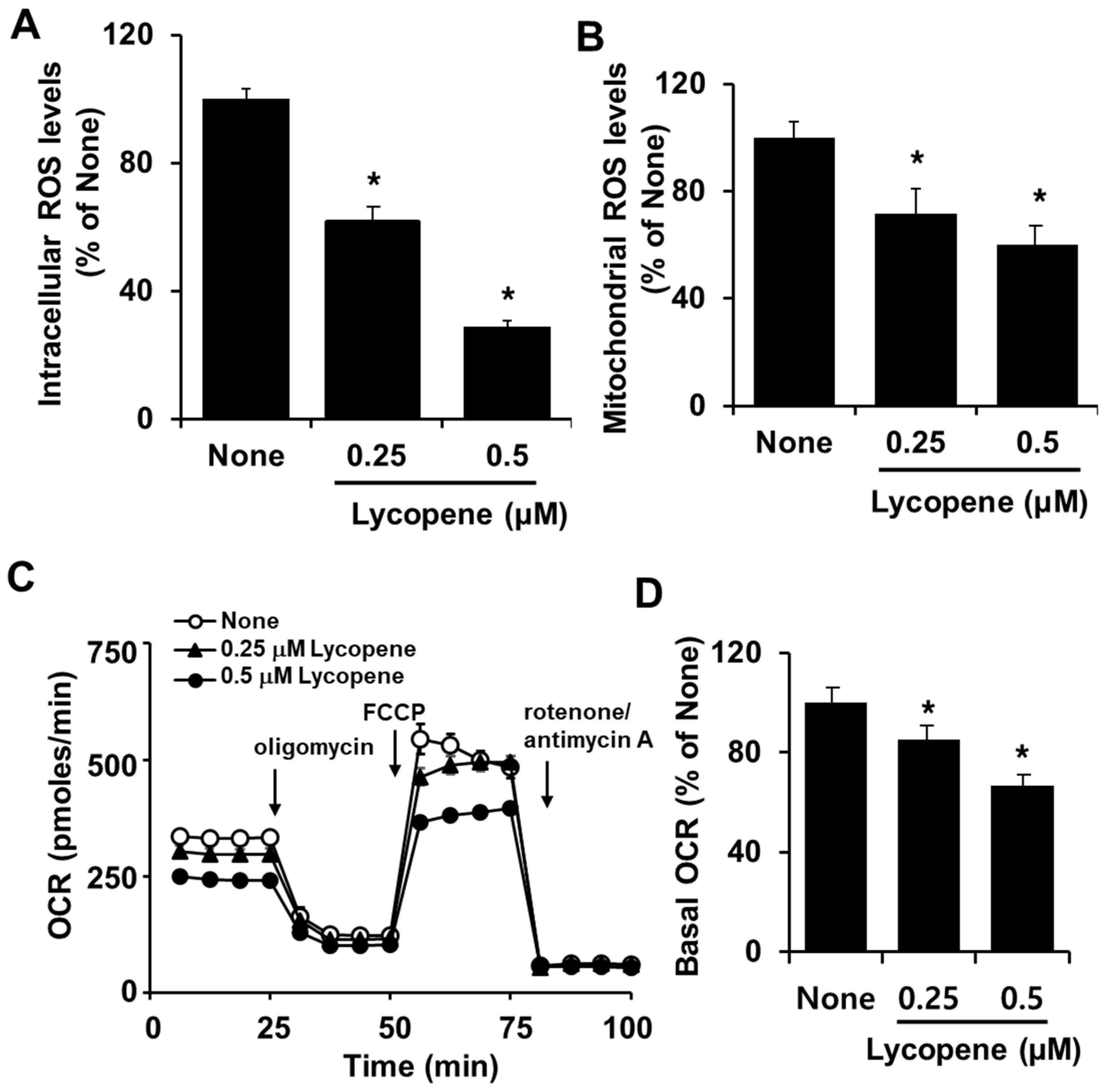
3.2. Lycopene Decreases Intracellular and Mitochondrial ROS Levels and OCR in PANC-1 Cells
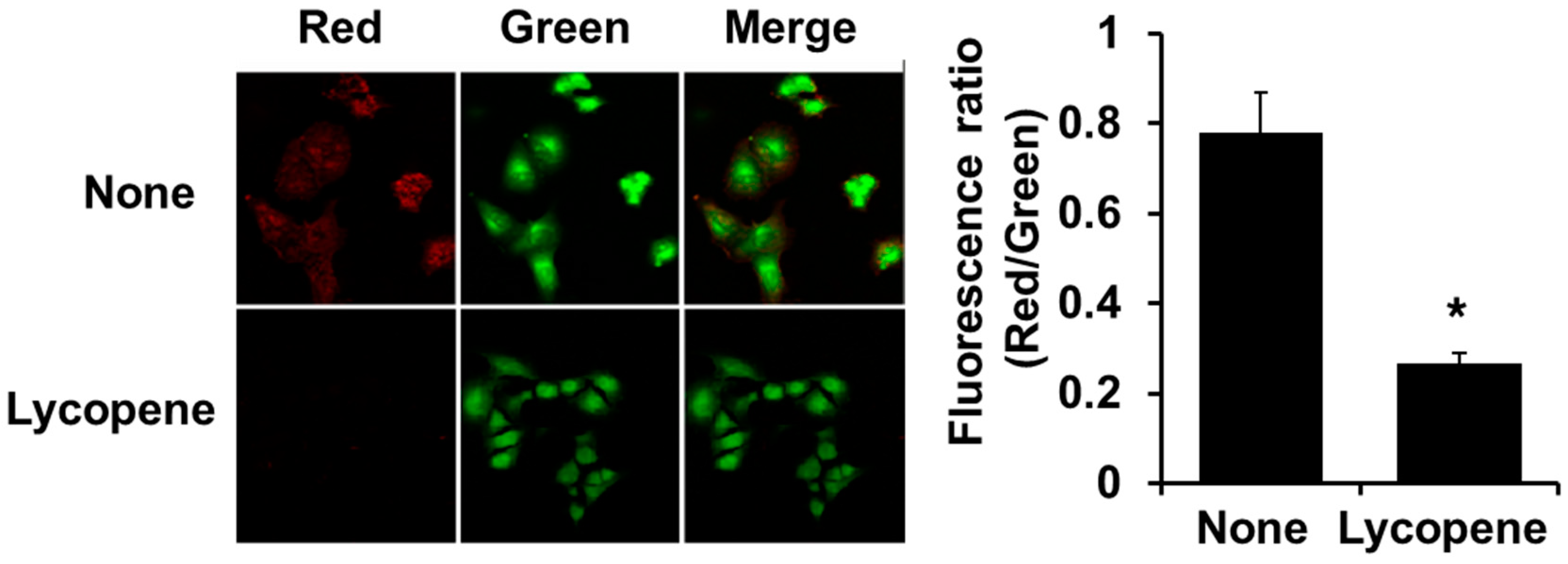
3.3. Lycopene Decreases MMP in PANC-1 Cells
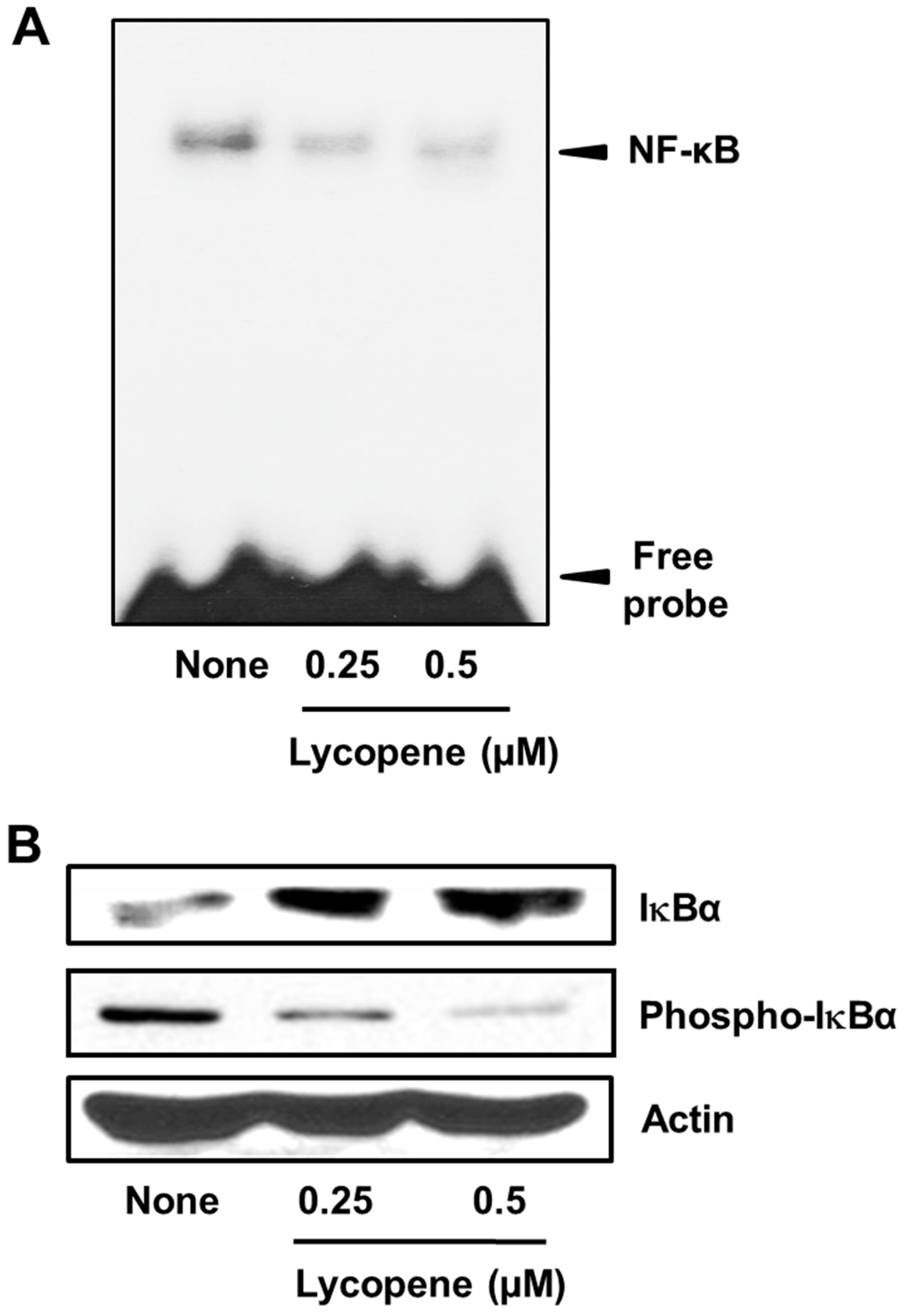
3.4. Lycopene Suppresses NF-κB Activation in PANC-1 Cells
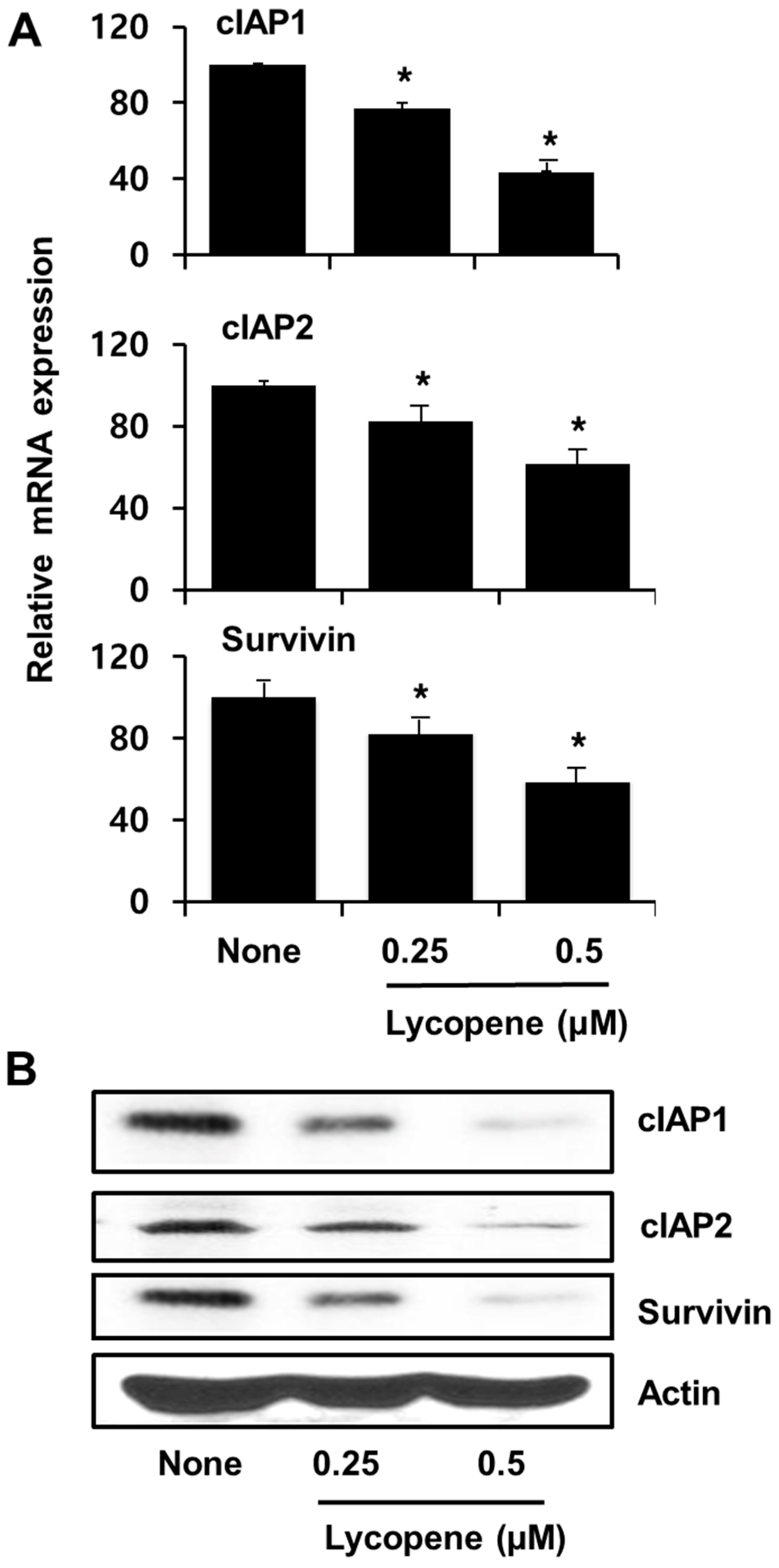
3.5. Lycopene Decreases Expression of cIAP1, cIAP2, and Survivin in PANC-1 Cells
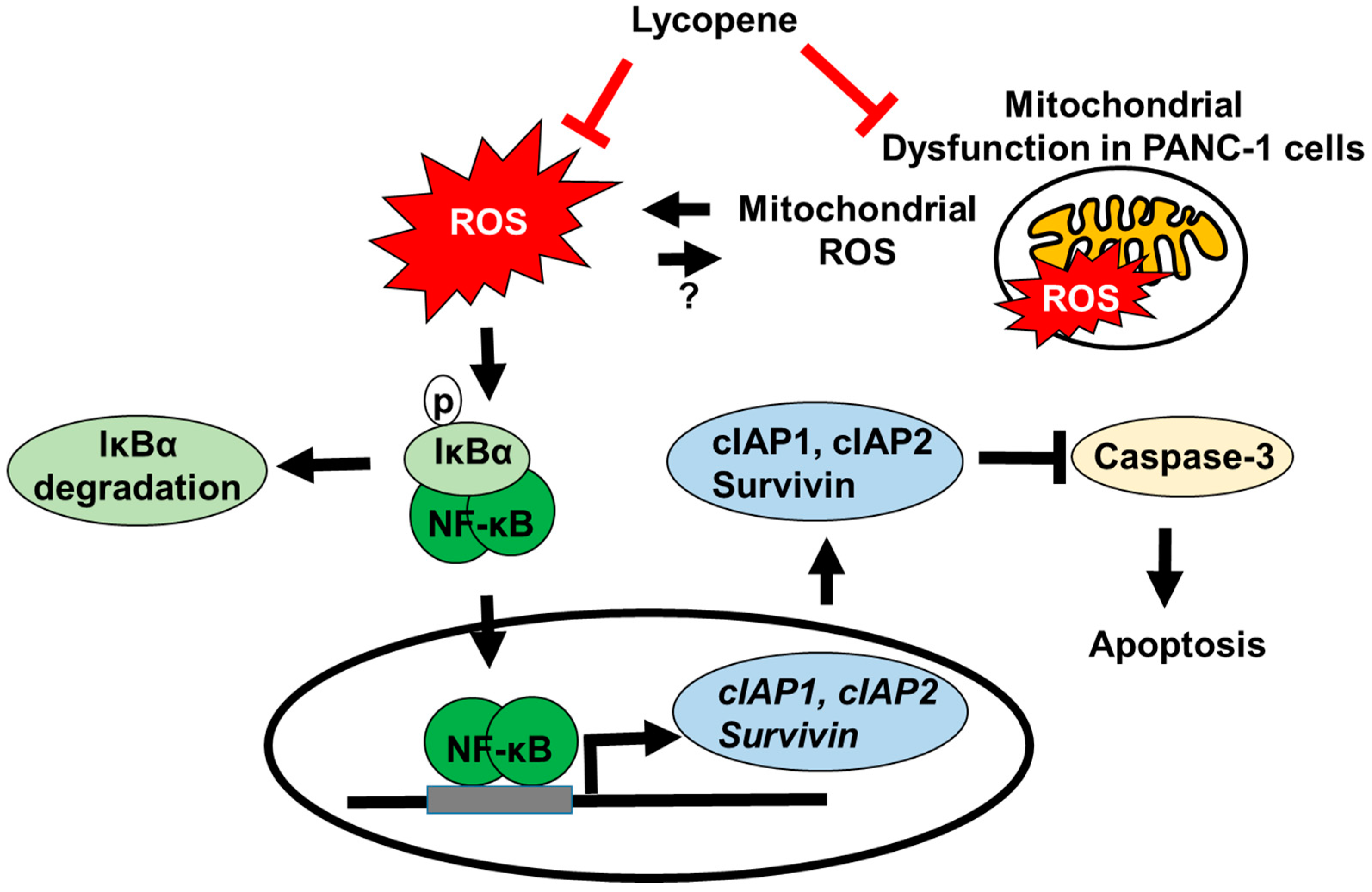
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, D.; Xie, K.; Wolff, R.; Abbruzzese, J.L. Pancreatic cancer. Lancet 2004, 363, 1049–1057. [Google Scholar] [CrossRef]
- Maitra, A.; Hruban, R.H. Pancreatic cancer. Annu. Rev. Pathol. 2008, 3, 157–188. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Lee, E.S.; Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2015. Cancer Res. Treat. 2018, 50, 303–316. [Google Scholar] [CrossRef]
- Fulda, S. Apoptosis pathways and their therapeutic exploitation in pancreatic cancer. J. Cell Mol. Med. 2009, 13, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Komori, S.; Osada, S.; Yoshida, K. Novel strategy with gemcitabine for advanced pancreatic cancer. ISRN Oncol. 2011, 2011, 936893. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Yang, Y.; Zhao, Y.P.; Zhang, T.P.; Liao, Q.; Shu, H. Recent studies of 5-fluorouracil resistance in pancreatic cancer. World J. Gastroenterol. 2014, 20, 15682–15690. [Google Scholar] [CrossRef]
- Noble, S.; Goa, K.L. Gemcitabine. A review of its pharmacology and clinical potential in non-small cell lung cancer and pancreatic cancer. Drugs 1997, 54, 447–472. [Google Scholar] [CrossRef]
- Lee, K.H. Chemotherapy and targeted therapy with management of related complications in pancreatic cancer. Korean J. Pancreas Biliary Tract 2015, 20, 5–13. [Google Scholar] [CrossRef]
- Yokoyama, C.; Sueyoshi, Y.; Ema, M.; Mori, Y.; Takaishi, K.; Hisatomi, H. Induction of oxidative stress by anticancer drugs in the presence and absence of cells. Oncol. Lett. 2017, 14, 6066–6070. [Google Scholar] [CrossRef]
