Coffee Consumption and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies
Abstract
1. Introduction
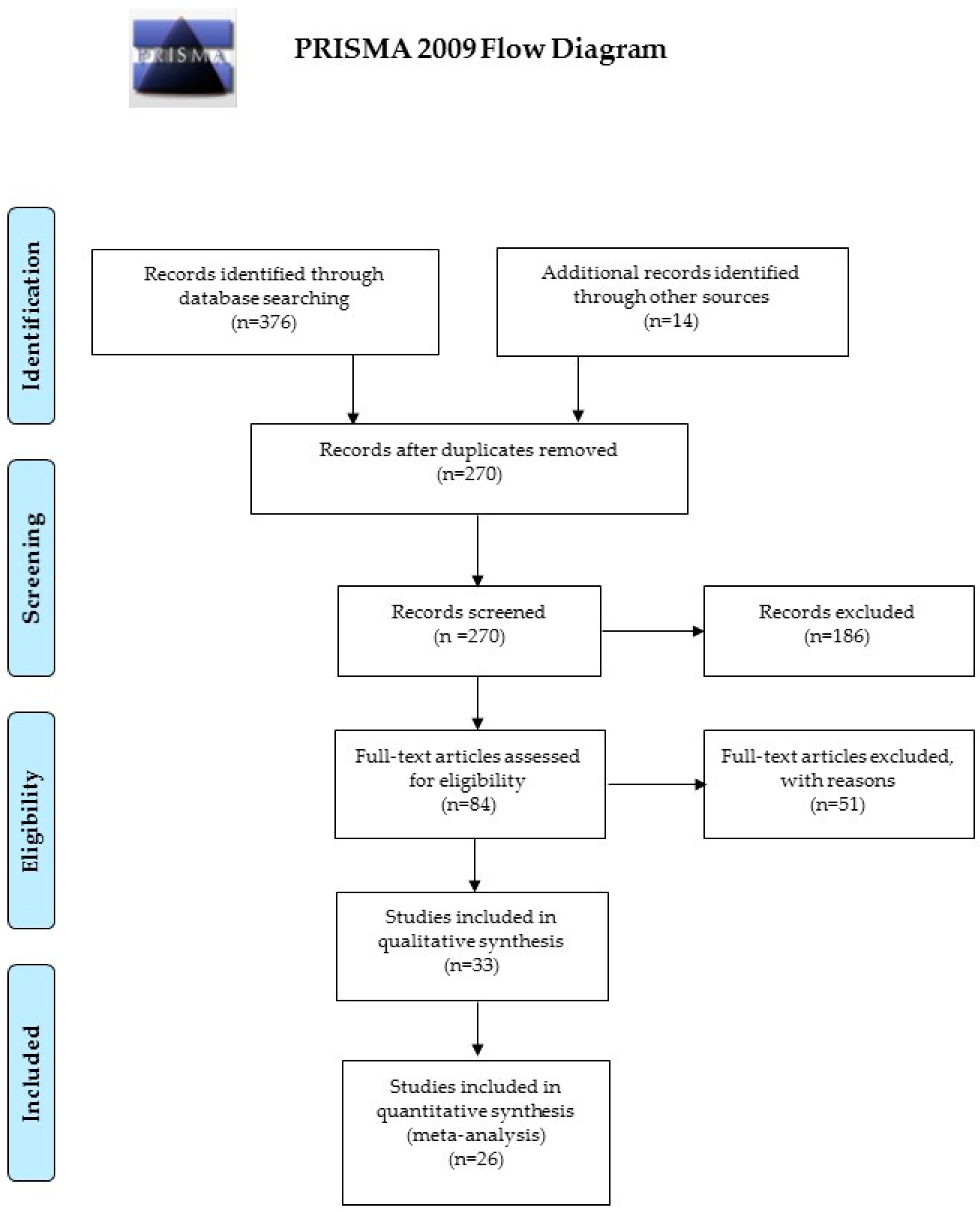
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Rustan, A.C.; Halvorsen, B.; Huggett, A.C.; Ranheim, T.; Drevon, C.A. Effect of coffee lipids (cafestol and kahweol) on regulation of cholesterol metabolism in HepG2 cells. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2140–2149. [Google Scholar] [CrossRef]
- Gaascht, F.; Dicato, M.; Diederich, M. Coffee provides a natural multitarget pharmacopeia against the hallmarks of cancer. Genes Nutr. 2015, 10, 51. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. The enigmatic effects of caffeine in cell cycle and cancer. Cancer Lett. 2007, 247, 26–39. [Google Scholar] [CrossRef]
- Nagao, M.; Fujita, Y.; Sugimura, T.; Kosuge, T. Methylglyoxal in beverages and foods: Its mutagenicity and carcinogenicity. IARC Sci. Publ. 1986, 70, 283–291. [Google Scholar]
- Nagao, M.; Fujita, Y.; Wakabayashi, K.; Nukaya, H.; Kosuge, T.; Sugimura, T. Mutagens in coffee and other beverages. Environ. Health Perspect. 1986, 67, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Wakabayashi, K.; Fujita, Y.; Tahira, T.; Ochiai, M.; Sugimura, T. Mutagenic compounds in soy sauce, Chinese cabbage, coffee and herbal teas. Prog. Clin. Biol. Res. 1986, 206, 55–62. [Google Scholar] [PubMed]
- Pericleous, M.; Mandair, D.; Caplin, M.E. Diet and supplements and their impact on colorectal cancer. J. Gastrointest. Oncol. 2013, 4, 409–423. [Google Scholar]
- Savolainen, H. Tannin content of tea and coffee. J. Appl. Toxicol. 1992, 12, 191–192. [Google Scholar] [CrossRef]
- Niseteo, T.; Komes, D.; Belščak-Cvitanović, A.; Horžić, D.; Budeč, M. Bioactive composition and antioxidant potential of different commonly consumed coffee brews affected by their preparation technique and milk addition. Food Chem. 2012, 134, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Valle, I.; Tramalloni, D.; Bragazzi, N.L. Cancer prevention: State of the art and future prospects. J. Prev. Med. Hyg. 2015, 56, E21–E27. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.K.; Bjelke, E.; Kvåle, G.; Heuch, I. Coffee drinking, mortality, and cancer incidence: Results from a Norwegian prospective study. J. Natl. Cancer Inst. 1986, 76, 823–831. [Google Scholar]
