Vitamin D Deficiency is Associated with Increased Use of Antimicrobials among Preschool Girls in Ethiopia
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Clinical Examination and Socio-Economic Status
2.3. Sample Collection
2.4. Hemagglutination Inhibition Assay
2.5. Streptococcus FP23 Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Ex Vivo Interferon Gamma (IFNγ) Enzyme-Linked ImmunoSpot (ELISpot) Assay
2.7. Pneumoslide IgG Antibody Detection of Selected Viruses and Bacteria
2.8. Vitamin Analyses
2.9. Statistics
3. Results
3.1. High Prevalence of Antimicrobial Use during Follow-Up
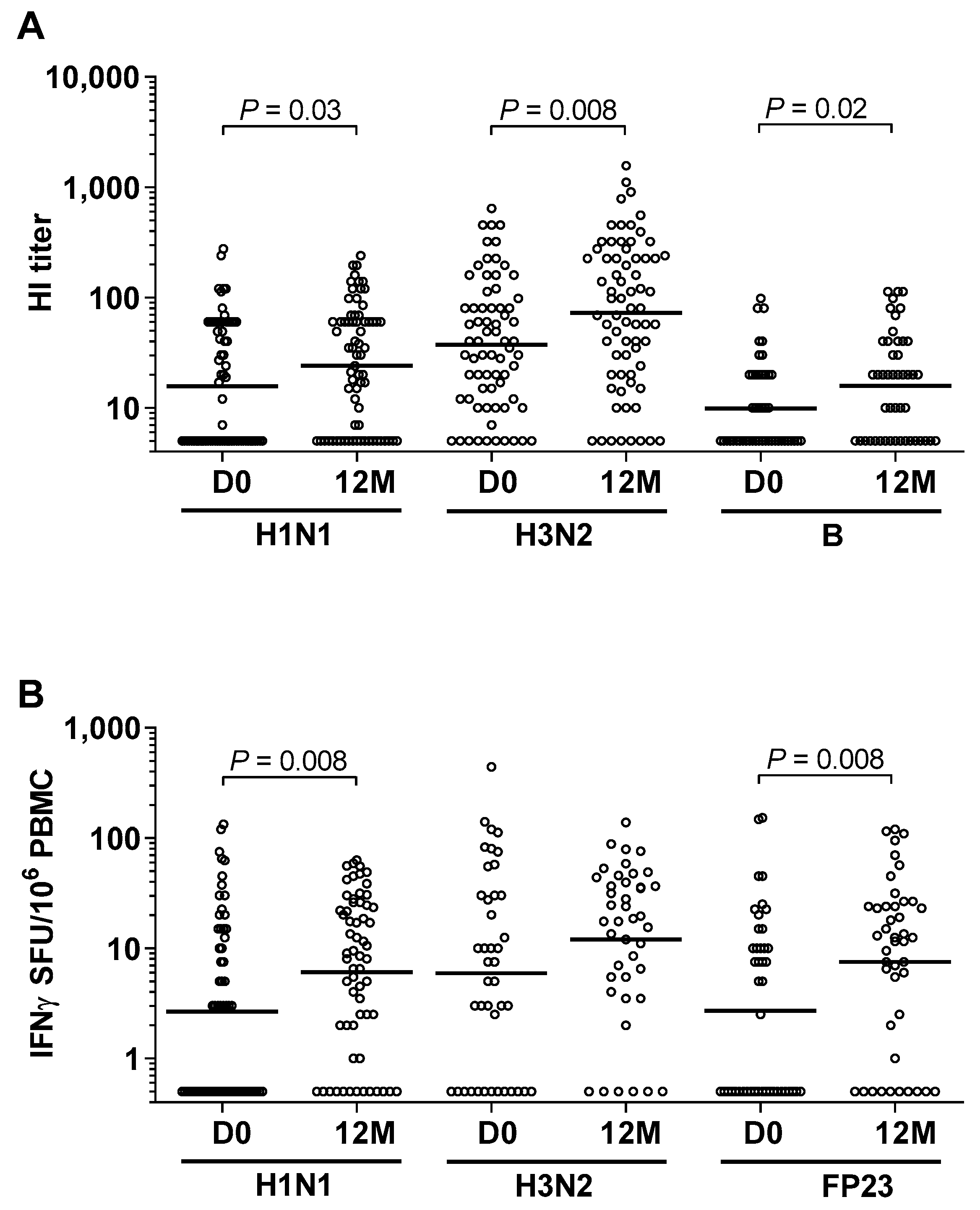
3.2. High Prevalence of Influenza and Pneumococcal Exposure during Follow-Up
3.3. High Exposure to Other Airway Pathogenic Viruses and Bacteria
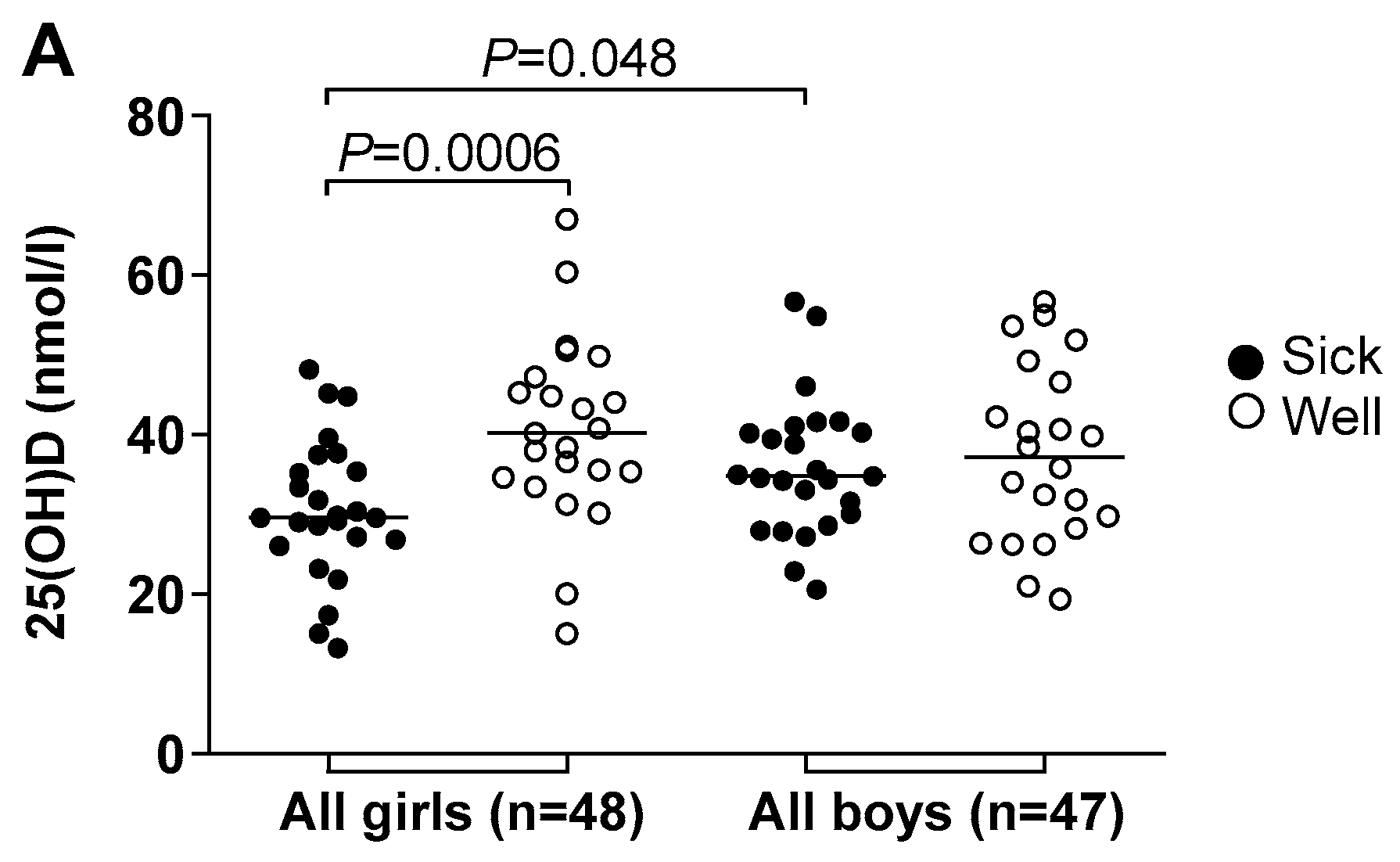
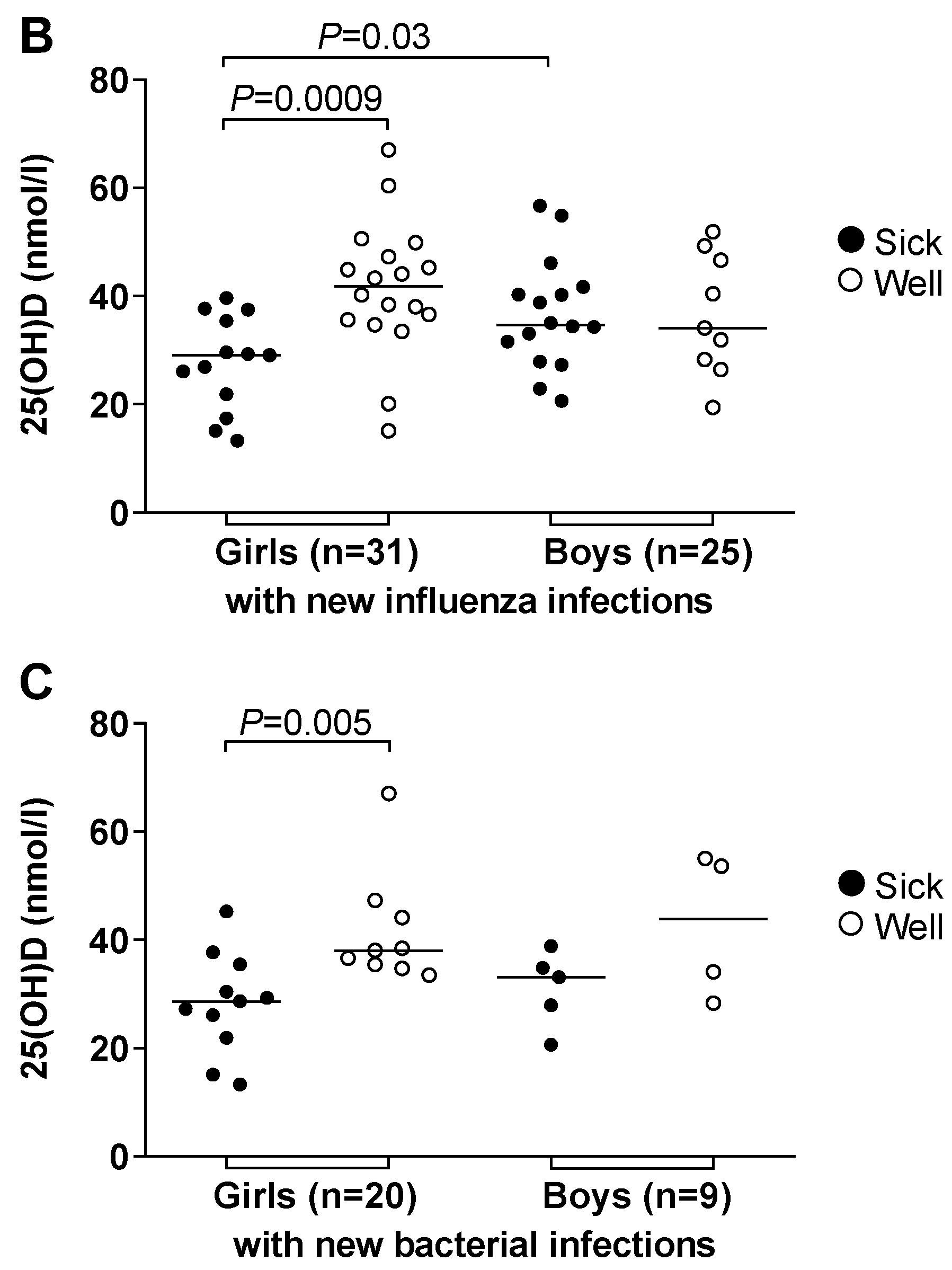
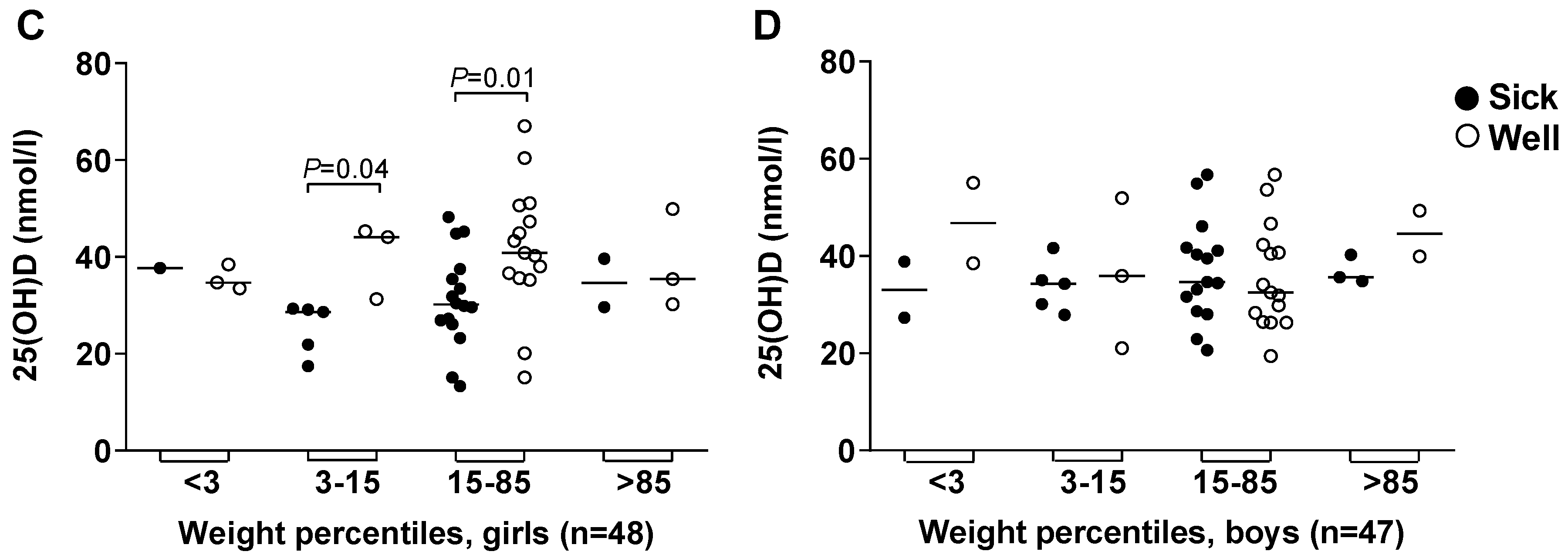
3.4. Vitamin D Deficiency Correlated to Illness and Antimicrobial Treatment
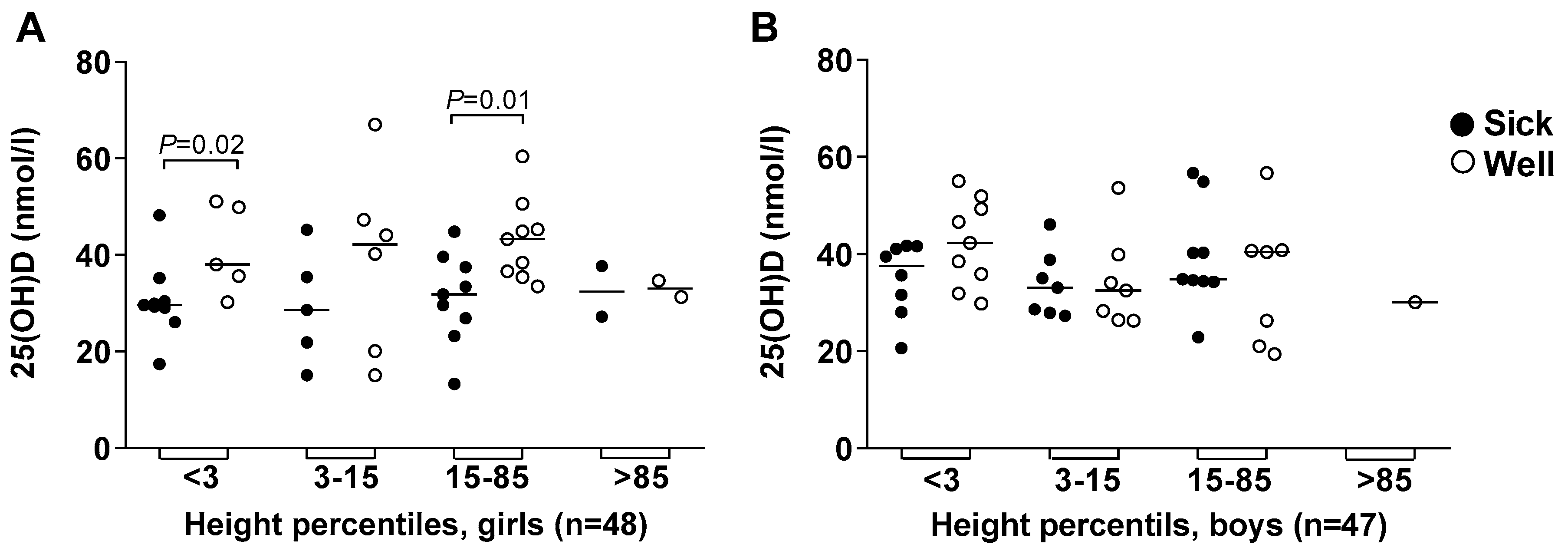
3.5. Body Height and Weight
3.6. Vitamins A and B12
3.7. Socio-Economic Status
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dembinski, J.L.; Mihret, A.; Yimer, S.A.; Tessema, B.; Trieu, M.C.; Tarekegn, A.; Getachew, N.; Cox, R.J.; Oftung, F.; Haneberg, B.; et al. High Prevalence of Humoral and Cellular Immunity to Influenza Viruses in Preschool Children Living in Addis Ababa, Ethiopia. Open Forum Infect. Dis. 2017, 4, ofx026. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.L.; Walters, M.I.; Sellman, J.; Quinlisk, P.; Regnery, H.; Schwartz, B.; Dowell, S.F. Severe pneumococcal pneumonia in previously healthy children. the role of preceding influenza infection. Clin. Infect. Dis. 2000, 30, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Kolls, J.K.; Alcorn, J.F. The immunology of influenza virus-associated bacterial pneumonia. Curr. Opin. Immunol. 2015, 34, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, K.; Bender, J.; Sheng, X.; Korgenski, K.; Daly, J.; Pavia, A.T.; Byington, C.L. Seasonal invasive pneumococcal disease in children. role of preceding respiratory viral infection. Pediatrics 2008, 122, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Grijalva, C.G.; Griffin, M.R.; Edwards, K.M.; Williams, J.V.; Gil, A.I.; Verastegui, H.; Hartinger, S.M.; Vidal, J.E.; Klugman, K.P.; Lanata, C.F. The role of influenza and parainfluenza infections in nasopharyngeal pneumococcal acquisition among young children. Clin. Infect. Dis. 2014, 58, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Klugman, K.P. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat. Med. 2004, 10, 811–813. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Children: Reducing Mortality; WHO Media Centre: Geneva, Switzerland, September 2018; Volume 1. [Google Scholar]
- Brooks, W.A.; Goswami, D.; Rahman, M.; Nahar, K.; Fry, A.M.; Balish, A.; Iftekharuddin, N.; Azim, T.; Xu, X.; Klimov, A.; et al. Influenza is a major contributor to childhood pneumonia in a tropical developing country. Pediatr. Infect. Dis. J. 2010, 29, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Emukule, G.O.; Paget, J.; van der Velden, K.; Mott, J.A. Influenza-Associated Disease Burden in Kenya. A Systematic Review of Literature. PLoS ONE 2015, 10, e0138708. [Google Scholar] [CrossRef] [PubMed]
- Adinew, Y.M.; Feleke, S.A.; Mengesha, Z.B.; Workie, S.B. Childhood Mortality: Trends and Determinants in Ethiopia from 1990 to 2015—A Systematic Review. Adv. Public Health 2017, 2017, 10. [Google Scholar]
- De Oliveira, L.H.; Camacho, L.A.; Coutinho, E.S.; Martinez-Silveira, M.S.; Carvalho, A.F.; Ruiz-Matus, C.; Toscano, C.M. Impact and Effectiveness of 10 and 13-Valent Pneumococcal Conjugate Vaccines on Hospitalization and Mortality in Children Aged Less than 5 Years in Latin American Countries: A Systematic Review. PLoS ONE 2016, 11, e0166736. [Google Scholar] [CrossRef] [PubMed]
- Olarte, L.; Barson, W.J.; Barson, R.M.; Romero, J.R.; Bradley, J.S.; Tan, T.Q.; Givner, L.B.; Hoffman, J.A.; Lin, P.L.; Hulten, K.G.; et al. Pneumococcal Pneumonia Requiring Hospitalization in US Children in the 13-Valent Pneumococcal Conjugate Vaccine Era. Clin. Infect. Dis. 2017, 64, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Muhe, L.; Lulseged, S.; Mason, K.E.; Simoes, E.A. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet 1997, 349, 1801–1804. [Google Scholar] [CrossRef]
- Wakayo, T.; Belachew, T.; Vatanparast, H.; Whiting, S.J. Vitamin D deficiency and its predictors in a country with thirteen months of sunshine: The case of school children in central Ethiopia. PLoS ONE 2015, 10, e0120963. [Google Scholar] [CrossRef] [PubMed]