- Conklin, K.A. Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr. Cancer. 2000, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.Q.; Gocho, T.; Aguilar, M.; Wu, M.; Zhuang, Z.N.; Fu, J.; Yanaga, K.; Huang, P.; Chiao, P.J. Mechanisms of overcoming intrinsic resistance to gemcitabine in pancreatic ductal adenocarcinoma through the redox modulation. Mol. Cancer Ther. 2015, 14, 789–798. [Google Scholar] [CrossRef]
- Yasued, A.; Urushima, H.; Ito, T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: A systematic review. Integr. Cancer Ther. 2016, 15, 17–39. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, G.; Zhu, F.; Peng, C.; Li, W.; Li, H.; Kim, H.G.; Bode, A.M.; Dong, Z.; Dong, Z. Antioxidants decrease the apoptotic effect of 5-Fu in colon cancer by regulating Src-dependent caspase-7 phosphorylation. Cell Death Dis. 2014, 5, e983. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, V.; Hay, N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef]
- Durackova, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar]
- Schraufstatter, I.; Hyslop, P.A.; Jackson, J.H.; Cochrane, C.G. Oxidant-induced DNA damage of target cells. J. Clin. Investig. 1988, 82, 1040–1050. [Google Scholar] [CrossRef]
- Poyton, R.O.; Ball, K.A.; Castello, P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 2009, 20, 332–340. [Google Scholar] [CrossRef]
- Epstein, T.; Gatenby, R.A.; Brown, J.S. The Warburg effect as an adaptation of cancer cells to rapid fluctuations in energy. PLoS ONE 2017, 12, e0185085. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, S. Metabolic changes in cancer: Beyond the Warburg effect. Acta Biochim. Biophys. Sin. (Shanghai) 2013, 45, 18–26. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Guda, M.R.; Asuthkar, S.; Labak, C.M.; Tsung, A.J.; Alexandrov, I.; Mackenzie, M.J.; Prasad, D.V.; Velpula, K.K. Targeting PDK4 inhibits breast cancer metabolism. Am. J. Cancer Res. 2018, 8, 1725–1738. [Google Scholar] [PubMed]
- Bauer, G. Tumor cell-protective catalase as a novel target for rational therapeutic approaches based on specific intercellular ROS signaling. Anticancer Res. 2012, 32, 2599–2624. [Google Scholar]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 2008, 320, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Afanas’ev, I. Reactive oxygen species signaling in cancer: Comparison with aging. Aging Dis. 2014, 2, 219–230. [Google Scholar]