- Wu, A.H.; Paganini-Hill, A.; Ross, R.K.; Henderson, B.E. Alcohol, physical activity and other risk factors for colorectal cancer: A prospective study. Br. J. Cancer 1987, 55, 687–694. [Google Scholar] [CrossRef]
- Klatsky, A.L.; Armstrong, M.A.; Friedman, G.D.; Hiatt, R.A. The relations of alcoholic beverage use to colon and rectal cancer. Am. J. Epidemiol. 1988, 128, 1007–1015. [Google Scholar] [CrossRef]
- Stensvold, I.; Jacobsen, B.K. Coffee and cancer: A prospective study of 43,000 Norwegian men and women. Cancer Causes Control 1994, 5, 401–408. [Google Scholar] [CrossRef]
- Terry, P.; Bergkvist, L.; Holmberg, L.; Wolk, A. Coffee consumption and risk of colorectal cancer in a population based prospective cohort of Swedish women. Gut 2001, 49, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Goto, R.; Kobayashi, K.; Suzumura, S.; Nagata, Y.; Sonoda, T.; Sakauchi, F.; Washio, M.; Mori, M. Dietary habits and cancer mortality among middle aged and older Japanese living in Hokkaido, Japan by cancer site and sex. Asian Pac. J. Cancer Prev. 2004, 5, 58–65. [Google Scholar] [PubMed]
- Michels, K.B.; Willett, W.C.; Fuchs, C.S.; Giovannucci, E. Coffee, tea, and caffeine consumption and incidence of colon and rectal cancer. J. Natl. Cancer Inst. 2005, 97, 282–292. [Google Scholar] [CrossRef]
- Larsson, S.C.; Bergkvist, L.; Giovannucci, E.; Wolk, A. Coffee consumption and incidence of colorectal cancer in two prospective cohort studies of Swedish women and men. Am. J. Epidemiol. 2006, 163, 638–644. [Google Scholar] [CrossRef]
- Mucci, L.A.; Adami, H.O.; Wolk, A. Prospective study of dietary acrylamide and risk of colorectal cancer among women. Int. J. Cancer 2006, 118, 169–173. [Google Scholar] [CrossRef]
- Oba, S.; Shimizu, N.; Nagata, C.; Shimizu, H.; Kametani, M.; Takeyama, N.; Ohnuma, T.; Matsushita, S. The relationship between the consumption of meat, fat, and coffee and the risk of colon cancer: A prospective study in Japan. Cancer Lett. 2006, 244, 260–267. [Google Scholar] [CrossRef]
- Lee, K.J.; Inoue, M.; Otani, T.; Iwasaki, M.; Sasazuki, S.; Tsugane, S. JPHC Study Group. Coffee consumption and risk of colorectal cancer in a population-based prospective cohort of Japanese men and women. Int. J. Cancer 2007, 121, 1312–1318. [Google Scholar] [CrossRef]