- Wakayo, T.; Whiting, S.J.; Belachew, T. Vitamin D Deficiency is Associated with Overweight and/or Obesity among Schoolchildren in Central Ethiopia: A Cross-Sectional Study. Nutrients 2016, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Madhun, A.S.; Akselsen, P.E.; Sjursen, H.; Pedersen, G.; Svindland, S.; Nostbakken, J.K.; Nilsen, M.; Mohn, K.; Jul-Larsen, A.; Smith, I.; et al. An adjuvanted pandemic influenza H1N1 vaccine provides early and long term protection in health care workers. Vaccine 2010, 29, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.J.; Chen, M.I.; Yap, J.; Ong, J.; Lim, W.Y.; Lin, R.T.; Barr, I.; Ong, J.B.; Mak, T.M.; Goh, L.G.; et al. Comparability of different methods for estimating influenza infection rates over a single epidemic wave. Am. J. Epidemiol. 2011, 174, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, J.; Aase, A.; Bergmann, S.; Herstad, T.K.; Rodal, G.; Frank, R.; Rohde, M.; Hammerschmidt, S. Streptococcus pneumoniae enolase is important for plasminogen binding despite low abundance of enolase protein on the bacterial cell surface. Microbiology 2006, 152, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Savic, M.; Dembinski, J.L.; Kim, Y.; Tunheim, G.; Cox, R.J.; Oftung, F.; Peters, B.; Mjaaland, S. Epitope specific T-cell responses against influenza A in a healthy population. Immunology 2016, 147, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Dembinski, J.L.; Hungnes, O.; Hauge, A.G.; Kristoffersen, A.C.; Haneberg, B.; Mjaaland, S. Hydrogen