- Li, N.; Michael, K. Is NF-κB the sensor of oxidative stress? FASEB J. 1999, 13, 1137–1143. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Dangerous liaisons: STAT3 and NF-κB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010, 21, 11–19. [Google Scholar] [CrossRef]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.W. NF-κB in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kim, M.O.; Kang, S.H.; Choi, Y.H.; Kim, G.Y. Sulforaphane suppresses TNF-α-mediated activation of NF-κB and induces apoptosis through activation of reactive oxygen species-dependent caspase-3. Cancer Lett. 2009, 274, 132–142. [Google Scholar] [CrossRef]
- Wang, C.Y.; Mayo, M.W.; Korneluk, R.G.; Goeddel, D.V.; Baldwin, A.S. NF-κB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998, 281, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Theodore, T.S.; Xu, L. Overcoming cancer therapy resistance by targeting inhibitors of apoptosis proteins and nuclear factor-kappa B. Am. J. Transl. Res. 2009, 1, 1. [Google Scholar]
- Vucic, D.; Fairbrother, W.J. The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin. Cancer Res. 2007, 13, 5995–6000. [Google Scholar] [CrossRef]
- Lopes, R.B.; Gangeswaran, R.; McNeish, I.A.; Wang, Y.; Lemoine, N.R. Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. Int. J. Cancer 2007, 120, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Arlt, A.; Müerköster, S.S.; Schäfer, H. Targeting apoptosis pathways in pancreatic cancer. Cancer Lett. 2013, 332, 346–358. [Google Scholar] [CrossRef]
- Salman, H.; Bergman, M.; Djaldetti, M.; Bessler, H. Lycopene affects proliferation and apoptosis of four malignant cell lines. Biomed. Pharmacother. 2007, 61, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, A.J.; Oliveira, F.L.; Martins, N.B.; Maia Gde, A.; Martucci, R.B.; Borojevic, R. Effect of lycopene on cell viability and cell cycle progression in human cancer cell lines. Cancer Cell Int. 2012, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, V.; Venier, N.A.; Colquhoun, A.J.; Fleshner, N.E.; Klotz, L.H. Lycopene enhances the anti-proliferative and pro-apoptotic effects of capsaicin in prostate cancer in vitro. J. Cancer Res. 2012, 1, 30. [Google Scholar]
