Nutrition and Risk of Stroke
Abstract
1. Introduction and Background
2. Issues to Be Discussed
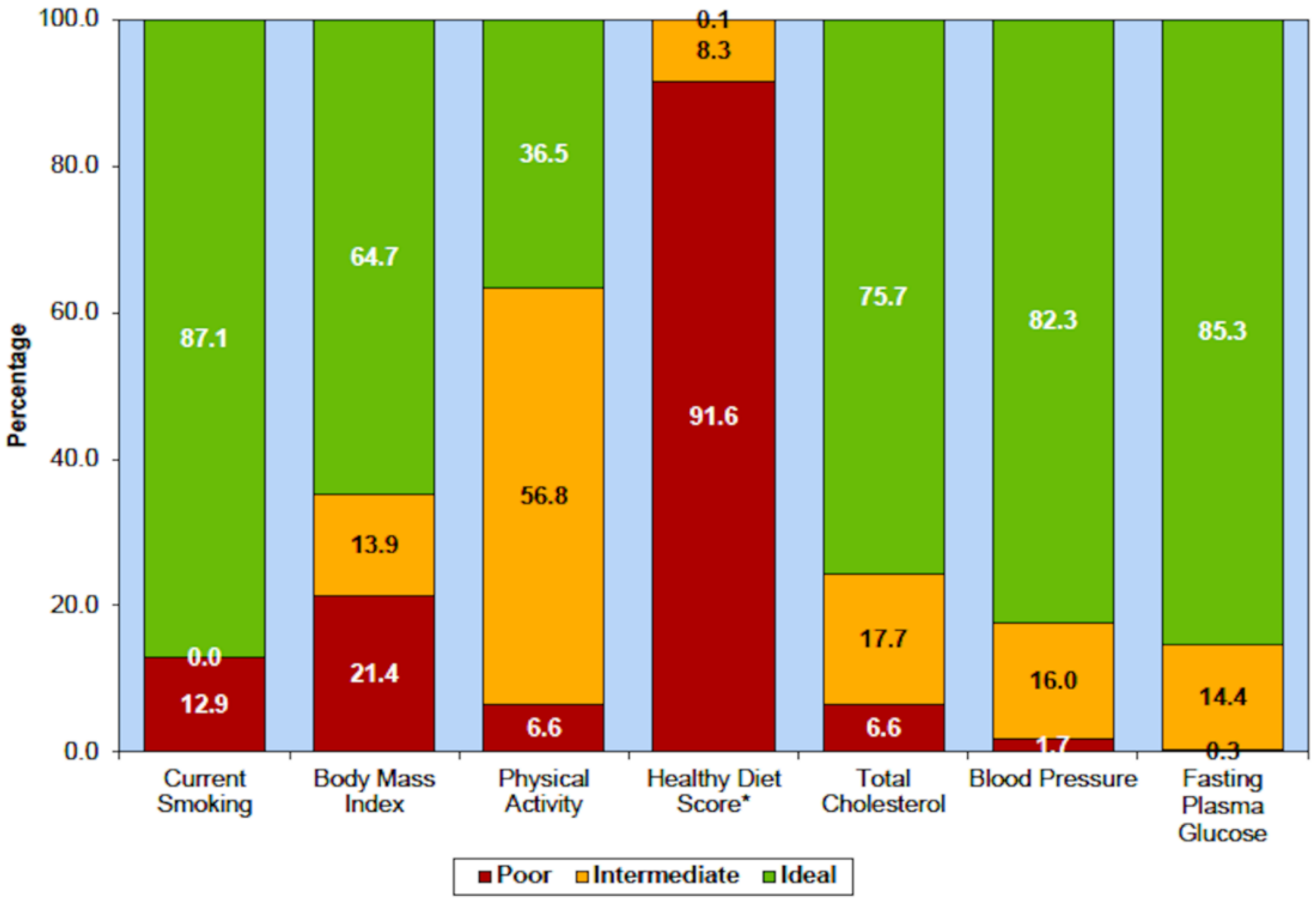
2.1. Diet
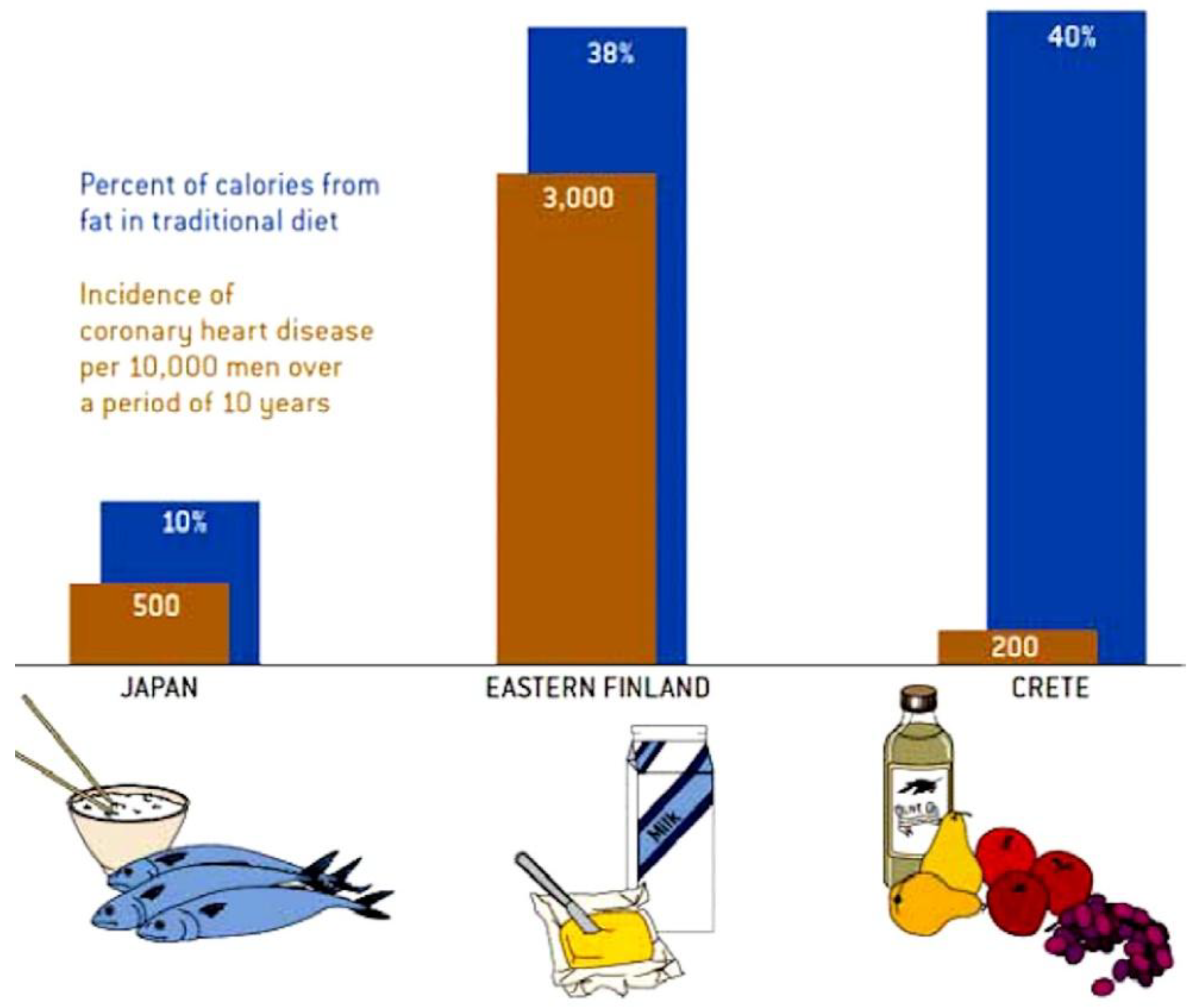
2.1.1. Mediterranean Diet
2.1.2. Maintenance of a Healthy Weight
2.2. Dietary Cholesterol
2.3. Intestinal Microbiome
Consumption of Egg Yolk
2.4. Salt Intake
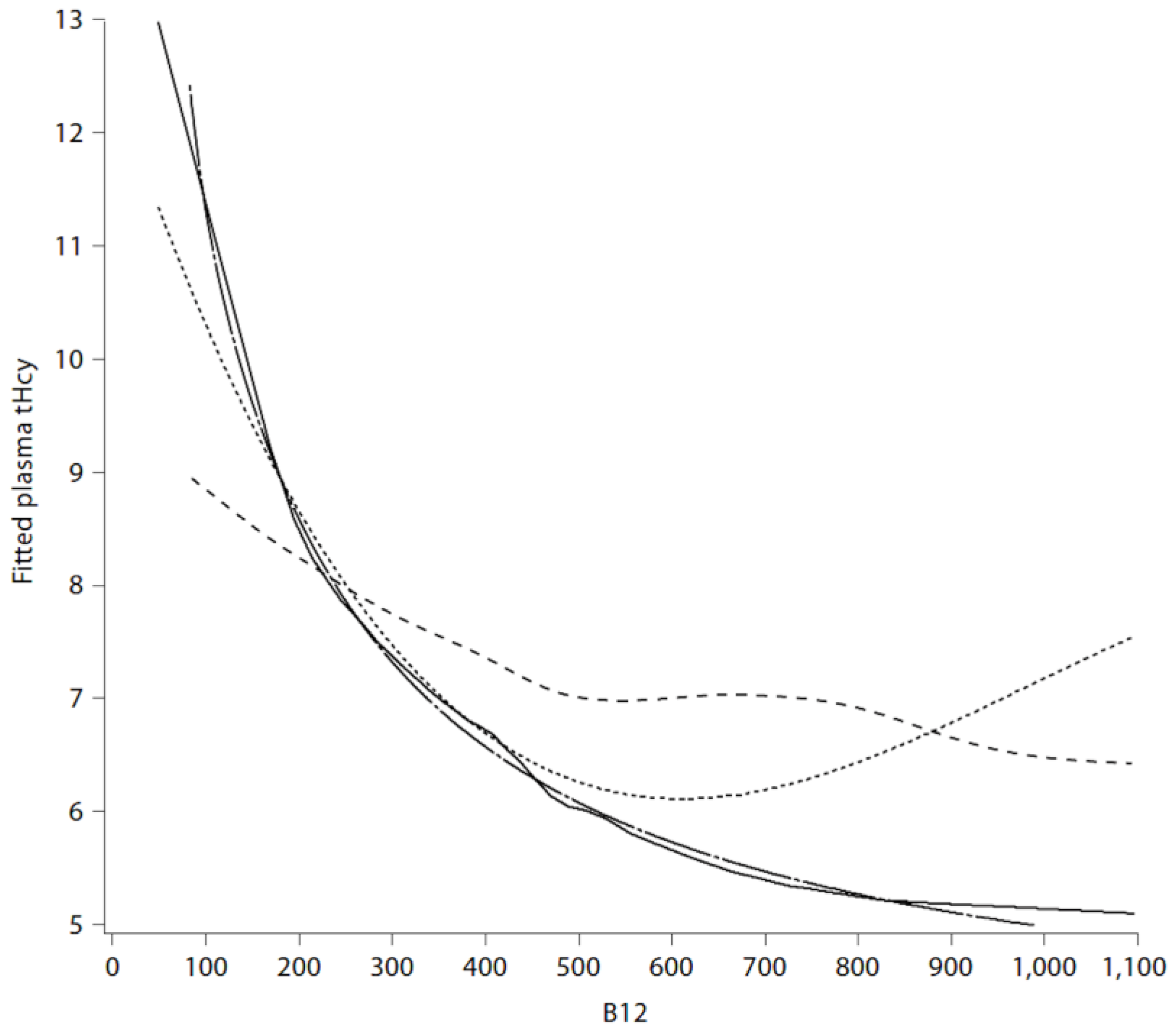
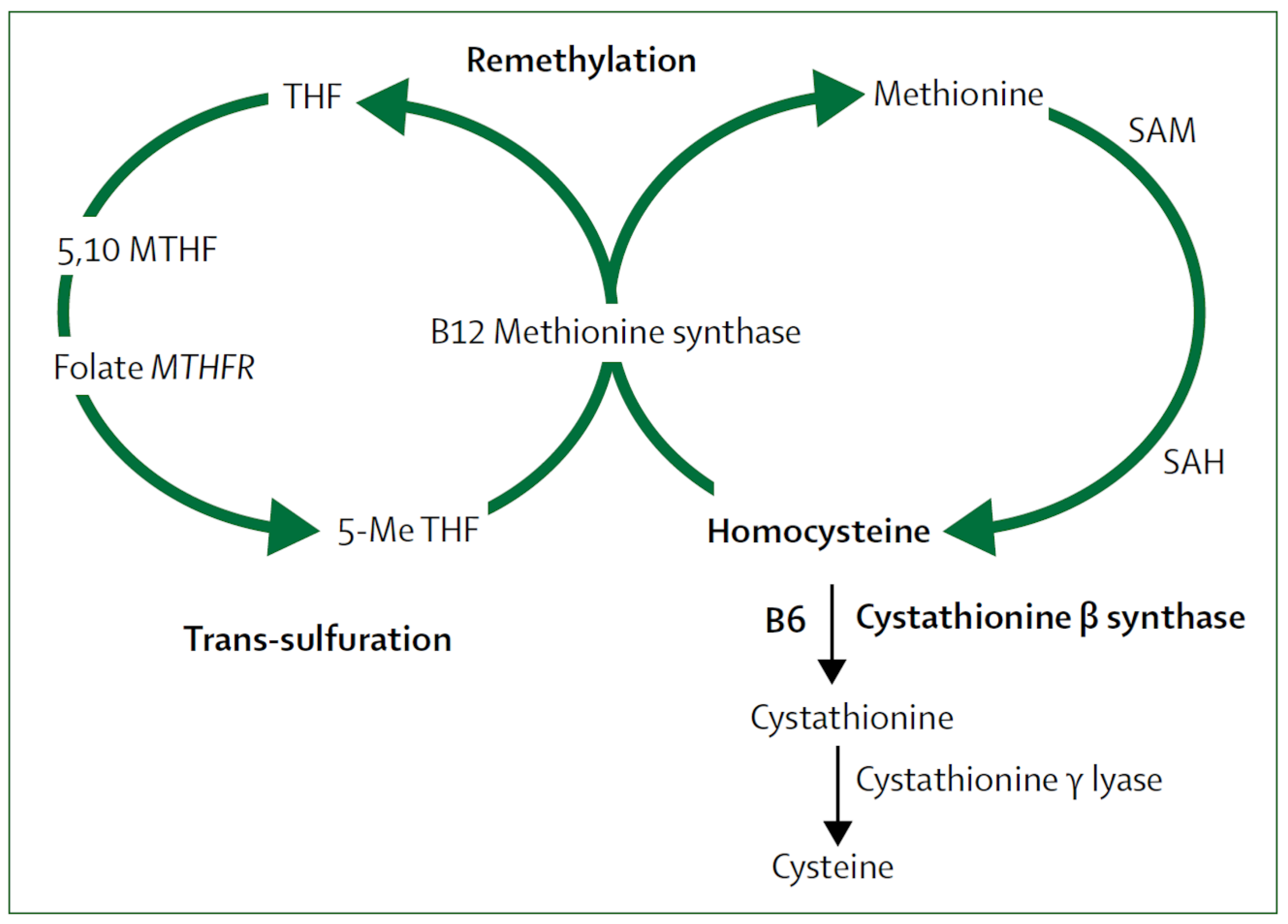
2.5. Metabolic B12 Deficiency
2.6. B Vitamins for Stroke Prevention
2.7. Other Vitamins and Supplements Including Omega 3 Fatty Acids
3. Discussion
4. Conclusions
Funding
Conflicts of Interest
References
- Chiuve, S.E.; Rexrode, K.M.; Spiegelman, D.; Logroscino, G.; Manson, J.E.; Rimm, E.B. Primary prevention of stroke by healthy lifestyle. Circulation 2008, 118, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Akesson, A.; Larsson, S.C.; Discacciati, A.; Wolk, A. Low-risk diet and lifestyle habits in the primary prevention of myocardial infarction in men: A population-based prospective cohort study. J. Am. Coll. Cardiol. 2014, 64, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gao, R.; Liu, L.; Zhu, M.; Wang, W.; Wang, Y.; Wu, Z.; Li, H.; Zheng, Z.; Jiang, L.; et al. Outline of the report on cardiovascular diseases in China, 2014. Eur. Heart J. Suppl. 2016, 18, F2–F11. [Google Scholar]
- Jenkins, D.J.A.; Spence, J.D.; Giovannucci, E.L.; Kim, Y.I.; Josse, R.; Vieth, R.; Blanco Mejia, S.; Viguiliouk, E.; Nishi, S.; Sahye-Pudaruth, S.; et al. Supplemental Vitamins and Minerals for CVD Prevention and Treatment. J. Am. Coll. Cardiol. 2018, 71, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Stampfer, M.J. Rebuilding the food pyramid. Sci. Am. 2003, 288, 64–71. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M.; Delaye, J.; Guidollet, J.; Jacquard, F.; Mamelle, N.; Martin, J.L.; Monjaud, I.; Salen, P.; Toubol, P. Cretan Mediterranean diet for prevention of coronary heart disease. Am. J. Clin. Nutr. 1995, 61, 1360S–1367S. [Google Scholar] [CrossRef]
- Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4,444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389. [Google Scholar]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Neelakantan, N.; Martin-Calvo, N.; Koh, W.P.; Yuan, J.M.; Bonaccio, M.; Iacoviello, L.; Martinez-Gonzalez, M.A.; Qin, L.Q.; van Dam, R.M. Adherence to the Mediterranean diet and risk of stroke and stroke subtypes. Eur. J. Epidemiol. 2019. [Google Scholar] [CrossRef]
- Dinsa, G.D.; Goryakin, Y.; Fumagalli, E.; Suhrcke, M. Obesity and socioeconomic status in developing countries: A systematic review. Obes. Rev. 2012, 13, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.M.; Sung, J.; Davey Smith, G.; Ebrahim, S. Body mass index and ischemic and hemorrhagic stroke: A prospective study in Korean men. Stroke 2004, 35, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Prospective Studies, C.; Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef]
- Viera, A.J.; Antonelli, R. Potential effect of physical activity calorie equivalent labeling on parent fast food decisions. Pediatrics 2015, 135, e376–e382. [Google Scholar] [CrossRef]
- Willcox, D.C.; Scapagnini, G.; Willcox, B.J. Healthy aging diets other than the Mediterranean: A focus on the Okinawan diet. Mech. Ageing Dev. 2014, 136–137, 148–162. [Google Scholar] [CrossRef]