peroxide inactivation of influenza virus preserves antigenic structure and immunogenicity. J. Virol. Methods 2014, 207, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Herrador, Z.; Sordo, L.; Gadisa, E.; Buno, A.; Gomez-Rioja, R.; Iturzaeta, J.M.; de Armas, L.F.; Benito, A.; Aseffa, A.; Moreno, J.; et al. Micronutrient deficiencies and related factors in school-aged children in Ethiopia: A cross-sectional study in Libo Kemkem and Fogera districts, Amhara Regional State. PLoS ONE 2014, 9, e112858. [Google Scholar] [CrossRef] [PubMed]
- Jovanovich, A.J.; Ginde, A.A.; Holmen, J.; Jablonski, K.; Allyn, R.L.; Kendrick, J.; Chonchol, M. Vitamin D level and risk of community-acquired pneumonia and sepsis. Nutrients 2014, 6, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Usuf, E.; Bottomley, C.; Adegbola, R.A.; Hall, A. Pneumococcal carriage in sub-Saharan Africa--a systematic review. PLoS ONE 2014, 9, e85001. [Google Scholar] [CrossRef] [PubMed]
- Trzcinski, K.; Thompson, C.M.; Srivastava, A.; Basset, A.; Malley, R.; Lipsitch, M. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infect. Immun. 2008, 76, 2678–2684. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Cohen, J.M.; Jose, R.J.; de Vogel, C.; Baxendale, H.; Brown, J.S. Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal Immunol. 2015, 8, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Moe, N.; Pedersen, B.; Nordbo, S.A.; Skanke, L.H.; Krokstad, S.; Smyrnaios, A.; Dollner, H. Respiratory Virus Detection and Clinical Diagnosis in Children Attending Day Care. PLoS ONE 2016, 11, e0159196. [Google Scholar] [CrossRef] [PubMed]
