Iron Deficiency and Neuroendocrine Regulators of Basal Metabolism, Body Composition and Energy Expenditure in Rats
Abstract
1. Introduction
2. Material and Methods
2.1. Study Animals
2.2. Study Design and Diet
2.3. Dry Matter
2.4. Iron Determination
2.5. Haematological Test
2.6. Serum Iron, Total Iron Binding Capacity and Transferrin Saturation
2.7. Serum Ferritin
2.8. Serum Hepcidin
2.9. Biochemical Parameters
2.10. Assessment of Body Composition
2.11. Respiratory Gas Collection, Energy Expenditure Calculation and Analysis
2.12. Thyroid Hormones, Ghrelin, Glucose-Dependent Insulinotropic Polypeptide, Glucagon, Insulin, Adrenocorticotropic Hormone and Corticosterone Measurement
2.13. Statistical Analysis
3. Results
3.1. Haematological and Bichemical Parameters
3.2. Hepatosomatic Index
3.3. Body Composition
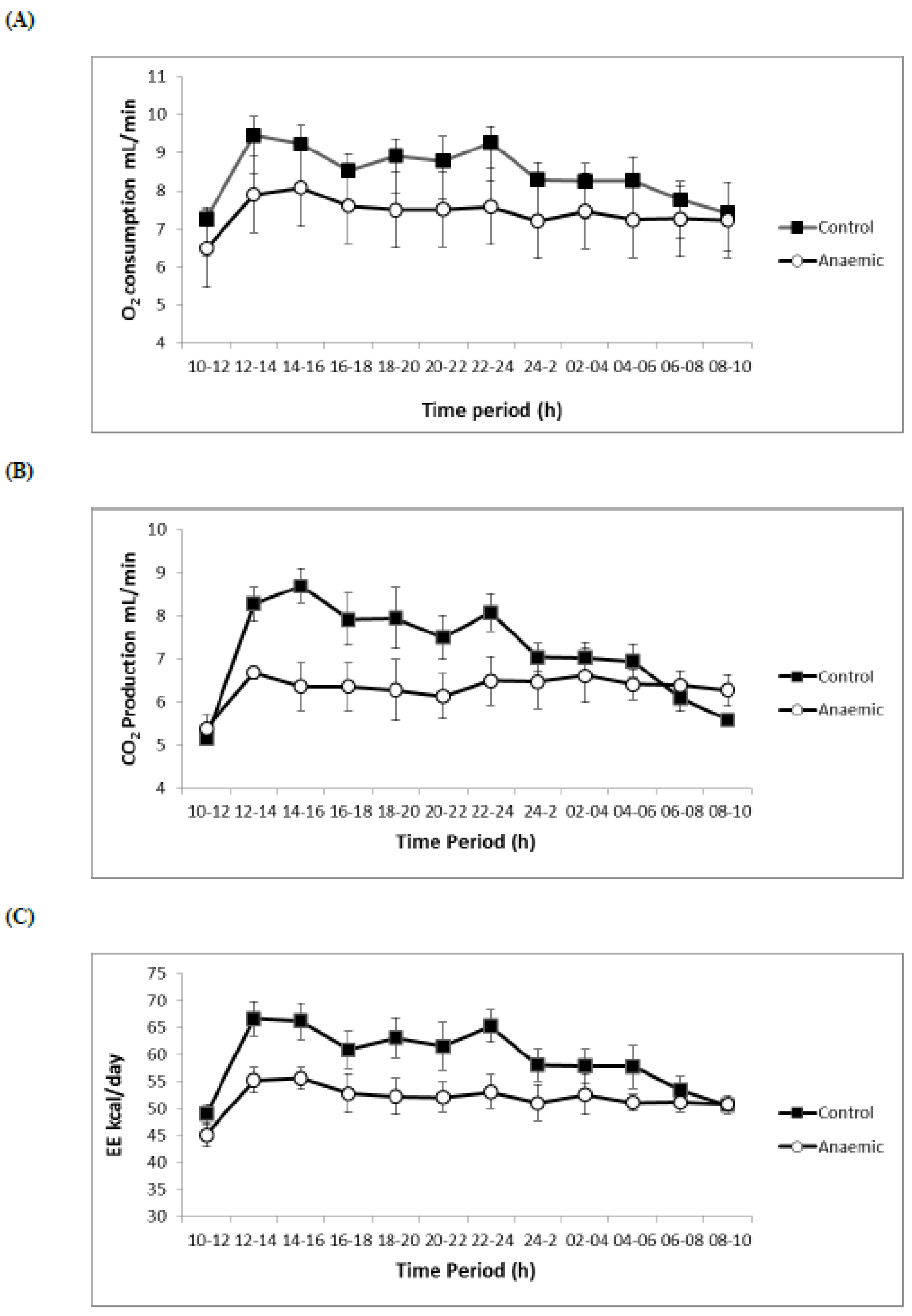
3.4. Respiratory Volumes and Flows, Energy Expenditure, O2 Consumption
3.5. Neuroendocrine Regulators of Basal Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeLoughery, T.G. Microcytic anemia. N. Engl. J. Med. 2014, 371, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.C.; Heshka, S.; Gallagher, D.; Wang, J.; Pi-Sunyer, F.X.; Pierson, R.N., Jr. Serum iron and body fat distribution in a multiethnic cohort of adults living in New York City. J. Am. Diet. Assoc. 2006, 106, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Zafon, C.; Lecube, A.; Simo, R. Iron in obesity. An ancient micronutrient for a modern disease. Obes. Rev. 2010, 11, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F. Bioavailability of iron. Eur. J. Clin. Nutr. 1997, 51, S4–S8. [Google Scholar] [PubMed]
- Popovic, V.; Duntas, L.H. Brain somatic cross-talk: Ghrelin, leptin and ultimate challengers of obesity. Nutr. Neurosci. 2005, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, O.R.; Pisa, P.T.; Vorster, H.H.; Kruger, S.H. The relationship between iron status and adiposity in women from developing countries: A review. Crit. Rev. Food. Sci. Nutr. 2014, 54, 553–560. [Google Scholar] [CrossRef]
- Park, C.Y.; Chung, J.; Koo, K.O.; Kim, M.S.; Han, S.N. Hepatic iron storage is related to body adiposity and hepatic inflammation. Nutr. Metab. (Lond.) 2017, 14:14. [Google Scholar] [CrossRef] [PubMed]