- Nitsche, C.; Simon, P.; Weiss, F.U.; Fluhr, G.; Weber, E.; Gärtner, S.; Behn, C.O.; Kraft, M.; Ringel, J.; Aghdassi, A.; et al. Environmental risk factors for chronic pancreatitis and pancreatic cancer. Dig. Dis. 2011, 29, 235–242. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, W.; Shao, L.; Zhong, D.; Wu, Y.; Cai, J. Association between intake of antioxidants and pancreatic cancer risk: a meta-analysis. Int. J. Food Sci. Nutr. 2016, 67, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Burney, P.G.; Comstock, G.W.; Morris, J.S. Serologic precursors of cancer: Serum micronutrients and the subsequent risk of pancreatic cancer. Am. J. Clin. Nutr. 1989, 49, 895–900. [Google Scholar] [CrossRef]
- Han, X.; Li, J.; Brasky, T.M.; Xun, P.; Stevens, J.; White, E.; Gammon, M.D.; He, K. Antioxidant intake and pancreatic cancer risk: The Vitamins and Lifestyle (VITAL) Study. Cancer 2013, 119, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Dhakal, I.B.; Gross, M.D.; Kadlubar, F.F.; Anderson, K.E. Sequence variants in antioxidant defense and DNA repair genes, dietary antioxidants, and pancreatic cancer risk. Int. J. Mol. Epidemiol. Genet. 2011, 2, 236–244. [Google Scholar]
- Palozza, P.; Parrone, N.; Simone, R.; Catalano, A. Role of lycopene in the control of ROS-mediated cell growth: Implications in cancer prevention. Curr. Med. Chem. 2011, 18, 1846–1860. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Simone, R.; Catalano, A.; Monego, G.; Barini, A.; Mele, M.C.; Parrone, N.; Trombino, S.; Picci, N.; Ranelletti, F.O. Lycopene prevention of oxysterol-induced proinflammatory cytokine cascade in human macrophages: inhibition of NF-κB nuclear binding and increase in PPARγ expression. J. Nutr. Biochem. 2011, 22, 259–268. [Google Scholar] [CrossRef]
- Huang, C.S.; Fan, Y.E.; Lin, C.Y.; Hu, M.L. Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1. J. Nutr. Biochem. 2007, 18, 449–456. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef]