- Naganuma, T.; Kuriyama, S.; Akhter, M.; Kakizaki, M.; Nakaya, N.; Matsuda-Ohmori, K.; Shimazu, T.; Fukao, A.; Tsuji, I. Coffee consumption and the risk of colorectal cancer: A prospective cohort study in Japan. Int. J. Cancer 2007, 120, 1542–1547. [Google Scholar] [CrossRef]
- Bidel, S.; Hu, G.; Jousilahti, P.; Antikainen, R.; Pukkala, E.; Hakulinen, T.; Tuomilehto, J. Coffee consumption and risk of colorectal cancer. Eur. J. Clin. Nutr. 2010, 64, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.M.; Johansson, I.; Lenner, P.; Lindahl, B.; Van Guelpen, B. Consumption of filtered and boiled coffee and the risk of incident cancer: A prospective cohort study. Cancer Causes Control 2010, 21, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Simons, C.C.; Leurs, L.J.; Weijenberg, M.P.; Schouten, L.J.; Goldbohm, R.A.; van den Brandt, P.A. Fluid intake and colorectal cancer risk in the Netherlands Cohort Study. Nutr. Cancer 2010, 62, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Albanes, D.; Beeson, W.L.; van den Brandt, P.A.; Buring, J.E.; Flood, A.; Freudenheim, J.L.; Giovannucci, E.L.; Goldbohm, R.A.; Jaceldo-Siegl, K.; et al. Risk of colon cancer and coffee, tea, and sugar-sweetened soft drink intake: Pooled analysis of prospective cohort studies. J. Natl. Cancer Inst. 2010, 102, 771–783. [Google Scholar] [CrossRef]
- Sinha, R.; Cross, A.J.; Daniel, C.R.; Graubard, B.I.; Wu, J.W.; Hollenbeck, A.R.; Gunter, M.J.; Park, Y.; Freedman, N.D. Caffeinated and decaffeinated coffee and tea intake and risk of colorectal cancer in a large prospective study. Am. J. Clin. Nutr. 2012, 96, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Dominianni, C.; Huang, W.Y.; Berndt, S.; Hayes, R.B.; Ahn, J. Prospective study of the relationship between coffee and tea with colorectal cancer risk: The PLCO Cancer Screening Trial. Br. J. Cancer 2013, 109, 1352–1359. [Google Scholar] [CrossRef]
- Perrigue, M.M.; Kantor, E.D.; Hastert, T.A.; Patterson, R.; Potter, J.D.; Neuhouser, M.L.; White, E. Eating frequency and risk of colorectal cancer. Cancer Causes Control 2013, 24, 2107–2115. [Google Scholar] [CrossRef]