- Stankowiak-Kulpa, H.; Kargulewicz, A.; Styszyński, A.; Swora-Cwynar, E.; Grzymisławski, M. Iron status in obese women. Ann. Agric. Environ. Med. 2017, 24, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Pallarés, I.; Lisbona, F.; López-Aliaga, I.; Barrionuevo, M.; Alférez, M.J.M. Effects of iron deficiency on the digestive utilization of iron, phosphorus, calcium and magnesium in rats. Br. J. Nutr. 1993, 70, 609–620. [Google Scholar] [CrossRef]
- Tang, H.; Vasselli, J.; Wu, E.; Gallagher, D. In vivo determination of body composition of rats using magnetic resonance imaging. Ann. N. Y. Acad. Sci. 2000, 904, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.S.; Barrionuevo, M.; Alferez, M.J.; Gomez-Ayala, A.E.; Rodriguez-Matas, M.C.; Lopez Aliaga, I.; Lisbona, F. Interactions among iron, calcium, phosphorus and magnesium in the nutritionally iron-deficient rat. Exp. Physiol. 1998, 83, 771–781. [Google Scholar] [CrossRef]
- Thomas, C.; Thomas, L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin. Chem. 2002, 48, 1066–1076. [Google Scholar] [PubMed]
- Cook, J.D. Diagnosis and management of iron-deficiency anaemia. Best Pract. Res. Clin. Haematol. 2005, 18, 319–332. [Google Scholar] [CrossRef]
- Finch, C.A.; Bellotti, V.; Stray, S.; Lipschitz, D.A.; Cook, J.D.; Pippard, M.J.; Huebers, H.A. Plasma ferritin determination as a diagnostic tool. West. J. Med. 1986, 145, 657–663. [Google Scholar] [PubMed]
- Pasricha, S.R.; McQuilten, Z.; Westerman, M.; Keller, A.; Nemeth, E.; Ganz, T.; Wood, E. Serum hepcidin as a diagnostic test of iron deficiency in premenopausal female blood donors. Haematologica 2011, 96, 1099–1105. [Google Scholar] [CrossRef]
- Tolman, K.G.; Re, J.R. Liver function. In Tietz Textbook of Clinical Chemistry; Burtis, C.A., Ashwood, E.R., Eds.; Saunders: Philadelpia, PA, USA, 1999; pp. 1125–1177. [Google Scholar]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of astaxanthin in obese mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2007, 71, 893–899. [Google Scholar] [CrossRef]
- Theurl, I.; Aigner, E.; Theurl, M.; Nairz, M.; Seifert, M.; Schroll, A.; Sonnweber, T.; Eberwein, L.; Witcher, D.R.; Murphy, A.T.; et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: Diagnostic and therapeutic implications. Blood 2009, 113, 5277–5286. [Google Scholar] [CrossRef]
- Viatte, L.; Vaulont, S. Hepcidin, the iron watcher. Biochimie 2009, 91, 1223–1228. [Google Scholar] [CrossRef]
- Finberg, K.E. Striking the target in iron overload disorders. J. Clin. Investig. 2013, 123, 1424–1427. [Google Scholar] [CrossRef]