- Green, D.R. Death and NF-kappaB in T cell activation: Life at the edge. Mol. Cell 2003, 11, 551–552. [Google Scholar] [CrossRef]
- Lee, J.K.; Edderkaoui, M.; Truong, P.; Ohn, I.; Jang, K.T. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology 2007, 133, 1637–1648. [Google Scholar] [CrossRef]
- Fukuda, A.; Wang, S.C.; Morris, J.P., 4th; Folias, A.E.; Liou, A.; Kim, G.E.; Akira, S.; Boucher, K.M.; Firpo, M.A.; Mulvihill, S.J.; et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell 2011, 19, 441–455. [Google Scholar] [CrossRef]
- Lesina, M.; Kurkowski, M.U.; Ludes, K.; Rose-John, S.; Treiber, M.; Klöppel, G.; Yoshimura, A.; Reindl, W.; Sipos, B.; Akira, S.; et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011, 19, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.D.; Lim, J.W.; Kim, K.H.; Kim, H. Potential role of NADPH oxidase-mediated activation of Jak2/Stat3 and mitogen-activated protein kinases and expression of TGF-β1 in the pathophysiology of acute pancreatitis. Inflamm. Res. 2011, 60, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, E.C.; Edderkaoui, M.; Pandol, S.J.; Gukovsky, I.; Gukovskaya, A.S. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cell. J. Biol. Chem. 2004, 279, 34643–34654. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Mehrotra, A.; Kamboj, S.S. Lycopene prevents 3-nitropropionic acid-induced mitochondrial oxidative stress and dysfunctions in nervous system. Neurochem. Int. 2010, 57, 579–587. [Google Scholar] [CrossRef]
- Hantz, H.L.; Young, L.F.; Martin, K.R. Physiologically attainable concentrations of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Exp. Biol. Med. (Maywood) 2005, 230, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Galluzzi, L.; Kroemer, G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Leber, B.; Lin, J.; Andrews, D.W. Still embedded together binding to membranes regulates Bcl-2 protein interactions. Oncogene 2010, 29, 5221–5230. [Google Scholar] [CrossRef]