- Dik, V.K.; Bueno-de-Mesquita, H.B.; Van Oijen, M.G.; Siersema, P.D.; Uiterwaal, C.S.; Van Gils, C.H.; Van Duijnhoven, F.J.; Cauchi, S.; Yengo, L.; Froguel, P.; et al. Coffee and tea consumption, genotype-based CYP1A2 and NAT2 activity and colorectal cancer risk-results from the EPIC cohort study. Int. J. Cancer 2014, 135, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Hartman, T.J.; Tangrea, J.A.; Pietinen, P.; Malila, N.; Virtanen, M.; Taylor, P.R.; Albanes, D. Tea and coffee consumption and risk of colon and rectal cancer in middle-aged Finnish men. Nutr. Cancer 1998, 31, 41–48. [Google Scholar] [CrossRef]
- Peterson, S.; Yuan, J.M.; Koh, W.P.; Sun, C.L.; Wang, R.; Turesky, R.J.; Yu, M.C. Coffee intake and risk of colorectal cancer among Chinese in Singapore: The Singapore Chinese Health Study. Nutr. Cancer 2009, 62, 21–29. [Google Scholar] [CrossRef]
- Yamada, H.; Kawado, M.; Aoyama, N.; Hashimoto, S.; Suzuki, K.; Wakai, K.; Suzuki, S.; Watanabe, Y.; Tamakoshi, A.; JACC Study Group. Coffee consumption and risk of colorectal cancer: The Japan Collaborative Cohort Study. J. Epidemiol. 2014, 24, 370–378. [Google Scholar] [CrossRef]
- Groessl, E.J.; Allison, M.A.; Larson, J.C.; Ho, S.B.; Snetslaar, L.G.; Lane, D.S.; Tharp, K.M.; Stefanick, M.L. Coffee Consumption and the Incidence of Colorectal Cancer in Women. J. Cancer Epidemiol. 2016, 2016, 6918431. [Google Scholar] [CrossRef]
- Lukic, M.; Licaj, I.; Lund, E.; Skeie, G.; Weiderpass, E.; Braaten, T. Coffee consumption and the risk of cancer in the Norwegian Women and Cancer (NOWAC) Study. Eur. J. Epidemiol. 2016, 31, 905–916. [Google Scholar] [CrossRef]
- Kashino, I.; Akter, S.; Mizoue, T.; Sawada, N.; Kotemori, A.; Matsuo, K.; Oze, I.; Ito, H.; Naito, M.; Nakayama, T.; et al. Coffee drinking and colorectal cancer and its subsites: A pooled analysis of 8 cohort studies in Japan. Int. J. Cancer 2018, 143, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef] [PubMed]
- Platt, D.E.; Ghassibe-Sabbagh, M.; Salameh, P.; Salloum, A.K.; Haber, M.; Mouzaya, F.; Gauguier, D.; Al-Sarraj, Y.; El-Shanti, H.; Zalloua, P.A.; et al. Caffeine Impact on Metabolic Syndrome Components Is Modulated by a CYP1A2 Variant. Ann. Nutr. Metab. 2016, 68, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.M.; Paing, S.S.; Li, H.; Pavanni, R.; Yuen, Y.; Zhao, Y.; Tan, E.K. Differential effect of caffeine intake in subjects with genetic susceptibility to Parkinson’s Disease. Sci. Rep. 2015, 5, 15492. [Google Scholar] [CrossRef]