- Weinstein, D.A.; Roy, C.N.; Fleming, M.D.; Loda, M.F.; Wolfsdorf, J.I.; Andrews, N.C. Inappropriate expression of hepcidin is associated with iron refractory anemia: Implications for the anemia of chronic disease. Blood 2002, 100, 3776–3781. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Chauvet, C.; Viatte, L.; Danan, J.L.; Bigard, X.; Devaux, I.; Beaumont, C.; Kahn, A.; Vaulont, S. The gene encoding the iron regulatory peptide hepcidinis regulated by anemia, hypoxia, and inflammation. J. Clin. Investig. 2002, 110, 1037–1044. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006, 26, 323–342. [Google Scholar] [CrossRef]
- Tselepis, C.; Ford, S.J.; McKie, A.T.; Vogel, W.; Zoller, H.; Simpson, R.J.; Diaz Castro, J.; Iqbal, T.H.; Ward, D.G. Characterization of the transition-metal-binding properties of hepcidin. Biochem. J. 2010, 427, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Strube, Y.N.; Beard, J.L.; Ross, A.C. Iron deficiency and marginal vitamin A deficiency affect growth, hematological indices and the regulation of iron metabolism genes in rats. J. Nutr. 2002, 132, 3607–3615. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L.; Brigham, D.E.; Kelley, S.K.; Green, M.H. Plasma thyroid hormone kinetics are altered in iron-deficient rats. J. Nutr. 1998, 128, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Castro, J.; Lopez-Frias, M.R.; Campos, M.S.; Lopez-Frias, M.; Alferez, M.J.; Nestares, T.; Ojeda, M.L.; Lopez-Aliaga, I. Severe nutritional iron-deficiency anaemia has a negative effect on some bone turnover biomarkers in rats. Eur. J. Nutr. 2012, 51, 241–247. [Google Scholar] [CrossRef]
- Wilson, A.; Yu, H.T.; Goodnough, L.T.; Nissenson, A.R. Prevalence and outcomes of anemia in rheumatoid arthritis: A systematic review of the literature. Am. J. Med. 2004, 116, 50S–57S. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef]
- Newton, C.J.; Ran, G.; Xie, Y.X.; Bilko, D.; Burgoyne, C.H.; Adams, I.; Abidia, A.; McCollum, P.T.; Atkin, S.L. Statin-induced apoptosis of vascular endothelial cells is blocked by dexamethasone. J. Endocrinol. 2002, 174, 7–16. [Google Scholar] [CrossRef]
- Akarsu, S.; Ustundag, B.; Gurgoze, M.K.; Sen, Y.; Aygun, A.D. Plasma ghrelin levels in various stages of development of iron deficiency anemia. J. Pediatr. Hematol. Oncol. 2007, 29, 384–387. [Google Scholar] [CrossRef]