- Muenchhoff, M.; Goulder, P.J. Sex differences in pediatric infectious diseases. J. Infect. Dis. 2014, 209 (Suppl. 3), S120–S126. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.D.; Nama, N.; O’Hearn, K.; Sampson, M.; Amrein, K.; Iliriani, K.; McIntyre, L.; Fergusson, D.; Menon, K. Vitamin D deficiency in critically ill children: A systematic review and meta-analysis. Crit. Care 2017, 21, 287. [Google Scholar] [CrossRef] [PubMed]
- Mamani, M.; Muceli, N.; Ghasemi Basir, H.R.; Vasheghani, M.; Poorolajal, J. Association between serum concentration of 25-hydroxyvitamin D and community-acquired pneumonia: A case-control study. Int. J. Gen. Med. 2017, 10, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cheng, X.; Guo, L.; Li, H.; Sun, C.; Cui, X.; Zhang, Q.; Song, G. Association between serum 25-hydroxyvitamin D concentration and pulmonary infection in children. Medicine (Baltimore) 2018, 97, e9060. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Arihiro, S.; Nakashima, A.; Matsuoka, M.; Suto, S.; Uchiyama, K.; Kato, T.; Mitobe, J.; Komoike, N.; Itagaki, M.; Miyakawa, Y.; et al. Randomized Trial of Vitamin D Supplementation to Prevent Seasonal Influenza and Upper Respiratory Infection in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The WHO Child Growth Standards; WHO Media Centre: Geneva, Switzerland, 2018; Available online: https://www.who.int/childgrowth/standards/en/ (accessed on 11 May 2018).