- Renda, G.; Zimarino, M.; Antonucci, I.; Tatasciore, A.; Ruggieri, B.; Bucciarelli, T.; Prontera, T.; Stuppia, L.; De Caterina, R. Genetic determinants of blood pressure responses to caffeine drinking. Am. J. Clin. Nutr. 2012, 95, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.H.; Lill, C.M.; Lee, P.C.; Hansen, J.; Lassen, C.F.; Bertram, L.; Greene, N.; Sinsheimer, J.S.; Ritz, B. Gene-Environment Interaction in Parkinson’s Disease: Coffee, ADORA2A, and CYP1A2. Neuroepidemiology 2016, 47, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, J.; Vitonis, A.F.; Terry, K.L.; De Vivo, I.; Cramer, D.W.; Hankinson, S.E.; Tworoger, S.S. Coffee intake, variants in genes involved in caffeine metabolism, and the risk of epithelial ovarian cancer. Cancer Causes Control 2009, 20, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, J.; Ghadirian, P.; El-Sohemy, A.; Lynch, H.T.; Snyder, C.; Daly, M.; Domchek, S.; Randall, S.; Karlan, B.; Zhang, P.; et al. The CYP1A2 genotype modifies the association between coffee consumption and breast cancer risk among BRCA1 mutation carriers. Cancer Epidemiol. Biomark. Prev. 2007, 16, 912–916. [Google Scholar] [CrossRef]
- Cornelis, M.C.; El-Sohemy, A.; Kabagambe, E.K.; Campos, H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA 2006, 295, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Coffee and Health: A review of recent Human Research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, K.; Kiedrowski, M.; Gajewska, D.; Deptała, A.; Włodarek, D. Wpływ spożycia warzyw, owoców, herbaty oraz kawy na rozwój raka jelita grubego [The impact of the consumption of vegetables, fruits, coffee and tea on the development of colorectal carcinoma]. Pol. Merkur. Lekarski. 2016, 41, 205–208. (In Polish) [Google Scholar] [PubMed]
| Search Strategy | Details |
|---|---|
| Search string | (coffee OR caffeine) AND (tumor OR cancer OR neoplasm) AND (colon OR rectal OR colorectal) |
| Databases | PubMed/MEDLINE, Scopus |
| Inclusion criteria | P (patients/population): general population/patients suffering from colorectal cancer I (intervention/exposure): subjects consuming coffee C (comparisons/comparators): coffee consumers versus non-consumers; different kinds of coffee (caffeinated/decaffeinated) O (outcome): incidence of colorectal cancer S (study design): prospective study |