- Otto, B.; Tschop, M.; Heldwein, W.; Pfeiffer, A.F.; Diederich, S. Endogenous and exogenous glucocorticoids decrease plasma ghrelin in humans. Eur. J. Endocrinol. 2004, 151, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Borel, M.J.; Beard, J.L.; Farrell, P.A. Hepatic glucose production and insulin sensitivity and responsiveness in iron-deficient anemic rats. Am. J. Physiol. 1993, 264, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, K.; Yamada, Y.; Yano, H.; Niwa, H.; Ban, N.; Ihara, Y.; Kubota, A.; Fujimoto, S.; Kajikawa, M.; Kuroe, A.; et al. Glucose intolerance caused by a defect in the entero-insular axis: A study in gastric inhibitory polypeptide receptor knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 14843–14847. [Google Scholar] [CrossRef] [PubMed]
- Gault, V.A.; McClean, P.L.; Cassidy, R.S.; Irwin, N.; Flatt, P.R. Chemical gastric inhibitory polypeptide receptor antagonism protects against obesity, insulin resistance, glucose intolerance and associated disturbances in mice fed high-fat and cafeteria diets. Diabetologia 2007, 50, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Nakajima, A.; Yoneda, M.; Yamada, Y.; Mukasa, K.; Fujita, K.; Fujisawa, N.; Wada, K.; Terauchi, Y. Serum ferritin is associated with visceral fat area and subcutaneous fat area. Diabetes Care 2005, 28, 2486–2491. [Google Scholar] [CrossRef] [PubMed]
- Yanoff, L.B.; Menzie, C.M.; Denkinger, B.; Sebring, N.G.; McHugh, T.; Remaley, A.T.; Yanovski, J.A. Inflammation and iron deficiency in the hypoferremia of obesity. Int. J. Obes. 2007, 31, 1412–1419. [Google Scholar] [CrossRef]
- Landsberg, L.; Young, J.B. Sympathoadrenal activity and obesity: Physiological rationale for the use of adrenergic thermogenic drugs. Int. J. Obes. Relat. Metab. Disord. 1993, 17, S29–S34. [Google Scholar]
- Arch, J.R.; Wilson, S. Prospects for beta 3-adrenoceptor agonists in the treatment of obesity and diabetes. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 191–199. [Google Scholar]
- Colin-Ramirez, E.; Castillo-Martinez, L.; Orea-Tejeda, A.; AsensioLafuente, E.; Torres Villanueva, F.; Rebollar Gonzalez, V.; Narvaez David, R.; Dorantes Garcia, J. Body composition and echocardiographic abnormalities associated to anemia and volume overload in heart failure patients. Clin. Nutr. 2006, 25, 746–757. [Google Scholar] [CrossRef]
- Schneider, J.M.; Fujii, M.L.; Lamp, C.L.; Lönnerdal, B.; Dewey, K.G. The use of multiple logistic regression to identify risk factors associated with anemia and iron deficiency in a convenience sample of 12–36-mo-old children from low-income families. Am. J. Clin. Nutr. 2008, 87, 614–620. [Google Scholar] [CrossRef] [PubMed]
