Miso (Fermented Soybean Paste) Suppresses Visceral Fat Accumulation in Mice, Especially in Combination with Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Test Diets
2.2. Animals
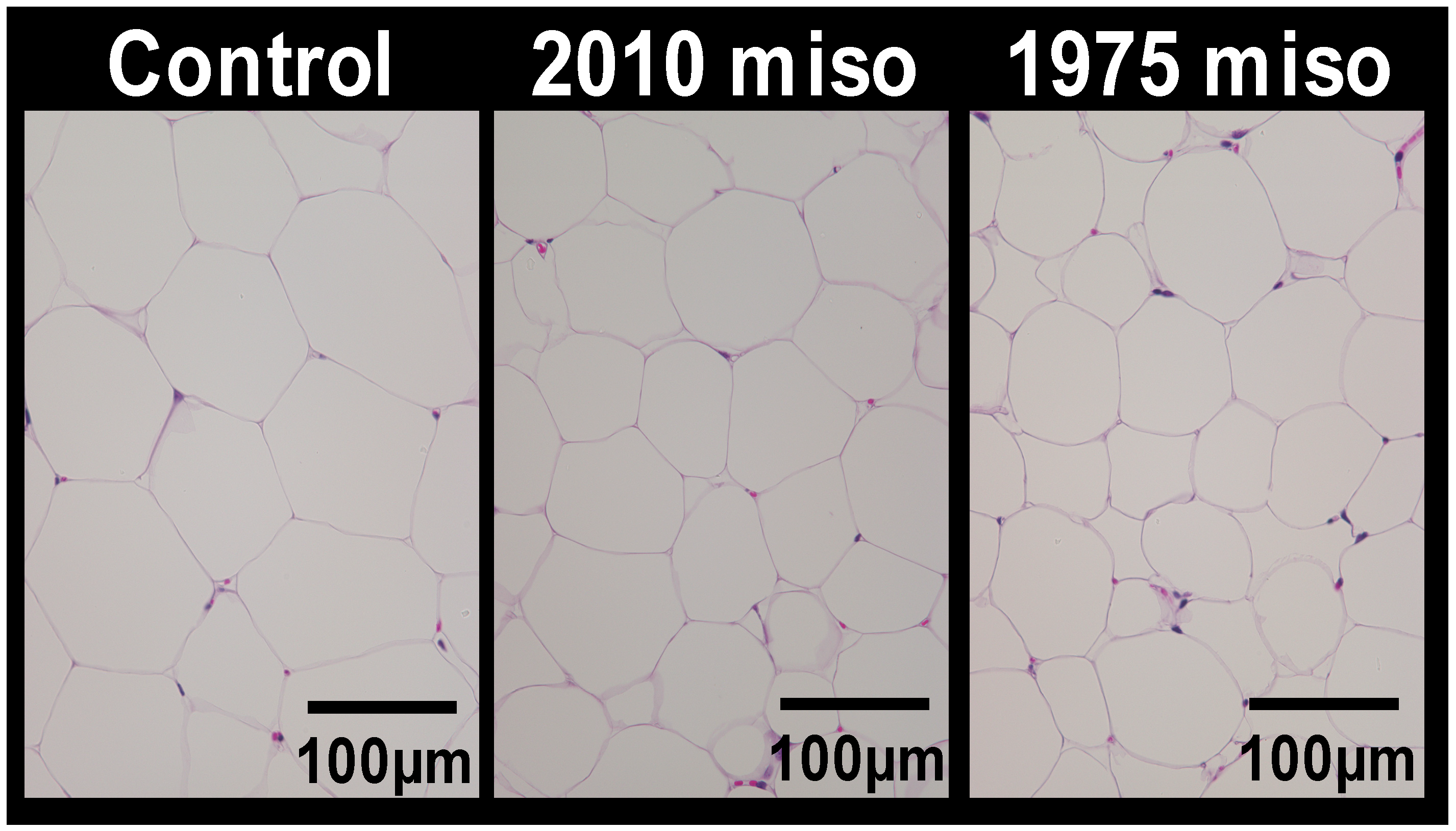
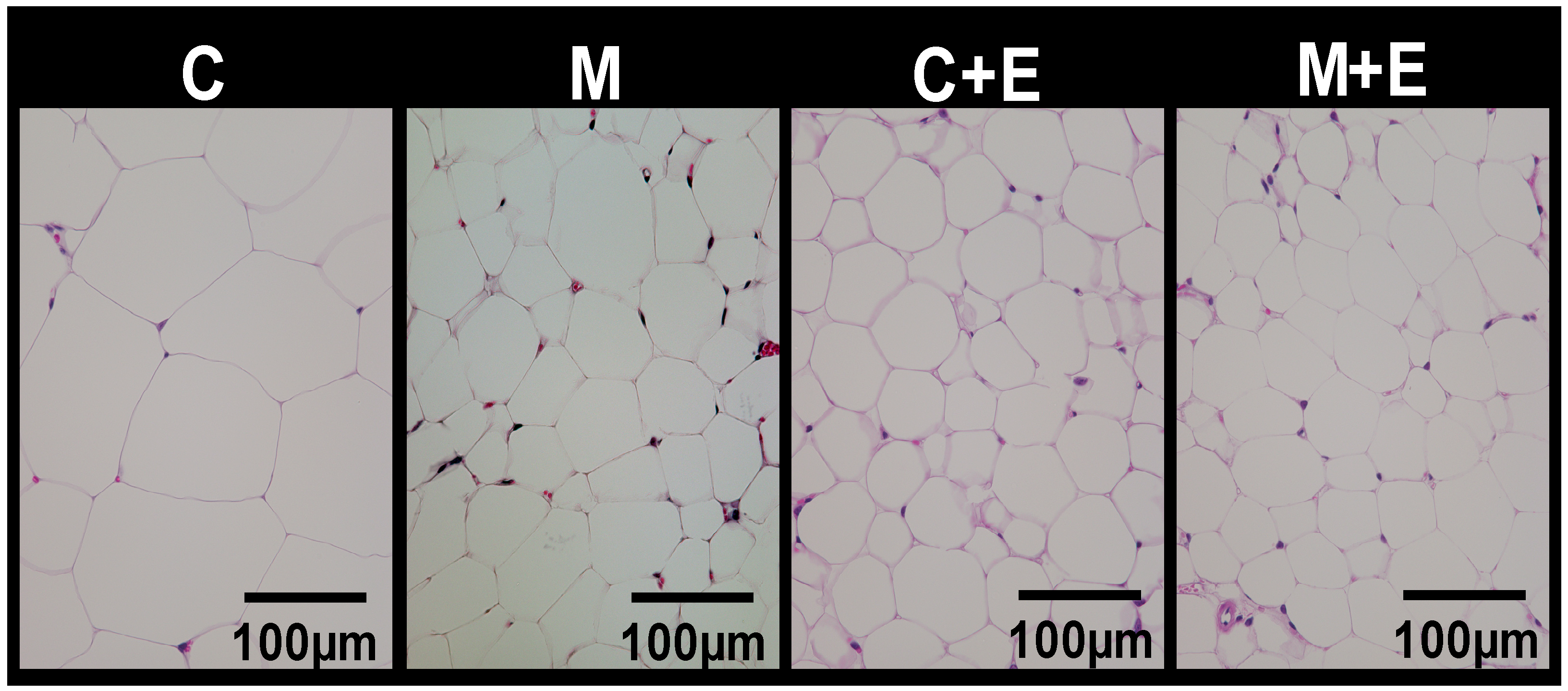
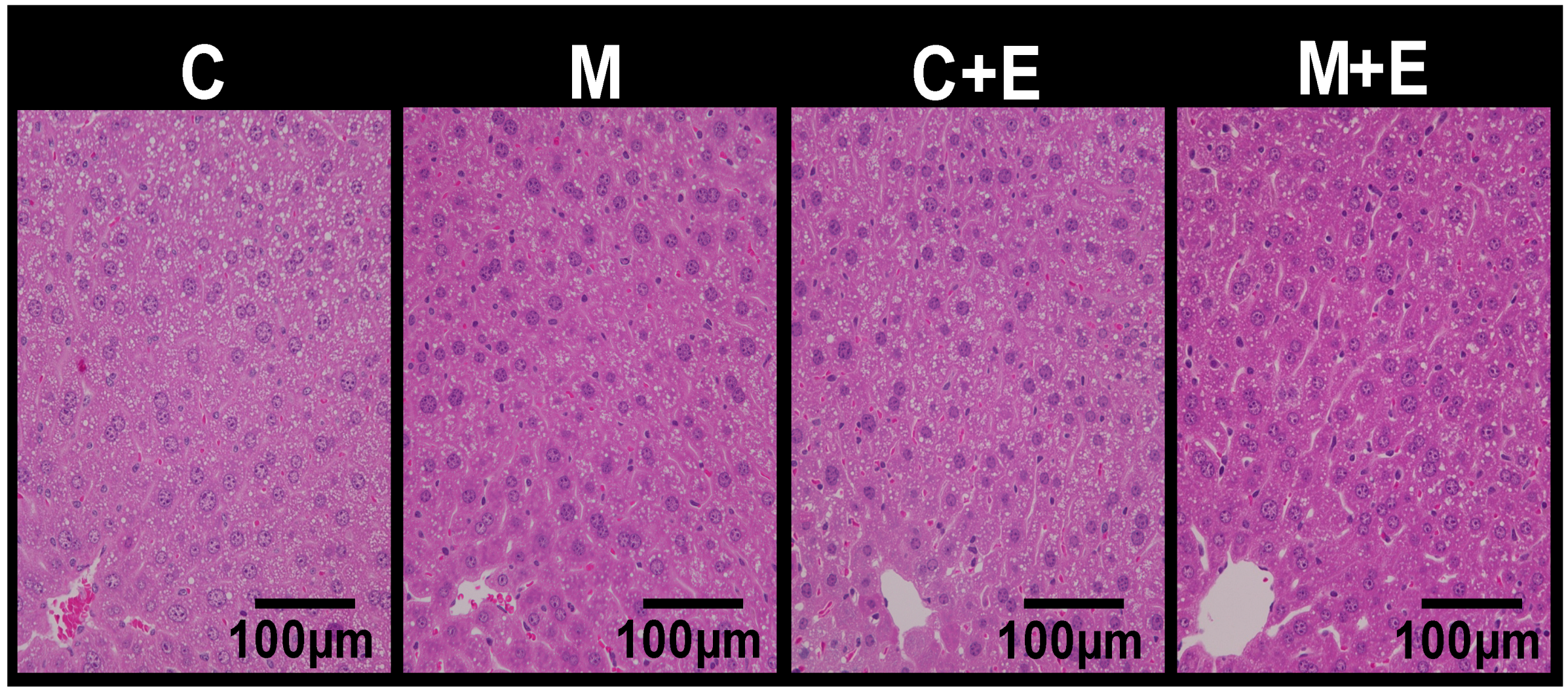
2.3. Histological Analysis
2.4. mRNA Expression Analysis
2.5. Serum and Liver Biochemical Analyses
2.6. Statistical Analysis
3. Results
3.1. Experiment 1
3.2. Growth Parameters (Experiment 2)
3.3. mRNA Expression Level in Epididymal Adipose Tissue (Experiment 2)
3.4. Biochemical Parameters in Serum and Liver (Experiment 2)
3.5. mRNA Expression Level in Liver (Experiment 2)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ministry of Health, Labour and Welfare. 2016 Abridged Life Table. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/life/life17/index.html (accessed on 1 May 2017).
- Kitano, Y.; Honma, T.; Hatakeyama, Y.; Jibu, Y.; Kawakami, Y.; Tsuduki, T.; Nakagawa, K.; Miyazawa, T. Effects of Historical Differences in Components of the Japanese Diet on the Risk of Obesity in Mice. Nippon Eiyo Shokuryo Gakkaishi 2014, 67, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health, Labor and Welfare. National Health and Nutrition Survey. Available online: http://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html (accessed on 1 May 2017).
- National Institute of Health and Nutrition. National Health and Nutrition Survey, Current State of National Nutrition. Available online: http://www0.nih.go.jp/eiken/chosa/kokumin_eiyou/ (accessed on 1 May 2017).