| Exclusion criteria | Experimental studies investigating in vitro or animal models Study design: editorial, commentaries, expert opinions, letters to editor, review articles, original non-prospective studies, articles with insufficient details |
| Time filter | None (from inception) |
| Language filter | None (any language) |
| First Authors (year) | Country | Study Subject | Coffee Consumption (“high” vs. “low”) | No. Cases |
|---|---|---|---|---|
| Jacobsen (1986) [12] | Sweden | All (16,555) F (2891); M (13,664) | ≥7 cups/d vs. ≤2 cups/d | 97 CC—63 RC |
| Wu (1987) [13] | USA | All (11,632) F (7456); M (4163) | ≥4 cups/d vs. ≤1 cup/d | NA (CRC) |
| Klatsky (1988) [14] | USA | All (10,572) | Continuous variable (cups/d) | 203 CC—66 RC |
| Stensvold (1994) [15] | Sweden | F (21,238); M (21,735) | ≥7 cups/d vs. ≤2 cups/d | F: 52 CC—38 RC; M: 78 CC—41 RC |
| Terry (2001) [16] | Sweden | F (61,463) | ≥4 cups/d vs. <1 cup/d | 291 CC—159 RC—460 CRC |
| Khan (2004) [17] | Japan | F (1634); M (1524) | Continuous variable (cups/d) | F: 14 CRC; M: 15 CRC |
| Michels (2005) [18] | USA | All (133,893) F (87,794); M (46,099) | >5 cups/d vs. none (caffeinated, decaffeinated) | Caffeinated: 1170 CC—260 RC—1431 CRC Decaffeinated: 913 CC—224 RC—1138 CRC |
| Larsson (2006) [19] | Sweden | All (106,739) | ≥4 cups/d vs. <1 cup/d | 843 CC—440 RC—1279 CRC |
| Mucci (2006) [20] | Sweden | F (61,467) | ≥4 cups/d vs. ≤1 cup/d | 504 CC—237 RC—741 CRC |
| Oba (2006) [21] | Japan | F (16,327); M (13,894) | ≥1 cups/d vs. none | F: 102 CC; M: 111 CC |
| Lee (2007) [22] | Japan | F (50,139); M (46,023) | ≥3 cups/d vs. none | F: 286 CC—151 RC—437 CRC M: 174 CC—102 RC—276 CRC |
| Naganuma (2007) [23] | Japan | All (47,605) F (24,769); M (22,836) | ≥3 cups/d vs. none | ALL: 281 CC—180 RC—457 CRC F: 106 CC—68 RC—173 CRC M: 175 CC—112 RC—284 CRC |
| Bidel (2010) [24] | Finland | All (60,041) F (30,882); M (29,159) | ≥10 cups/d vs. none | ALL: 333 CC—252 RC—538 CRC F: 167 CC—123 RC—271 CRC M: 166 CC—129 RC—267 CRC |
| Nilsson (2010) [25] | Sweden | All (64,603) | ≥4 cups/d vs. <1 cup/d | 321 CRC |
| Simons (2010) [26] | Netherlands | F (62,573) M (58,279) | >6 cups/d vs. ≤2 cups/d | F: 173 RC—939 CRC M: 322 RC—1260 CRC |
| Zhang (2010) [27] | Multi-center (conducted in USA and in Europe) | All (731,441) | High quintile vs. low quintile | 5,604 CC |