- Willcox, B.J.; Willcox, D.C. Caloric restriction, caloric restriction mimetics, and healthy aging in Okinawa: Controversies and clinical implications. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 51–58. [Google Scholar] [CrossRef]
- Prior, P.L.; Suskin, N. Exercise for stroke prevention. Stroke Vasc. Neurol. 2018, 3, 59–68. [Google Scholar] [CrossRef]
- Spence, J.D. Red meat intake and cardiovascular risk: it’s the events that matter; not the risk factors. J. Public Health Emerg. 2017, 1. [Google Scholar] [CrossRef]
- Spence, J.D. Fasting lipids: The carrot in the snowman. Can. J. Cardiol. 2003, 19, 890–892. [Google Scholar]
- Fielding, C.J.; Havel, R.J.; Todd, K.M.; Yeo, K.E.; Schloetter, M.C.; Weinberg, V.; Frost, P.H. Effects of dietary cholesterol and fat saturation on plasma lipoproteins in an ethnically diverse population of healthy young men. J. Clin. Investig. 1995, 95, 611–618. [Google Scholar] [CrossRef]
- Ghanim, H.; Abuaysheh, S.; Sia, C.L.; Korzeniewski, K.; Chaudhuri, A.; Fernandez-Real, J.M.; Dandona, P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: Implications for insulin resistance. Diabetes Care 2009, 32, 2281–2287. [Google Scholar] [CrossRef]
- Spence, J.D.; Jenkins, D.J.; Davignon, J. Dietary cholesterol and egg yolks: Not for patients at risk of vascular disease. Can. J. Cardiol. 2010, 26, e336–e339. [Google Scholar] [CrossRef]
- Kushi, L.H.; Lew, R.A.; Stare, F.J.; Ellison, C.R.; El Lozy, M.; Bourke, G.; Daly, L.; Graham, I.; Hickey, N.; Mulcahy, R.; et al. Diet and 20-year mortality from coronary heart disease. The Ireland-Boston Diet-Heart Study. N. Engl. J. Med. 1985, 312, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Shekelle, R.B.; Shryock, A.M.; Paul, O.; Lepper, M.; Stamler, J.; Liu, S.; Raynor, W.J., Jr. Diet, serum cholesterol, and death from coronary heart disease. The Western Electric study. N. Engl. J. Med. 1981, 304, 65–70. [Google Scholar] [CrossRef]