- Ohara, M.; Lu, H.; Shiraki, K.; Ishimura, Y.; Uesaka, T.; Katoh, O.; Watanabe, H. Prevention by long-term fermented miso of induction of colonic aberrant crypt foci by azoxymethane in F344 rats. Oncol. Rep. 2002, 9, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, M.; Toda, N.; Tamura, Y.; Terakado, S.; Ueno, M.; Otsuka, K.; Numabe, A.; Kawabata, Y.; Uehara, Y. Japanese traditional miso soup attenuates salt-induced hypertension and its organ damage in Dahl salt-sensitive rats. Nutrition 2012, 28, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Kashimoto, N.; Kajimura, J.; Kamiya, K. A miso (Japanese soybean paste) diet conferred greater protection against hypertension than a sodium chloride diet in Dahl salt-sensitive rats. Hypertens. Res. 2006, 29, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.A.; Hiramatsu, M.; Mori, A. Japanese soybean paste miso scavenges free radicals and suppresses lipid peroxidation. J. Nutr. Sci. Vitaminol. 1992, 38, 298–304. [Google Scholar] [CrossRef]
- Nakamura, T.; Tokunaga, K.; Shimomura, I.; Nishida, M.; Yoshida, S.; Kotani, K.; Islam, A.H.; Keno, Y.; Kobatake, T.; Nagai, Y. Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis 1994, 107, 239–246. [Google Scholar] [CrossRef]
- Bray, G.A.; Popkin, B.M. Dietary fat intake does affect obesity! Am. J. Clin. Nutr. 1998, 68, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, O. Prevention of Lifestyle-related Disease by Regular Exercise and Fish Oil Feeding. J. Jpn. Soc. Nutr. Food Sci. 2006, 59, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Honma, T.; Kitano, Y.; Kijima, R.; Jibu, Y.; Kawakami, Y.; Tsuduki, T.; Nakagawa, K.; Miyazawa, T. Comparison of the Health Benefits of Different Eras of Japanese Foods: Lipid and Carbohydrate Metabolism Focused Research. Nippon Shokuhin Kagaku Kogaku Kaishi 2013, 60, 541–553. [Google Scholar] [CrossRef]
- Nomura, S.; Kawanami, H.; Ueda, H.; Kizaki, T.; Ohno, H.; Izawa, T. Possible mechanisms by which adipocyte lipolysis is enhanced in exercise-trained rats. Biochem. Biophys. Res. Commun. 2002, 295, 236–242. [Google Scholar] [CrossRef]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Yasunaga, K.; Matsuo, N.; Katsuragi, Y.; Komikado, M.; Tokimitsu, I.; Wilder, D.; Jones, F.; et al. Green Tea Catechin Consumption Enhances Exercise-Induced Abdominal Fat Loss in Overweight and Obese Adults. J. Nutr. 2009, 139, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Ota, N.; Soga, S.; Shimotoyodome, A.; Haramizu, S.; Inaba, M.; Murase, T.; Tokimitsu, I. Effects of Combination of Regular Exercise and Tea Catechins Intake on Energy Expenditure in Humans. J. Health Sci. 2005, 51, 233–236. [Google Scholar] [CrossRef]
- Ota, N.; Soga, S.; Murase, T.; Shimotoyodome, A.; Hase, T. Consumption of Coffee Polyphenols Increases Fat Utilization in Humans. J. Health Sci. 2010, 56, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Mizowaki, Y.; Sugawara, S.; Yamamoto, K.; Sakamoto, Y.; Iwagaki, Y.; Kawakami, Y.; Igarashi, M.; Tsuduki, T. Comparison of the effects of the 1975 Japanese diet and the modern Mediterranean diet on lipid metabolism in mice. J. Oleo Sci. 2017, 66, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Guo, X.; Sugawara, S.; Iwagaki, Y.; Yamamoto, K.; Tsuduki, T. Effect of the Japanese diet during pregnancy and lactation or post-weaningon the risk of metabolic syndrome in offspring. Biosci. Biotechnol. Biochem. 