- Yang, C.L.; Ma, Y.G.; Xue, Y.X.; Liu, Y.Y.; Xie, H.; Qiu, G.R. Curcumin induces small cell lung cancer NCI-H446 cell apoptosis via the reactive oxygen species-mediated mitochondrial pathway and not the cell death receptor pathway. DNA Cell Biol. 2012, 31, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis: An update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Liu, J.X.; Zhang, J.H.; Li, H.H.; Lai, F.J.; Chen, K.J.; Chen, H.; Luo, J.; Guo, H.C.; Wang, Z.H.; Lin, S.Z. Emodin induces Panc-1 cell apoptosis via declining the mitochondrial membrane potential. Oncol. Rep. 2012, 28, 1991–1996. [Google Scholar] [CrossRef]
- Kim, A.; Im, M.; Hwang, Y.H.; Yang, H.J.; Ma, J.Y. Jaeumganghwa-Tang induces apoptosis via the mitochondrial pathway and Lactobacillus fermentation enhances its anti-cancer activity in HT1080 human fibrosarcoma cells. PLoS ONE 2015, 10, e0127898. [Google Scholar] [CrossRef]
- Sakamuru, S.; Li, X.; Matias, S.; Attene-Ramos, M.S.; Huang, R.; Lu, J.; Shou, L.; Shen, M.; Tice, R.R.; Austin, C.P.; et al. Application of a homogenous membrane potential assay to assess mitochondrial function. Physiol. Genom. 2012, 44, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.B.; Evers, B.M. Induction of cIAP-2 in human colon cancer cells through PKCδ/NF-κB. J. Biol. Chem. 2003, 278, 51091–51099. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Cusack, J.C.; Liu, R.; Baldwin, A.S. Control of inducible chemoresistance: Enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-κB. Nat. Med. 1999, 5, 412–417. [Google Scholar] [CrossRef]
- Liu, Z.G.; Hsu, H.; Goeddel, D.V.; Karin, M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 1996, 87, 565–576. [Google Scholar] [CrossRef]
- Dohi, T.; Okada, K.; Xia, F.; Wilford, C.E.; Samuel, T.; Welsh, K.; Marusawa, H.; Zou, H.; Armstrong, R.; Matsuzawa, S.; et al. An IAP-IAP complex inhibits apoptosis. J. Biol. Chem. 2004, 279, 34087–34090. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.H.; Plenchette, S.; Kern, C.J.; Mahoney, D.J.; Korneluk, R.G. The RING domain of cIAP1 mediates the degradation of RING-bearing inhibitor of apoptosis proteins by distinct pathways. Mol. Biol. Cell 2008, 19, 2729–2740. [Google Scholar] [CrossRef]