- Wilkins, J.T.; Ning, H.; Berry, J.; Zhao, L.; Dyer, A.R.; Lloyd-Jones, D.M. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012, 308, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D. Effects of the intestinal microbiome on constituents of red meat and egg yolks: A new window opens on nutrition and cardiovascular disease. Can. J. Cardiol. 2014, 30, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Simenhoff, M.L.; Burke, J.F.; Saukkonen, J.J.; Ordinario, A.T.; Doty, R. Biochemical profile of uremic breath. N. Engl. J. Med. 1977, 297, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Bogiatzi, C.; Gloor, G.; Allen-Vercoe, E.; Reid, G.; Wong, R.G.; Urquhart, B.L.; Dinculescu, V.; Ruetz, K.N.; Velenosi, T.J.; Pignanelli, M.; et al. Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis 2018, 273, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D.; Urquhart, B.L.; Bang, H. Effect of renal impairment on atherosclerosis: Only partially mediated by homocysteine. Nephrol. Dial. Transplant. 2015. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.A.; Corbin, K.D.; da Costa, K.A.; Zhang, S.; Zhao, X.; Galanko, J.A.; Blevins, T.; Bennett, B.J.; O’Connor, A.; Zeisel, S.H. Effect of egg ingestion on trimethylamine-N-oxide production in humans: A randomized, controlled, dose-response study. Am. J. Clin. Nutr. 2014, 100, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.T.; Chien, Y.W.; Tsen, J.H.; Chang, C.C.; Chang, J.H.; Huang, S.Y. Taurine supplementation improves the utilization of sulfur-containing amino acids in rats continually administrated alcohol. J. Nutr. Biochem. 2009, 20, 132–139. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Rimm, E.B.; Manson, J.E.; Ascherio, A.; Colditz, G.A.; Rosner, B.A.; Spiegelman, D.; Speizer, F.E.; Sacks, F.M.; et al. A prospective study of egg consumption and risk of cardiovascular disease in men and women. JAMA 1999, 281, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Suri, F.K.; Ahmed, S.; Nasar, A.; Divani, A.A.; Kirmani, J.F. Regular egg consumption does not increase the risk of stroke and cardiovascular diseases. Med. Sci. Monit. 2007, 13, CR1–CR8. [Google Scholar]
- Rose, G. Sick individuals and sick populations. Int. J. Epidemiol. 2001, 30, 427–432. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Psaltopoulou, T.; Orfanos, P.; Trichopoulos, D. Diet and physical activity in relation to overall mortality amongst adult diabetics in a general population cohort. J. Intern. Med. 2006, 259, 583–591. [Google Scholar] [CrossRef]
- Djousse, L.; Gaziano, J.M.; Buring, J.E.; Lee, I.M. Egg consumption and risk of type 2 diabetes in men and women. Diabetes Care 2009, 32, 295–300. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.; Zhou, X.; Li, L. Egg consumption and risk of cardiovascular diseases and diabetes: A meta-analysis. Atherosclerosis 2013, 229, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Batis, C.; Wang, H.; Zhang, B.; Zhang, J.; Popkin, B.M. Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in China. Am. J. Clin. Nutr. 2014, 99, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Miller, E.R., 3rd; Weaver, C.M.; Appel, L.J. Effects of Sodium Reduction and the DASH Diet in Relation to Baseline Blood Pressure. J. Am. Coll. Cardiol. 2017, 70, 2841–2848. [Google Scholar] [CrossRef]