2018, 82, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Iwagaki, Y.; Sugawara, S.; Huruya, Y.; Sato, M.; Wu, Q.; E, S.; Yamamoto, K.; Tsuduki, T. The 1975 Japanese diet has a stress reduction effect in mice: Search for physiological effects using metabolome analysis. Biosci. Biotechnol. Biochem. 2018, 82, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.; Iwagaki, Y.; Sugawara, S.; Kushida, M.; Okouchi, R.; Yamamoto, K.; Tsuduki, T. Effects of the Japanese diet in combination with exercise on visceral fataccumulation. Nutrition 2018, 57, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Iwagaki, Y.; Sakamoto, Y.; Sugawara, S.; Mizowaki, Y.; Yamamoto, K.; Sugawara, T.; Kimura, K.; Tsuduki, T. Identification of characteristic components and foodstuffs in healthy Japanesediet and the health effects of a diet with increased use frequency of thesefoodstuffs. Mol. Nutr. Food Res. 2017, 61, 1700430. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Yamamoto, K.; Hatakeyama, Y.; Tsuduki, T. Effects of fatty acid quality and quantity in the Japanese diet on thesuppression of lipid accumulation. J. Oleo Sci. 2016, 65, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Tsuduki, T.; Yamamoto, K.; Hatakeyama, Y.; Sakamoto, Y. High dietary cholesterol intake during lactation promotes development offatty liver in offspring of mice. Mol. Nutr. Food Res. 2016, 60, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Mizowaki, Y.; Iwagaki, Y.; Sakamoto, Y.; Yamamoto, K.; Tsuduki, T. Standardization of the Japanese diet for use in animal experiments. Br. J. Nutr. 2017, 118, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Iwagaki, Y.; Watanabe, K.; Nochi, T.; Aso, H.; Tsuduki, T. Effects of a moderate-fat diet enriched with fish oil on intestinal lipid absorption in a senescence-accelerated prone mouse model. Nutrition 2018, 50, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Rouser, G.; Fleischer, S.; Yamamoto, A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 1970, 5, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Fukui, K.; Takamatsu, K.; Hashimoto, Y.; Yamamoto, T. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow KK). Nutrition 2000, 16, 349–354. [Google Scholar] [CrossRef]
- Davis, J.; Higginbotham, A.; O’Connor, T.; Moustaid-Moussa, N.; Tebbe, A.; Kim, Y.C.; Cho, K.W.; Shay, N.; Adler, S.; Peterson, R.; et al. Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann. Nutr. Metab. 2007, 51, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kushida, M.; Okouchi, R.; Iwagaki, Y.; Asano, M.; Du, M.X.; Yamamoto, K.; Tsuduki, T. Fermented soybean suppresses visceral fat accumulation in mice. Mol. Nutr. Food Res. 2018, 62, e1701054. [Google Scholar] [CrossRef] [PubMed]
- Kushida, M.; Sugawara, S.; Asano, M.; Yamamoto, K.; Fukuda, S.; Tsuduki, T. Effects of the 1975 Japanese diet on the gut microbiota in younger adults. J. Nutr. Biochem. 2018, 64, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Janani, C.; Kumari, B.R. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Okuno, A.; Tamemoto, H.; Tobe, K.; Ueki, K.; Mori, Y.; Iwamoto, K.; Umesono, K.; Akanuma, Y.; Fujiwara, T.; Horikoshi, H.; et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J. Clin. Investig. 1998, 101, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Gogg, S.; Hedjazifar, S.; Jenndahl, L.; Hammarstedt, A.; Smith, U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am. J. Physiol. Endocrinol. Metab. 