- Jelenkovic, A.; Sund, R.; Hur, Y.-M.; Yokoyama, Y.; Hjelmborg, J.v.B.; Möller, S.; Honda, C.; Magnusson, P.K.E.; Pedersen, N.L.; Ooki, S.; et al. Genetic and environmental influences on height from infancy to early adulthood: An individual-based pooled analysis of 45 twin cohorts. Sci. Rep. 2016, 6, 28496. [Google Scholar] [CrossRef] [PubMed]
- Fish, E.N. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Roberts, C.W. Sex and Gender Differences in Infection and Treatments for Infectious Diseases; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Lang, P.O.; Aspinall, R. Vitamin D Status and the Host Resistance to Infections: What It Is Currently (Not) Understood. Clin. Ther. 2017, 39, 930–945. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.R.; Mascarenhas, L.P.; Satler, F.; Boguszewski, M.C.; Spritzer, P.M. Vitamin D deficiency in girls from South Brazil: A cross-sectional study on prevalence and association with vitamin D receptor gene variants. BMC Pediatr. 2012, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Greiller, C.L.; Mein, C.A.; Hoti, M.; Bakhsoliani, E.; Telcian, A.G.; Simpson, A.; Barnes, N.C.; Curtin, J.A.; Custovic, A.; et al. Vitamin D receptor genotype influences risk of upper respiratory infection. Br. J. Nutr. 2018, 120, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-triggering response. Science 2006, 5768, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Stukes, T.M.; Shary, J.R.; Wei, W.; Ebeling, M.D.; Dezsi, K.B.; Shary, F.S.; Forestieri, N.E.; Hollis, B.W.; Wagner, C.L. Circulating Cathelicidin Concentrations in a Cohort of Healthy Children: Influence of Age, Body Composition, Gender and Vitamin D Status. PLoS ONE 2016, 11, e0152711. [Google Scholar] [CrossRef] [PubMed]
- Krementsov, D.N.; Asarian, L.; Fang, Q.; McGill, M.M.; Teuscher, C. Sex-Specific Gene-by-Vitamin D Interactions Regulate Susceptibility to Central Nervous System Autoimmunity. Front. Immunol. 2018, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.J.; Fisker, A.B.; Andersen, A.; Sartono, E.; Yazdanbakhsh, M.; Aaby, P.; Erikstrup, C.; Benn, C.S. The effects of vitamin A supplementation with measles vaccine on leucocyte counts and in vitro cytokine production. Br. J. Nutr. 2016, 115, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Vom Steeg, L.G.; Klein, S.L. SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog. 2016, 12, e1005374. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Lelii, M. Vitamin D and respiratory tract infections in childhood. BMC Infect. Dis. 2015, 15, 487. [Google Scholar] [CrossRef] [PubMed]
- Howie, S.R.; Morris, G.A.; Tokarz, R.; Ebruke, B.E.; Machuka, E.M.; Ideh, R.C.; Chimah, O.; Secka, O.; Townend, J.; Dione, M.; et al. Etiology of severe childhood pneumonia in the Gambia, West Africa, determined by conventional and molecular microbiological analyses of lung and pleural aspirate samples. Clin. Infect. Dis. 2014, 59, 682–685. [Google Scholar] [CrossRef] [PubMed]
| All Children | Girls | Boys | |
|---|---|---|---|
| All children at study start | 95 | 48 | 47 |
| Age (months) 1 | 51 | 50 | 54 |
| Weight (kg) 1 | 14.8 | 14.2 | 15.0 |
| Height (m) 1 | 1.0 | 1.0 | 0.99 |
| BMI (kg/m2) 1 | 15.1 | 14.8 | 15.2 |
| Serum 25(OH)D (nmol/l) 1 | 35.2 | 35.3 | 35.0 |
| Serum vitamin A (µmol/l) 1 | 0.58 | 0.60 | 0.55 |
| Serum vitamin B12 (pmol/l) 1 | 472 | 430 | 502 |