- Conze, D.B.; Albert, L.; Ferrick, D.A.; Goeddel, D.V.; Yeh, W.C.; Mak, T.; Ashwell, J.D. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol. Cell Biol. 2005, 25, 3348–3356. [Google Scholar] [CrossRef]
- LaCasse, E.C.; Baird, S.; Korneluk, R.G.; MacKenzie, A.E. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene 1998, 17, 3247–3259. [Google Scholar] [CrossRef]
- Roy, N.; Deveraux, Q.L.; Takahashi, R.; Salvesen, G.S.; Reed, J.C. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997, 16, 6914–6925. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Li, M. The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res. 2000, 10, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Dubrez-Daloz, L.; Dupoux, A.; Cartier, J. IAPs: More than just inhibitors of apoptosis proteins. Cell Cycle 2008, 7, 1036–1046. [Google Scholar] [CrossRef]
- Eckelman, B.P.; Salvesen, G.S.; Scott, F.L. Human inhibitor of apoptosis proteins: Why XIAP is the black sheep of the family. EMBO Rep. 2006, 7, 988–994. [Google Scholar] [CrossRef]
- Deveraux, Q.L.; Roy, N.; Stennicke, H.R.; Van Arsdale, T.; Zhou, Q.; Srinivasula, S.M.; Alnemri, E.S.; Salvesen, G.S.; Reed, J.C. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998, 17, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.; Dowling, C.; O’Neill, A.J.; Watson, R.W.G. Effects of cIAP-1, cIAP-2 and XIAP triple knockdown on prostate cancer cell susceptibility to apoptosis cell survival and proliferation. Mol. Cancer 2009, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lin, F.; Wang, X.; Gao, P.; Dong, K.; Zou, A.M.; Cheng, S.Y.; Wei, S.H.; Zhang, H.Z. Silencing the livin gene expression to inhibit proliferation and enhance chemosensitivity in tumor cells. Cancer Gene Ther. 2008, 15, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; Carvalho, L.; Bernstein, P.S.; Muir, G.J.; Zhao, D.Y.; Katz, N.B. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp. Biol. Med. (Maywood) 2002, 227, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Cooney, R.V.; Bertram, J.S. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: Relationship to their cancer chemopreventive action. Carcinogenesis 1991, 12, 2109–2114. [Google Scholar] [CrossRef]