2009, 279, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogasawara, J.; Sakurai, T.; Kizaki, T.; Ishibashi, Y.; Izawa, T.; Sumitani, Y.; Ishida, H.; Radak, Z.; Haga, S.; Ohno, H. Higher levels of ATGL are associated with exercise-induced enhancement of lipolysis in rat epididymal adipocytes. PLoS ONE 2012, 7, e40876. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Shan, S.; Li, P.P.; Shen, Z.F.; Lu, X.P.; Cheng, J.; Ning, Z.Q. Peroxisome proliferator-activated receptor-gamma transcriptionally up-regulates hormone-sensitive lipase via the involvement of specificity protein-1. Endocrinology 2006, 147, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Sato, K.; Iemitsu, M. Exercise-inducible factors to activate lipolysis in adipocytes. J. Appl. Physiol. 2013, 115, 260–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcelin, G.; Chua, S., Jr. Contributions of adipocyte lipid metabolism to body fat content and implications for the treatment of obesity. Curr. Opin. Pharmacol. 2010, 10, 588–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, T.F.; Moreira, J.B.; Paixão, N.A.; Campos, J.C.; Monteiro, A.W.; Bacurau, A.V.; Bueno, C.R., Jr.; Ferreira, J.C.; Brum, P.C. Aerobic exercise training upregulates skeletal muscle calpain and ubiquitin-proteasome systems in healthy mice. J. Appl. Physiol. 2012, 112, 1839–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chibalin, A.V.; Yu, M.; Ryder, J.W.; Song, X.M.; Galuska, D.; Krook, A.; Wallberg-Henriksson, H.; Zierath, J.R. Exercise-induced changes in expression and activity of proteins involved in insulin signal transduction in skeletal muscle: Differential effects on insulin-receptor substrates 1 and 2. Proc. Natl. Acad. Sci. USA 2000, 97, 38–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewitt, M.; Estell, K.; Davis, I.C.; Schwiebert, L.M. Repeated bouts of moderate-intensity aerobic exercise reduce airway reactivity in a murine asthma model. Am. J. Respir. Cell Mol. Biol. 2010, 42, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Pastva, A.; Estell, K.; Schoeb, T.R.; Atkinson, T.P.; Schwiebert, L.M. Aerobic exercise attenuates airway inflammatory responses in a mouse model of atopic asthma. J. Immunol. 2004, 172, 4520–4526. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exerciseintensity and duration. Am. J. Physiol. 1993, 265, 380–391. [Google Scholar]
- Andrade, J.M.; Paraíso, A.F.; de Oliveira, M.V.; Martins, A.M.; Neto, J.F.; Guimarães, A.L.; de Paula, A.M.; Qureshi, M.; Santos, S.H. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition 2014, 30, 915–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudbrandsen, O.A.; Wergedahl, H.; Mørk, S.; Liaset, B.; Espe, M.; Berge, R.K. Dietary soya protein concentrate enriched with isoflavones reduced fatty liver, increased hepatic fatty acid oxidation and decreased the hepatic mRNA level of VLDL receptor in obese Zucker rats. Br. J. Nutr. 2006, 96, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peluso, M.R.; Winters, T.A.; Shanahan, M.F.; Banz, W.J. A cooperative interaction between soy protein and its isoflavone-enriched fraction lowers hepatic lipids in male obese Zucker rats and reduces blood platelet sensitivity in male Sprague-Dawley rats. J. Nutr. 2000, 130, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Kushida, M.; Iwagaki, Y.; Asano, M.; Yamamoto, K.; Tomata, Y.; Tsuji, I.; Tsuduki, T. The 1975 type Japanese diet improves lipid metabolic parameters in youngeradults: A randomized controlled trial. J. Oleo Sci. 2018, 67, 599–607. [Google Scholar] [CrossRef] [PubMed]