| Number of children providing additional samples | |||
| Paired plasma samples at study start/end | 70 | 39 | 31 |
| Paired PBMC samples at study start/end | 63 | 34 | 29 |
| Plasma samples for measurement of 25(OH)D and vitamin A at study start | 95 | 48 | 47 |
| Plasma samples for measurement of vitamin B12 at study start | 93 | 47 | 46 |
| Number of children receiving vaccines | |||
| BCG vaccine | 88 | 46 | 42 |
| Measles vaccine | 78 | 39 | 39 |
| DTP 2 vaccine × 3 | 81 | 42 | 39 |
| HepB 3 and HiB 4 vaccines | 77 | 39 | 38 |
| Oral polio vaccine × 3 | 86 | 44 | 42 |
| Pneumococcal vaccine 1–3 doses | 3 | 2 | 1 |
| All Children | Girls | Boys | |
|---|---|---|---|
| Treated with systemic antimicrobials | 50 | 25 | 25 |
| 1–2 courses of antimicrobials | 31 | 16 | 15 |
| 3–6 courses of antimicrobials | 19 | 9 | 10 |
| 4–6 visits Medical Doctor | 16 | 9 | 7 |
| Any airway infection | 43 | 22 | 21 |
| Pneumonia | 14 | 7 | 7 |
| Pulmonary tuberculosis | 2 | 0 | 2 |
| Intestinal parasites/amoebiasis | 7 | 5 | 2 |
| Acute gastroenteritis | 9 | 5 | 4 |
| Urinary tract infection | 2 | 2 | 0 |
| Not treated with systemic antimicrobials | 45 | 23 | 22 |
| No visit Medical Doctor | 34 | 17 | 17 |
| Any airway infection | 3 | 1 | 2 |
| Skin disorder | 8 | 5 | 3 |
| Herpes simplex | 1 | 1 | 0 |
| Antibodies at Start | New Antibodies | |||
|---|---|---|---|---|
| Pathogen | All Children | Girls/Boys | All Children | Girls/Boys |
| Parainfluenza virus 1, 2, 3 | 65 (93) | 39/26 | 4 | 0/4 |
| Adenovirus | 68 (97) | 37/31 | 2 | 2/0 |
| Respiratory syncytial virus | 65 (93) | 37/28 | 4 | 2/2 |
| Legionella pneumophila | 23 (33) | 14/9 | 12 | 8/4 |
| Mycoplasma pneumoniae | 22 (32) | 11/11 | 10 | 6/4 |
| Coxiella burnetii | 33 (47) | 16/17 | 5 | 5/0 |
| Chlamydophila pneumoniae | 15 (21) | 9/6 | 10 | 8/2 |
| Substance | Lower Limits | Children (Percent) | Girls/Boys |
|---|---|---|---|
| 25(OH)D | 50 nmol/L | 85 (89) | 44/41 |
| Vitamin A | 0.7 μmol/L | 70 (74) | 32/38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodin, J.; Mihret, A.; Holm-Hansen, C.; Dembinski, J.L.; Trieu, M.-C.; Tessema, B.; Tarekegne, A.; Yimer, S.A.; Cox, R.; Aseffa, A.; et al. Vitamin D Deficiency is Associated with Increased Use of Antimicrobials among Preschool Girls in Ethiopia. Nutrients 2019, 11, 575. https://doi.org/10.3390/nu11030575
Bodin J, Mihret A, Holm-Hansen C, Dembinski JL, Trieu M-C, Tessema B, Tarekegne A, Yimer SA, Cox R, Aseffa A, et al. Vitamin D Deficiency is Associated with Increased Use of Antimicrobials among Preschool Girls in Ethiopia. Nutrients. 2019; 11(3):575. https://doi.org/10.3390/nu11030575
Chicago/Turabian StyleBodin, Johanna, Adane Mihret, Carol Holm-Hansen, Jennifer L. Dembinski, Mai-Chi Trieu, Bamlak Tessema, Azeb Tarekegne, Solomon A. Yimer, Rebecca Cox, Abraham Aseffa, and et al. 2019. "Vitamin D Deficiency is Associated with Increased Use of Antimicrobials among Preschool Girls in Ethiopia" Nutrients 11, no. 3: 575. https://doi.org/10.3390/nu11030575
APA StyleBodin, J., Mihret, A., Holm-Hansen, C., Dembinski, J. L., Trieu, M.-C., Tessema, B., Tarekegne, A., Yimer, S. A., Cox, R., Aseffa, A., Haneberg, B., & Mjaaland, S. (2019). Vitamin D Deficiency is Associated with Increased Use of Antimicrobials among Preschool Girls in Ethiopia. Nutrients, 11(3), 575. https://doi.org/10.3390/nu11030575