- Drai, J.; Borel, P.; Faure, H.; Galabert, C.; Le Moël, G.; Laromiguière, M.; Fayol, V. Fasting plasma carotenoids concentrations in Crohn’s and pancreatic cancer patients compared to control subjects. Int. J. Vitam. Nutr. Res. 2009, 79, 87–94. [Google Scholar] [CrossRef]
- Abiaka, C.D.; Al-Awadi, F.M.; Al-Sayer, H.; Gulshan, S.; Behbehani, A.; Farghaly, M. Plasma micronutrient antioxidant in cancer patients. Cancer Detect. Prev. 2001, 25, 245–253. [Google Scholar]
- Huang, X.; Gao, Y.; Zhi, X.; Ta, N.; Jiang, H.; Zheng, J. Association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk: Evidence from epidemiologic studies. Sci. Rep. 2016, 6, 38936. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, C.J.; Trumbo, P.R.; Ellwood, K.C. The U.S. Food and Drug Administration’s evidence-based review for qualified health claims: Tomatoes, lycopene, and cancer. Natl. Cancer Inst. 2007, 99, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Nkondjock, A.; Ghadirian, P.; Johnson, K.C.; Krewski, D. Canadian Cancer Registries Epidemiology Research Group. Dietary intake of lycopene is associated with reduced pancreatic cancer risk. J. Nutr. 2005, 135, 592–597. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.; Lim, J.W.; Kim, H. Lycopene Inhibits Reactive Oxygen Species-Mediated NF-κB Signaling and Induces Apoptosis in Pancreatic Cancer Cells. Nutrients 2019, 11, 762. https://doi.org/10.3390/nu11040762
Jeong Y, Lim JW, Kim H. Lycopene Inhibits Reactive Oxygen Species-Mediated NF-κB Signaling and Induces Apoptosis in Pancreatic Cancer Cells. Nutrients. 2019; 11(4):762. https://doi.org/10.3390/nu11040762
Chicago/Turabian StyleJeong, Yoonseon, Joo Weon Lim, and Hyeyoung Kim. 2019. "Lycopene Inhibits Reactive Oxygen Species-Mediated NF-κB Signaling and Induces Apoptosis in Pancreatic Cancer Cells" Nutrients 11, no. 4: 762. https://doi.org/10.3390/nu11040762
APA StyleJeong, Y., Lim, J. W., & Kim, H. (2019). Lycopene Inhibits Reactive Oxygen Species-Mediated NF-κB Signaling and Induces Apoptosis in Pancreatic Cancer Cells. Nutrients, 11(4), 762. https://doi.org/10.3390/nu11040762