| Component | g/Kg Diet (Dry Weight) |
|---|---|
| Protein (casein) | 210 |
| Fat (olive oil) | 100 |
| Fibre (micronized cellulose) | 50 |
| Mineral supplement a | 35 |
| Vitamin supplement b | 10 |
| Choline chloride | 2 |
| Wheat starch | 491 |
| Sucrose | 100 |
| L-cystine | 2 |
| Energy (kJ/kg) | 17940 |
| Haematological Parameters | Normal-Fe Control Group (n = 20) | Low-Fe Anaemic Group (n = 20) |
|---|---|---|
| Hb concentration (g/L) | 138.83 ± 3.19 | 60.36 ± 3.22 * |
| RBCs (1012/L) | 7.05 ± 0.19 | 3.02 ± 0.24 * |
| Haematocrit (%) | 39.87 ± 1.14 | 11.61 ± 1.32 * |
| MCV (fL) | 56.40 ± 0.55 | 38.26 ± 0.37 * |
| MCH (pg) | 19.91 ± 0.14 | 14.32 ± 0.68 * |
| MCHC (g/dL) | 35.24 ± 0.36 | 30.88 ± 0.87 * |
| RDW (%) | 16.67 ± 0.34 | 19.31 ± 0.42 * |
| Platelets (109/L) | 745 ± 73.19 | 2251 ± 118 * |
| WBCs (109/L) | 8.88 ± 0.38 | 8.26 ± 0.97 |
| Lymphocytes (106/mL) | 8.01 ± 0.61 | 5.88 ± 0.85 * |
| Serum Fe (µg/L) | 1350 ± 106 | 598 ± 57.01 * |
| TIBC (µg/L) | 2756 ± 197 | 18235± 676 * |
| Transferrin saturation (%) | 48.55 ± 6.49 | 3.75 ± 0.41 * |
| Serum ferritin (µg/L) | 81.45 ± 2.37 | 49.12 ± 1.48 * |
| Serum hepcidin (ng/mL) | 16.98 ± 0.45 | 12.51 ± 0.62 * |
| Biochemical parameters | ||
| Total protein (g/dL) | 4.97 ± 0.15 | 5.27 ± 0.13 |
| Albumin (g/dL) | 2.84 ± 0.04 | 3.34 ± 0.12 * |
| Total cholesterol (mg/dL) | 88.92 ± 7.45 | 109.27 ± 9.69 * |
| LDL-cholesterol (U/L) | 2815 ± 444 | 2829 ± 446 |
| Triglycerides (mg/dL) | 73.92 ± 3.37 | 217.52 ± 28.46 * |
| Glucose (mg/dL) | 69.61 ± 3.97 | 86.13 ± 3.68 * |
| AST (IU/L) | 103.58 ± 8.93 | 228.04 ± 18.45 * |
| ALT (IU/L) | 24.57 ± 1.16 | 52.28 ± 2.73 * |
| Bilirubin (mg/dL) | 0.81 ± 0.09 | 1.25 ± 0.13 * |
| Urea (mg/dL) | 33.32 ± 1.77 | 42.47 ±2.01 * |
| Creatinine (mg/dL) | 0.048 ± 0.017 | 0.043 ± 0.015 |
| Amilase (U/L) | 76.40 ± 5.16 | 379.35 ± 16.81 * |
| Cortisol (µg/L) | 21.51± 1.07 | 42.21± 1.88 * |
| Creatine kinase-MB (U/L) | 1531 ± 112 | 1391 ± 112 |
| Normal-Fe Control Group (n = 20) | Low-Fe Anaemic Group (n = 20) | |
|---|---|---|
| Body weight (g) | 242.7 ± 4.6 | 200.8 ± 2.6 ** |
| Liver weight (g) | 6.415 ± 0.26 | 6.032 ± 0.34 |
| Liver weight/body weight (%) | 2.59 ± 0.06 | 2.83 ± 0.09 * |
| Liver Fe content (µg/g dry weight) | 609.26 ± 34.12 | 424.12 ± 23.10 ** |
| Normal-Fe Control Group (n = 20) | Low-Fe Anaemic Group (n = 20) | |
|---|---|---|
| Fat (%) | 7.29 ± 0.36 | 6.38 ± 0.41 ** |
| Fat (g) 1 | 22.20 ± 2.35 | 15.62 ±1.57 ** |