- Bang, H.; Mazumdar, M.; Spence, J.D. Tutorial in biostatistics: Analyzing associations between total plasma homocysteine and B vitamins using optimal categorization and segmented regression. Neuroepidemiology 2006, 27, 188–200. [Google Scholar] [CrossRef]
- Spence, J.D. Nutrition and stroke prevention. Stroke 2006, 37, 2430–2435. [Google Scholar] [PubMed]
- Spence, D. Mechanisms of thrombogenesis in atrial fibrillation. Lancet 2009, 373, 1006–1007. [Google Scholar] [CrossRef]
- Ahmed, S.; Bogiatzi, C.; Hackam, D.G.; Rutledge, A.C.; Sposato, L.A.; Khaw, A.; Mandzia, J.; Azarpazhoo, M.R.; Hachinski, V.; Spence, J.D. Vitamin B 12 deficiency and hyperhomocysteinaemia in outpatients with stroke or transient ischaemic attack: A cohort study at an academic medical centre. BMJ Open 2019, 9, e026564. [Google Scholar] [CrossRef] [PubMed]
- Toole, J.F.; Malinow, M.R.; Chambless, L.E.; Spence, J.D.; Pettigrew, L.C.; Howard, V.J.; Sides, E.G.; Wang, C.H.; Stampfer, M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004, 291, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Bønaa, K.H.; Njolstad, I.; Ueland, P.M.; Schirmer, H.; Tverdal, A.; Steigen, T.; Wang, H.; Nordrehaug, J.E.; Arnesen, E.; Rasmussen, K. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N. Engl. J. Med. 2006, 354, 1578–1588. [Google Scholar] [CrossRef]
- Lonn, E.; Yusuf, S.; Arnold, M.J.; Sheridan, P.; Pogue, J.; Micks, M.; McQueen, M.J.; Probstfield, J.; Fodor, G.; Held, C.; et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N. Engl. J. Med. 2006, 354, 1567–1577. [Google Scholar]
- Loscalzo, J. Homocysteine trials-clear outcomes for complex reasons. N. Engl. J. Med. 2006, 354, 1629–1632. [Google Scholar] [CrossRef]