| Experiment 1 | Experiment 2 | ||||
|---|---|---|---|---|---|
| Control Diet | 2010 Miso Diet | 1975 Miso Diet | Control Diet | Miso Diet | |
| (g/100 g) | |||||
| Casein | 19.48 | 19.48 | 19.48 | 19.48 | 19.48 |
| Soybean oil | 6.82 | 6.82 | 6.82 | 6.82 | 6.82 |
| Lard | 19.48 | 19.48 | 19.48 | 19.48 | 19.48 |
| Cornstarch | 19.24 | 19.24 | 19.24 | 19.24 | 19.24 |
| α-Cornstarch | 12.86 | 12.86 | 12.86 | 12.86 | 12.86 |
| Sucrose | 9.74 | 9.74 | 9.74 | 9.74 | 9.74 |
| Cellulose | 4.87 | 4.87 | 4.87 | 4.87 | 4.87 |
| Mineral mix (AIN-93G-MX) | 3.41 | 3.41 | 3.41 | 3.41 | 3.41 |
| Vitamin mix (AIN-93-VX) | 0.97 | 0.97 | 0.97 | 0.97 | 0.97 |
| L-Cysteine | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 |
| Choline bitartrate | 0.24 | 0.24 | 0.24 | 0.24 | 0.24 |
| tert-Butylhydroquinone | 0.0014 | 0.0014 | 0.0014 | 0.0014 | 0.0014 |
| Freeze-dried miso | 0.00 | 1.60 | 2.60 | 0.00 | 2.60 |
| Miso-replaced material 1 | 2.60 | 1.00 | 0.00 | 2.60 | 0.00 |
| (kcal/100 g) | |||||
| Energy | 491 | 491 | 491 | 491 | 491 |
| Control | 2010 Miso | 1975 Miso | |
|---|---|---|---|
| Initial body weight (g) | 30.1 ± 0.5 | 30.1 ± 0.5 | 30.0 ± 0.5 |
| Final body weight (g) | 46.5 ± 1.5 | 46.6 ± 2.2 | 42.4 ± 1.4 |
| Food intake (g/day) | 3.60 ± 0.04 | 3.64 ± 0.06 | 3.64 ± 0.08 |
| Energy intake (kcal/day) | 17.7 ± 0.2 | 17.9 ± 0.3 | 17.9 ± 0.4 |
| Tissue weight (g/100 g body weight) | |||
| Brain | 1.03 ± 0.04 | 1.03 ± 0.04 | 1.15 ± 0.04 |
| Heart | 0.41 ± 0.02 a | 0.41 ± 0.01 a | 0.48 ± 0.02 b |
| Kidney | 1.35 ± 0.04 a | 1.37 ± 0.05 a | 1.59 ± 0.04 b |
| Liver | 3.61 ± 0.07 | 3.60 ± 0.06 | 3.63 ± 0.10 |
| Lung | 0.63 ± 0.07 | 0.65 ± 0.06 | 0.55 ± 0.02 |
| Pancreas | 0.81 ± 0.04 | 0.80 ± 0.02 | 0.80 ± 0.03 |
| Spleen | 0.23 ± 0.02 | 0.22 ± 0.02 | 0.26 ± 0.02 |
| White adipose tissue | |||
| Epididymal | 3.75 ± 0.32 b | 3.66 ± 0.38 b | 2.64 ± 0.18 a |
| Mesenteric | 1.69 ± 0.14 b | 1.49 ± 0.16 b | 0.99 ± 0.11 a |
| Perinephric | 2.22 ± 0.20 b | 2.07 ± 0.19 b | 1.23 ± 0.18 a |
| C | M | C + E | M + E | Interaction | |
|---|---|---|---|---|---|
| Initial body weight (g) | 30.0 ± 0.5 | 30.0 ± 0.5 | 30.0 ± 0.5 | 30.1 ± 0.5 | |
| Final body weight (g) | 42.5 ± 1.2 | 40.4 ± 1.0 | 39.2 ± 1.0 | 38.7 ± 1.2 | |
| Food intake (g/day) | 3.62 ± 0.09 | 3.62 ± 0.06 | 3.53 ± 0.10 | 3.45 ± 0.10 | |
| Energy intake (kcal/day) | 17.8 ± 0.4 | 17.8 ± 0.3 | 17.3 ± 0.5 | 16.9 ± 0.5 | |
| Tissue weight (g/100 g body weight) | |||||
| Brain | 1.13 ± 0.03 | 1.18 ± 0.04 | 1.24 ± 0.03 | 1.25 ± 0.03 | E |
| Heart | 0.51 ± 0.02 | 0.51 ± 0.01 | 0.52 ± 0.02 | 0.53 ± 0.02 | |
| Kidney | 1.61 ± 0.04 | 1.65 ± 0.05 | 1.74 ± 0.05 | 1.76 ± 0.05 | E |
| Liver | 3.67 ± 0.08 | 3.63 ± 0.10 | 3.56 ± 0.06 | 3.66 ± 0.10 | |
| Lung | 0.69 ± 0.06 | 0.70 ± 0.10 | 0.77 ± 0.06 | 0.79 ± 0.09 | |
| Pancreas | 0.84 ± 0.05 | 0.83 ± 0.03 | 0.84 ± 0.02 | 0.85 ± 0.04 | |
| Spleen | 0.27 ± 0.02 | 0.28 ± 0.02 | 0.32 ± 0.03 | 0.27 ± 0.02 | |
| White adipose tissue | |||||
| Epididymal | 3.59 ± 0.39 c | 2.84 ± 0.13 b,c | 2.38 ± 0.23 a,b | 1.52 ± 0.19 a | M, E |
| Mesenteric | 1.88 ± 0.16 c | 1.64 ± 0.13 b,c | 1.25 ± 0.14 a,b | 0.81 ± 0.11 a | M, E |
| Perinephric | 2.29 ± 0.16 c | 1.73 ± 0.18 b,c | 1.42 ± 0.15 b | 0.70 ± 0.11 a | M, E |
| Skeletal muscle | |||||
| Gastrocnemius | 0.87 ± 0.02 a | 0.89 ± 0.02 a,b | 0.92 ± 0.02 a,b | 0.95 ± 0.02 b | E |
| Quadriceps | 0.95 ± 0.03 a | 1.02 ± 0.05 a,b | 1.08 ± 0.03 a,b | 1.10 ± 0.03 b | E |