| Sinha (2012) [28] | USA | All (489,706) | ≥6 cups/d vs. none (all, caffeinated, decaffeinated) | 5,072 CC—2863 Prox CC—1993 Distal CC—1874 RC—6946 CRC |
| Dominianni (2013) [29] | USA | All (57,398) | ≥4 cups/d vs. none | 681 CRC |
| Perrigue (2013) [30] | USA | All (67,912) | ≥7 cups/d vs. <7 cups/d | 409 CRC |
| Dik (2014) [31] | EPIC study (Europe) | All (521,448) F (365,014); M (156,434) | High quintile vs. low quintile (all, caffeinated, decaffeinated) | 2691 CC—1242 Prox CC—1202 Distal CC—1543 RC—4234 CRC |
| Hartmann (2014) [32] | Finland | M (27,111) | >6 cups/d vs. ≤4 cups/d | 106 CC—79 RC |
| Peterson (2014) [33] | Singapore | All (61,321) | ≥2 cups/d vs. <1 cup/d | 591 CC—370 RC |
| Yamada (2014) [34] | Japan | F (34,614) M (23,607) | ≥4 cups/d vs. <1 cup/d | F: 332 CC—112 RC—444 CRC M: 355 CC—202 RC—557 CRC |
| Groessl (2016) [35] | USA | F (83,972) | ≥4 cups/d vs. none | 1,083 CC—160 RC—12,852 CRC |
| Lukic (2016) [36] | Norway | F (79,461) | >7 cups/d vs. ≤1 cup/d | 1266 CRC |
| Kashino (2018) [37] | Japan | F (170,388) M (149,934) | ≥3 cups/d vs. <1 cup/d | F: 1963 CC—770 RC—2689 CRC M: 2619 CC—1402 RC—4022 CRC |
| Tumor and Geographic Provenience of the Studies | Men and Women | Men | Women | |
|---|---|---|---|---|
| RR [95%CI]; (N. Studies) | RR [95%CI]; (N. Studies) | RR [95%CI]; (N. Studies) | ||
| CRC | All | 0.96 [0.88–1.03]: (8) | 0.96 [0.88–1.04]; (9) | 1.06 [0.97–1.14]; (13) |
| EU studies only | 1.07 [0.96–1.17]; (4) | 0.93 [0.80–1.06]; (3) | 1.10 [0.98–1.22]; (6) | |
| Asian studies only | NA | 0.97 [0.87–1.08]; (5) | 0.94 [0.78–1.09]; (5) | |
| USA studies only | 0.83 [0.72–0.95]; (3) | NA | 1.14 [0.92–1.36]; (2) | |
| Caffeinated coffee | 0.96 [0.77–1.17]; (3) | NA | NA | |
| Decaffeinated coffee | 0.88 [0.78–0.97]; (3) | NA | NA | |
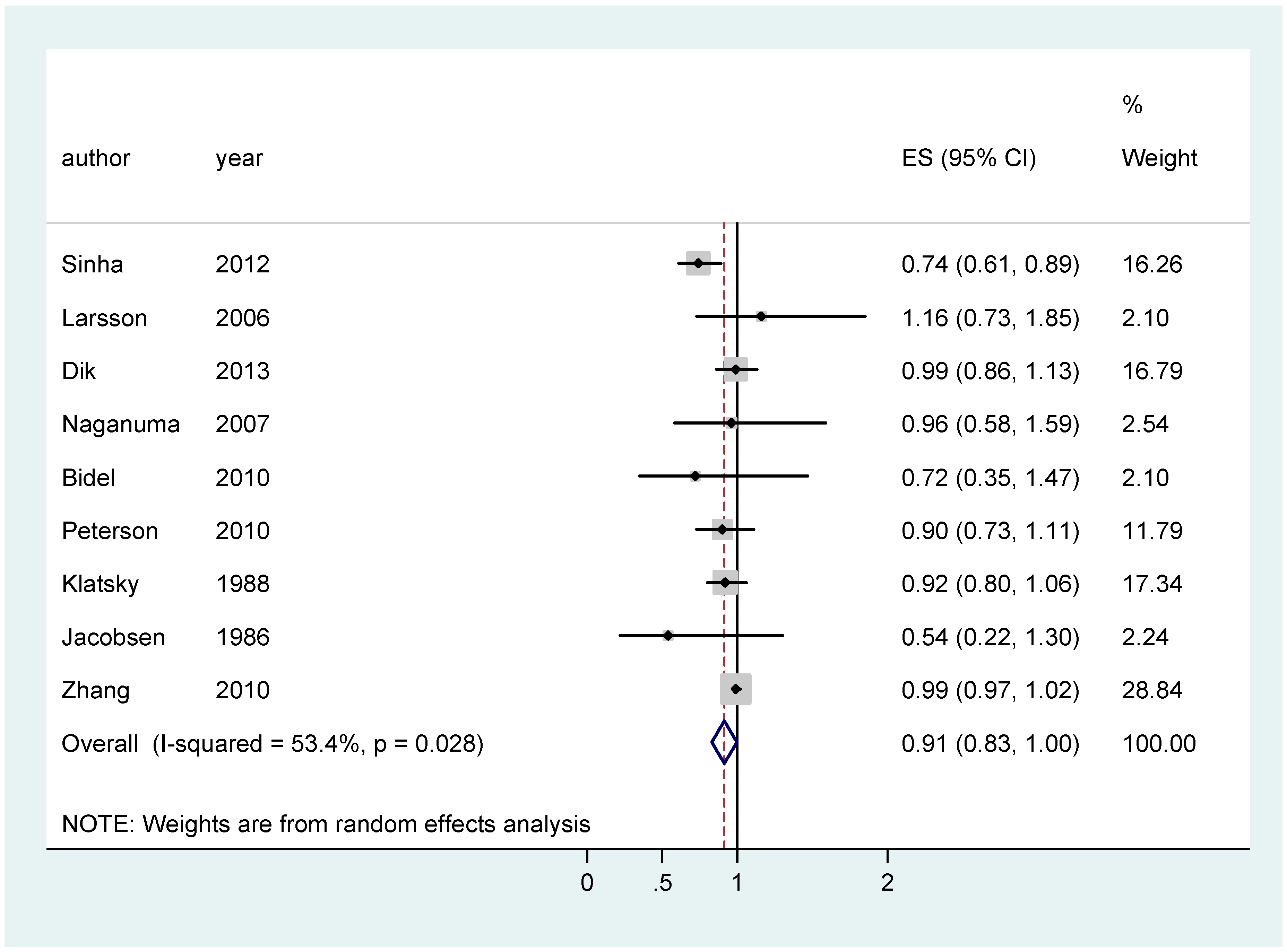
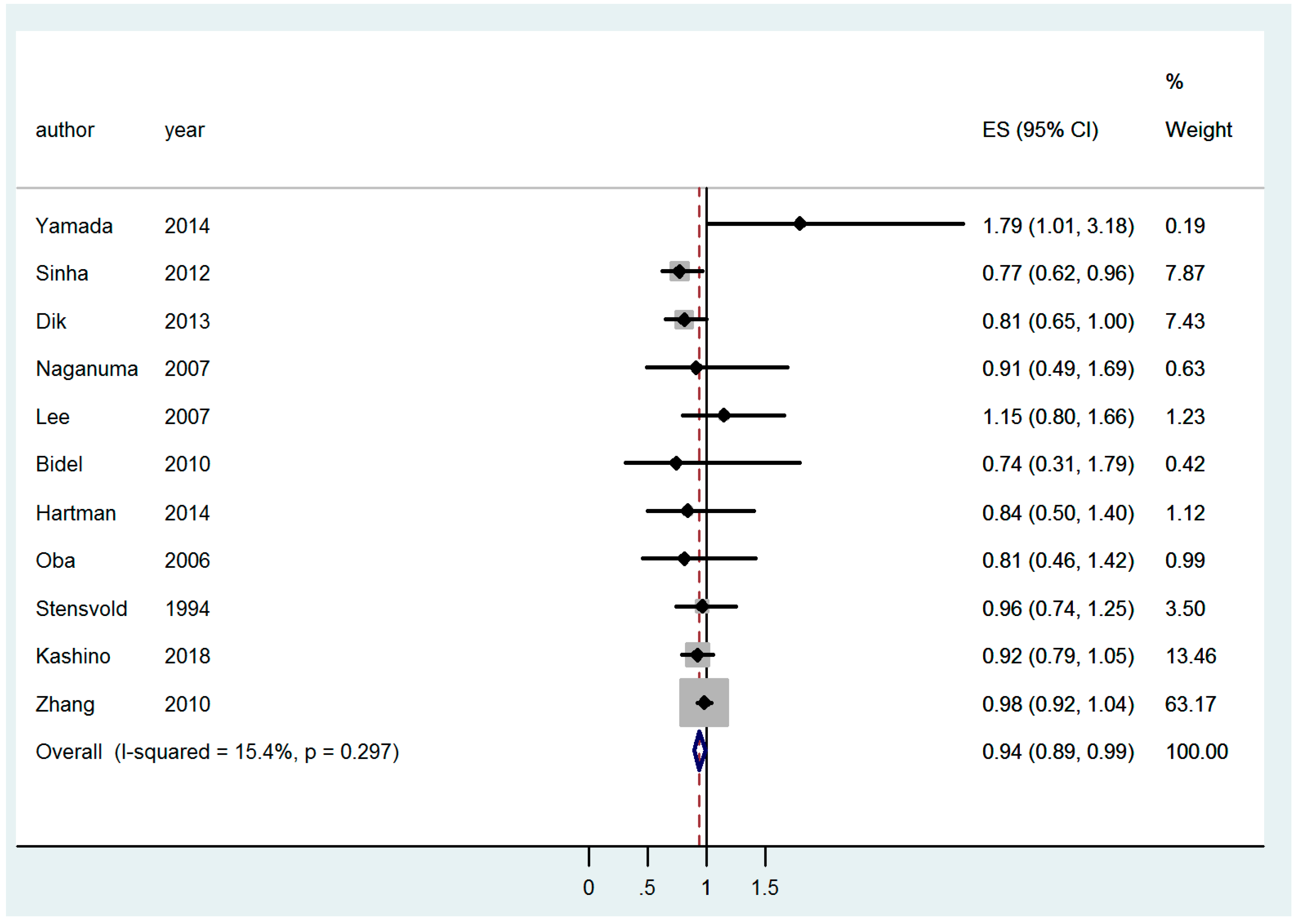
| CC | All | 0.91 [0.83–0.998]; (9) | 0.94 [0.89–0.99]; (11) | 0.92 [0.80–1.03]; (13) |
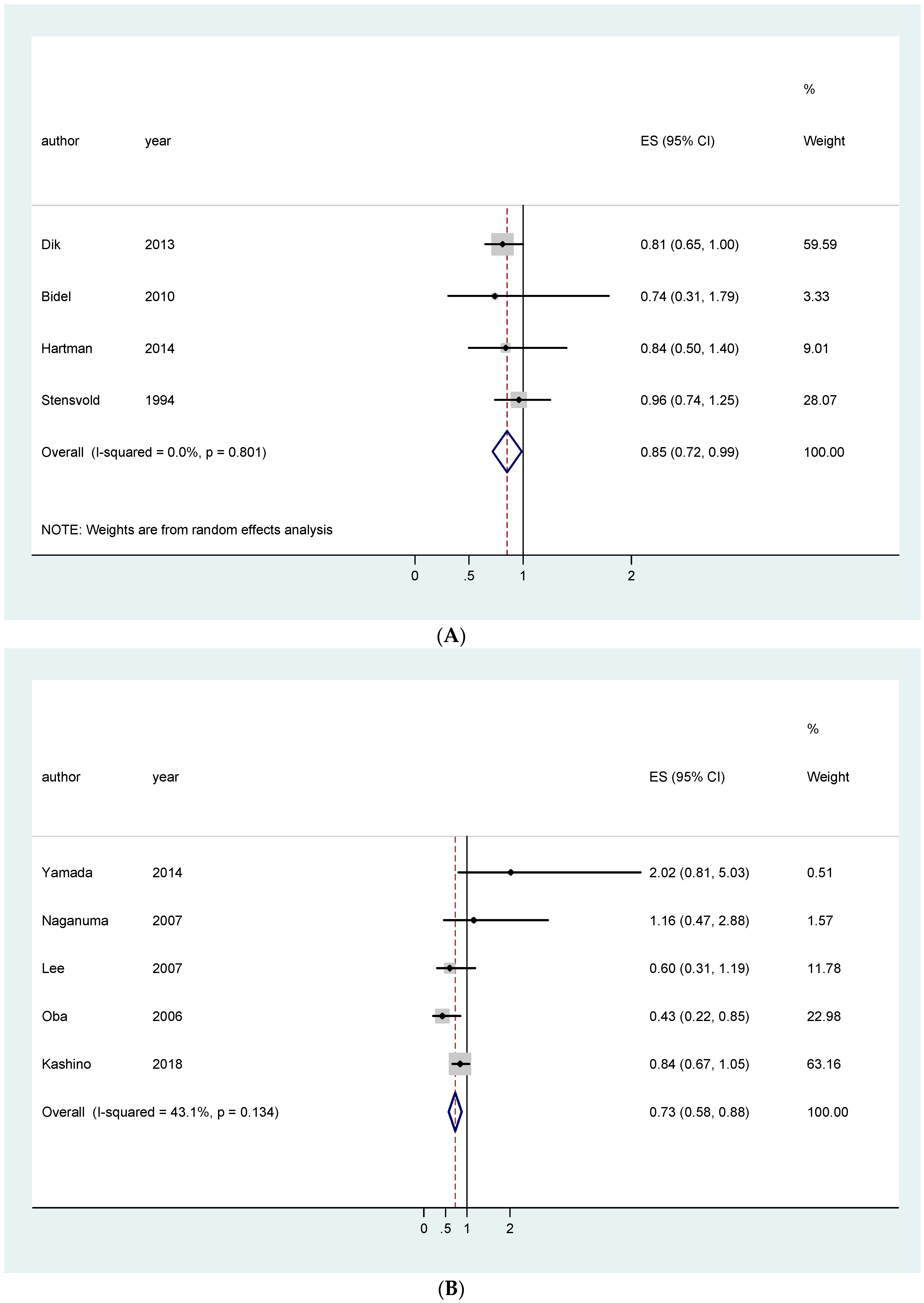
| EU studies only | 0.96 [0.84–1.09]; (4) | 0.85 [0.72–0.99]; (4) | 1.05 [0.93–1.18]; (5) | |
| Asian studies only | 0.91 [0.73–1.09]; (2) | 0.94 [0.82–1.06]; (5) | 0.73 [0.58–0.88]; (5) | |
| USA studies only | 0.83 [0.66–1.01]; (2) | NA | 0.90 [0.38–1.42]; (2) | |
| Caffeinated coffee | 0.92 [0.68–1.15]; (3) | NA | NA | |
| Decaffeinated coffee | 0.93 [0.81–1.05]; (3) | NA | NA | |