- Galan, P.; Kesse-Guyot, E.; Czernichow, S.; Briancon, S.; Blacher, J.; Hercberg, S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. BMJ 2010, 341, c6273. [Google Scholar] [CrossRef]
- House, A.A.; Eliasziw, M.; Cattran, D.C.; Churchill, D.N.; Oliver, M.J.; Fine, A.; Dresser, G.K.; Spence, J.D. Effect of B-vitamin therapy on progression of diabetic nephropathy: A randomized controlled trial. JAMA 2010, 303, 1603–1609. [Google Scholar] [CrossRef]
- Spence, J.D.; Stampfer, M.J. Understanding the complexity of homocysteine lowering with vitamins: The potential role of subgroup analyses. JAMA 2011, 306, 2610–2611. [Google Scholar] [CrossRef]
- Huo, Y.; Li, J.; Qin, X.; Huang, Y.; Wang, X.; Gottesman, R.F.; Tang, G.; Wang, B.; Chen, D.; He, M.; et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: The CSPPT randomized clinical trial. JAMA 2015, 313, 1325–1335. [Google Scholar] [CrossRef]
- Qin, X.; Li, J.; Spence, J.D.; Zhang, Y.; Li, Y.; Wang, X.; Wang, B.; Sun, N.; Chen, F.; Guo, J.; et al. Folic Acid Therapy Reduces the First Stroke Risk Associated with Hypercholesterolemia Among Hypertensive Patients. Stroke 2016, 47, 2805–2812. [Google Scholar] [CrossRef]
- Kong, X.; Huang, X.; Zhao, M.; Xu, B.; Xu, R.; Song, Y.; Yu, Y.; Yang, W.; Zhang, J.; Liu, L.; et al. Platelet Count Affects Efficacy of Folic Acid in Preventing First Stroke. J. Am. Coll. Cardiol. 2018, 71, 2136–2146. [Google Scholar] [CrossRef]
- Xu, X.; Qin, X.; Li, Y.; Sun, D.; Wang, J.; Liang, M.; Wang, B.; Huo, Y.; Hou, F.F.; Investigators of the Renal Substudy of the China Stroke Primary Prevention Trial. Efficacy of Folic Acid Therapy on the Progression of Chronic Kidney Disease: The Renal Substudy of the China Stroke Primary Prevention Trial. JAMA Intern. Med. 2016, 176, 1443–1450. [Google Scholar] [CrossRef]