| Soleus | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.12 ± 0.01 | M |
| Tibialis | 0.33 ± 0.01 | 0.33 ± 0.01 | 0.35 ± 0.01 | 0.36 ± 0.01 | E |
| Gene Function | Gene Name | C | M | C + E | M + E | Interaction |
|---|---|---|---|---|---|---|
| Fatty acid synthesis | Acc | 1.00 ± 0.15 a,b | 0.81 ± 0.14 a | 1.94 ± 0.27 c | 1.60 ± 0.23 b,c | E |
| Fasn | 1.00 ± 0.16 | 0.99 ± 0.11 | 0.82 ± 0.12 | 0.74 ± 0.10 | ||
| Me | 1.00 ± 0.27 | 0.93 ± 0.23 | 0.88 ± 0.27 | 0.74 ± 0.24 | ||
| G6pdx | 1.00 ± 0.09 a,b | 0.83 ± 0.10 a | 1.22 ± 0.09 b | 1.12 ± 0.11 a,b | E | |
| Srebp1c | 1.00 ± 0.13 a | 0.81 ± 0.14 a | 1.68 ± 0.29 b | 1.22 ± 0.08 a,b | E | |
| Lipolysis | Atgl | 1.00 ± 0.17 a | 1.22 ± 0.11 a,b | 1.95 ± 0.27 b,c | 2.61 ± 0.32 c | E |
| Hsl | 1.00 ± 0.13 a | 1.23 ± 0.08 a,b | 1.73 ± 0.24 b | 2.91 ± 0.22 c | M, E, M × E | |
| Differentiation | Pparγ | 1.00 ± 0.14 a | 1.24 ± 0.13 a | 2.04 ± 0.25 b | 3.07 ± 0.24 c | M, E |
| C | M | C + E | M + E | Interaction | |
|---|---|---|---|---|---|
| Serum | |||||
| (mmol/L) | |||||
| TG | 1.06 ± 0.07 | 1.01 ± 0.08 | 1.10 ± 0.12 | 0.93 ± 0.09 | |
| TC | 3.05 ± 0.21 | 2.81 ± 0.18 | 2.94 ± 0.09 | 2.77 ± 0.19 | |
| PL | 2.58 ± 0.05 | 2.51 ± 0.17 | 2.41 ± 0.14 | 2.46 ± 0.15 | |
| Glucose | 7.13 ± 0.42 b | 6.30 ± 0.19 b | 5.72 ± 0.63 a,b | 4.53 ± 0.22 a | M, E |
| (pmol/L) | |||||
| Insulin | 36.2 ± 5.4 | 36.2 ± 5.4 | 30.1 ± 5.4 | 25.2 ± 2.7 | |
| Liver | |||||
| (µmol/g) | |||||
| TG | 56.6 ± 6.7 b | 46.6 ± 3.9 b | 50.6 ± 6.4 b | 25.5 ± 2.5 a | M, E |
| TC | 20.5 ± 2.0 b | 19.4 ± 1.2 a,b | 19.0 ± 1.6 a,b | 14.4 ± 0.8 a | M, E |
| PL | 43.9 ± 1.1 | 43.8 ± 0.9 | 45.2 ± 0.6 | 44.8 ± 0.7 | |
| C | M | C + E | M + E | Interaction | ||
|---|---|---|---|---|---|---|
| Fatty acid synthesis | Acc | 1.00 ± 0.19 b | 0.77 ± 0.07 a,b | 0.85 ± 0.10 b | 0.39 ± 0.04 a | M, E |
| Fasn | 1.00 ± 0.12 | 0.84 ± 0.10 | 1.05 ± 0.17 | 0.88 ± 0.09 | ||
| Me | 1.00 ± 0.14 | 1.25 ± 0.14 | 1.28 ± 0.12 | 1.14 ± 0.10 | ||
| G6pdx | 1.00 ± 0.02 | 1.07 ± 0.10 | 1.21 ± 0.09 | 1.24 ± 0.10 | E | |
| Srebp1c | 1.00 ± 0.11 | 0.86 ± 0.11 | 0.95 ± 0.04 | 1.07 ± 0.12 | ||
| β-oxidation | Aco | 1.00 ± 0.09 b | 0.93 ± 0.07 b | 0.81 ± 0.07 b | 0.39 ± 0.05 a | M, E, M × E |
| Pparα | 1.00 ± 0.07 | 1.11 ± 0.12 | 1.02 ± 0.08 | 0.96 ± 0.05 | ||
| Cholesterol synthesis | Hmgcr | 1.00 ± 0.19 b | 0.63 ± 0.12 a,b | 0.92 ± 0.15 b | 0.39 ± 0.07 a | M |
| Cholesterol catabolism | Cyp7a1 | 1.00 ± 0.11 | 1.24 ± 0.10 | 1.02 ± 0.13 | 1.08 ± 0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okouchi, R.; Sakanoi, Y.; Tsuduki, T. Miso (Fermented Soybean Paste) Suppresses Visceral Fat Accumulation in Mice, Especially in Combination with Exercise. Nutrients 2019, 11, 560. https://doi.org/10.3390/nu11030560
Okouchi R, Sakanoi Y, Tsuduki T. Miso (Fermented Soybean Paste) Suppresses Visceral Fat Accumulation in Mice, Especially in Combination with Exercise. Nutrients. 2019; 11(3):560. https://doi.org/10.3390/nu11030560
Chicago/Turabian StyleOkouchi, Ran, Yuto Sakanoi, and Tsuyoshi Tsuduki. 2019. "Miso (Fermented Soybean Paste) Suppresses Visceral Fat Accumulation in Mice, Especially in Combination with Exercise" Nutrients 11, no. 3: 560. https://doi.org/10.3390/nu11030560
APA StyleOkouchi, R., Sakanoi, Y., & Tsuduki, T. (2019). Miso (Fermented Soybean Paste) Suppresses Visceral Fat Accumulation in Mice, Especially in Combination with Exercise. Nutrients, 11(3), 560. https://doi.org/10.3390/nu11030560