| Distal CC | All | 0.98 [0.95–1.02]; (5) | 0.94 [0.87–1.01]; (5) | 1.00 [0.96–1.04]; (6) |
| EU studies only | NA | 0.77 [0.57–0.98]; (2) | 1.09 [0.82–1.36]; (3) | |
| Asian studies only | NA | 0.83 [0.60–1.05]; (2) | 0.91 [0.56–1.25]; (2) | |
| USA studies only | 0.88 [0.65–1.12]; (2) | NA | NA | |
| Caffeinated coffee | 0.99 [0.79–1.91]; (2) | NA | NA | |
| Decaffeinated coffee | 1.05 [0.79–1.32]; (2) | NA | NA | |
| Proximal CC | All | 0.93 [0.73–1.15]; (5) | 0.98 [0.92–1.04]; (5) | 0.99 [0.96–1.03]; (6) |
| EU studies only | NA | 0.90 [0.66–1.14]; (2) | 1.17 [0.90–1.44]; (3) | |
| Asian studies only | NA | 1.08 [0.83–1.32]; (2) | 0.85 [0.57–1.13]; (2) | |
| USA studies only | 0.92 [0.23–1.60]; (2) | NA | NA | |
| Caffeinated coffee | 0.85 [0.32–1.36]; (2) | NA | NA | |
| Decaffeinated coffee | 0.86 [0.66–1.06]; (2) | NA | NA | |
| RC | All | 1.00 [0.89–1.11]; (9) | 1.01 [0.87–1.14]; (9) | 1.08 [0.94–1.23]; (11) |
| EU studies only | 1.17 [0.97–1.37]; (4) | 0.96 [0.78–1.14]; (5) | 1.04 [0.87–1.21]; (6) | |
| Asian studies only | 1.04 [0.79–1.29]; (2) | 1.07 [0.85–1.29]; (4) | 1.28 [0.96–1.60]; (4) | |
| USA studies only | 0.88 [0.72–1.04]; (3) | NA | NA | |
| Caffeinated coffee | 1.18 [0.98–1.38]; (3) | NA | NA | |
| Decaffeinated coffee | 0.71 [0.41–1.01]; (3) | NA | NA | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartini, M.; Bragazzi, N.L.; Spagnolo, A.M.; Schinca, E.; Ottria, G.; Dupont, C.; Cristina, M.L. Coffee Consumption and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2019, 11, 694. https://doi.org/10.3390/nu11030694
Sartini M, Bragazzi NL, Spagnolo AM, Schinca E, Ottria G, Dupont C, Cristina ML. Coffee Consumption and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients. 2019; 11(3):694. https://doi.org/10.3390/nu11030694
Chicago/Turabian StyleSartini, Marina, Nicola Luigi Bragazzi, Anna Maria Spagnolo, Elisa Schinca, Gianluca Ottria, Chiara Dupont, and Maria Luisa Cristina. 2019. "Coffee Consumption and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies" Nutrients 11, no. 3: 694. https://doi.org/10.3390/nu11030694
APA StyleSartini, M., Bragazzi, N. L., Spagnolo, A. M., Schinca, E., Ottria, G., Dupont, C., & Cristina, M. L. (2019). Coffee Consumption and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients, 11(3), 694. https://doi.org/10.3390/nu11030694