- Spence, J.D.; Yi, Q.; Hankey, G.J. B vitamins in stroke prevention: Time to reconsider. Lancet Neurol. 2017, 16, 750–760. [Google Scholar] [CrossRef]
- Koyama, K.; Ito, A.; Yamamoto, J.; Nishio, T.; Kajikuri, J.; Dohi, Y.; Ohte, N.; Sano, A.; Nakamura, H.; Kumagai, H.; et al. Randomized controlled trial of the effect of short-term coadministration of methylcobalamin and folate on serum ADMA concentration in patients receiving long-term hemodialysis. Am. J. Kidney Dis. 2010, 55, 1069–1078. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Rimm, E.B.; Merchant, A.; Rosner, B.A.; Stampfer, M.J.; Willett, W.C.; Ascherio, A. Fish consumption and risk of stroke in men. JAMA 2002, 288, 3130–3136. [Google Scholar] [CrossRef] [PubMed]
- Yamori, Y.; Taguchi, T.; Hamada, A.; Kunimasa, K.; Mori, H.; Mori, M. Taurine in health and diseases: Consistent evidence from experimental and epidemiological studies. J. Biomed. Sci 2010, 17, S6. [Google Scholar] [CrossRef]
- Veno, S.K.; Bork, C.S.; Jakobsen, M.U.; Lundbye-Christensen, S.; McLennan, P.L.; Bach, F.W.; Overvad, K.; Schmidt, E.B. Marine n-3 Polyunsaturated Fatty Acids and the Risk of Ischemic Stroke. Stroke 2019, 50, 274–282. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spence, J.D. Nutrition and Risk of Stroke. Nutrients 2019, 11, 647. https://doi.org/10.3390/nu11030647
Spence JD. Nutrition and Risk of Stroke. Nutrients. 2019; 11(3):647. https://doi.org/10.3390/nu11030647
Chicago/Turabian StyleSpence, J. David. 2019. "Nutrition and Risk of Stroke" Nutrients 11, no. 3: 647. https://doi.org/10.3390/nu11030647
APA StyleSpence, J. D. (2019). Nutrition and Risk of Stroke. Nutrients, 11(3), 647. https://doi.org/10.3390/nu11030647