| Lean mass (%) | 90.63 ± 0.47 | 92.51 ± 0.63 * |
| Lean mass (g) 1 | 263.52 ± 4.80 | 242.17 ± 3.69 * |
| Free water (%) | 0.42 ± 0.04 | 0.76 ± 0.09 * |
| Free water (g) 1 | 1.24 ± 0.21 | 1.12 ± 0.21 * |
| Total water (%) | 76.79 ± 0.57 | 79.89 ± 0.45 ** |
| Total water (g) 1 | 223.31 ± 4.09 | 206.47 ± 3.09 ** |
| Normal-Fe Control Group (n = 20) | Low-Fe Anaemic Group (n = 20) | |
|---|---|---|
| Day period | ||
| VO2 (mL/min) | 8.86 ± 0.15 | 7.43 ± 0.09 ** |
| VCO2 (mL/min) | 7.91 ± 0.20 | 6.07± 0.32 ** |
| EE (kcal/day) | 62.74 ± 0.95 | 51.59 ± 0.97 ** |
| RQ | 0.89 ± 0.02 | 0.82 ± 0.03* |
| Night period | ||
| VO2 (mL/min) | 8.31 ± 0.39 | 7.36 ± 0.26 ** |
| VCO2 (mL/min) | 7.12 ± 0.18 | 53.15 ± 1.34 ** |
| EE (kcal/day) | 59.49 ± 1.39 | 6.45 ± 0.37 * |
| RQ | 0.86 ± 0.01 | 0.81 ± 0.02 * |
| Normal-Fe Control Group (n = 20) | Low-Fe Anaemic Group (n = 20) | |
|---|---|---|
| TSH (pg/mL) | 29.43± 3.96 | 51.32 ± 5.21 ** |
| T3 (pg/mL) | 15,269.21 ± 432.21 | 10,123.29 ± 348.17 ** |
| T4 (pg/mL) | 1422.45 ± 98.77 | 1098.32 ± 95.37 ** |
| Ghrelin (pg/mL) | 36.42 ± 1.05 | 14.45 ± 0.81 ** |
| GIP (pg/mL) | 26.98 ± 1.24 | 33.28 ± 1.65 * |
| Glucagon (pg/mL) | 24.23 ± 1.13 | 30.18 ± 1.24 * |
| Insulin (pg/mL) | 726.43 ± 32.18 | 862.22 ± 29.98 * |
| Corticosterone (ng/mL) | 177.65 ± 27.45 | 341.65 ± 41.24 ** |
| ACTH (pg/mL) | 1252.11± 109.23 | 861.32± 87.54 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Fernandez, J.; Díaz-Castro, J.; Alférez, M.J.M.; López-Aliaga, I. Iron Deficiency and Neuroendocrine Regulators of Basal Metabolism, Body Composition and Energy Expenditure in Rats. Nutrients 2019, 11, 631. https://doi.org/10.3390/nu11030631
Moreno-Fernandez J, Díaz-Castro J, Alférez MJM, López-Aliaga I. Iron Deficiency and Neuroendocrine Regulators of Basal Metabolism, Body Composition and Energy Expenditure in Rats. Nutrients. 2019; 11(3):631. https://doi.org/10.3390/nu11030631
Chicago/Turabian StyleMoreno-Fernandez, Jorge, Javier Díaz-Castro, María J. M. Alférez, and Inmaculada López-Aliaga. 2019. "Iron Deficiency and Neuroendocrine Regulators of Basal Metabolism, Body Composition and Energy Expenditure in Rats" Nutrients 11, no. 3: 631. https://doi.org/10.3390/nu11030631
APA StyleMoreno-Fernandez, J., Díaz-Castro, J., Alférez, M. J. M., & López-Aliaga, I. (2019). Iron Deficiency and Neuroendocrine Regulators of Basal Metabolism, Body Composition and Energy Expenditure in Rats. Nutrients, 11(3), 631. https://doi.org/10.3390/nu11030631







