Association between Neonatal Whole Blood Iron Content and Cytokines, Adipokines, and Other Immune Response Proteins
Abstract
:1. Introduction
2. Materials and Methods
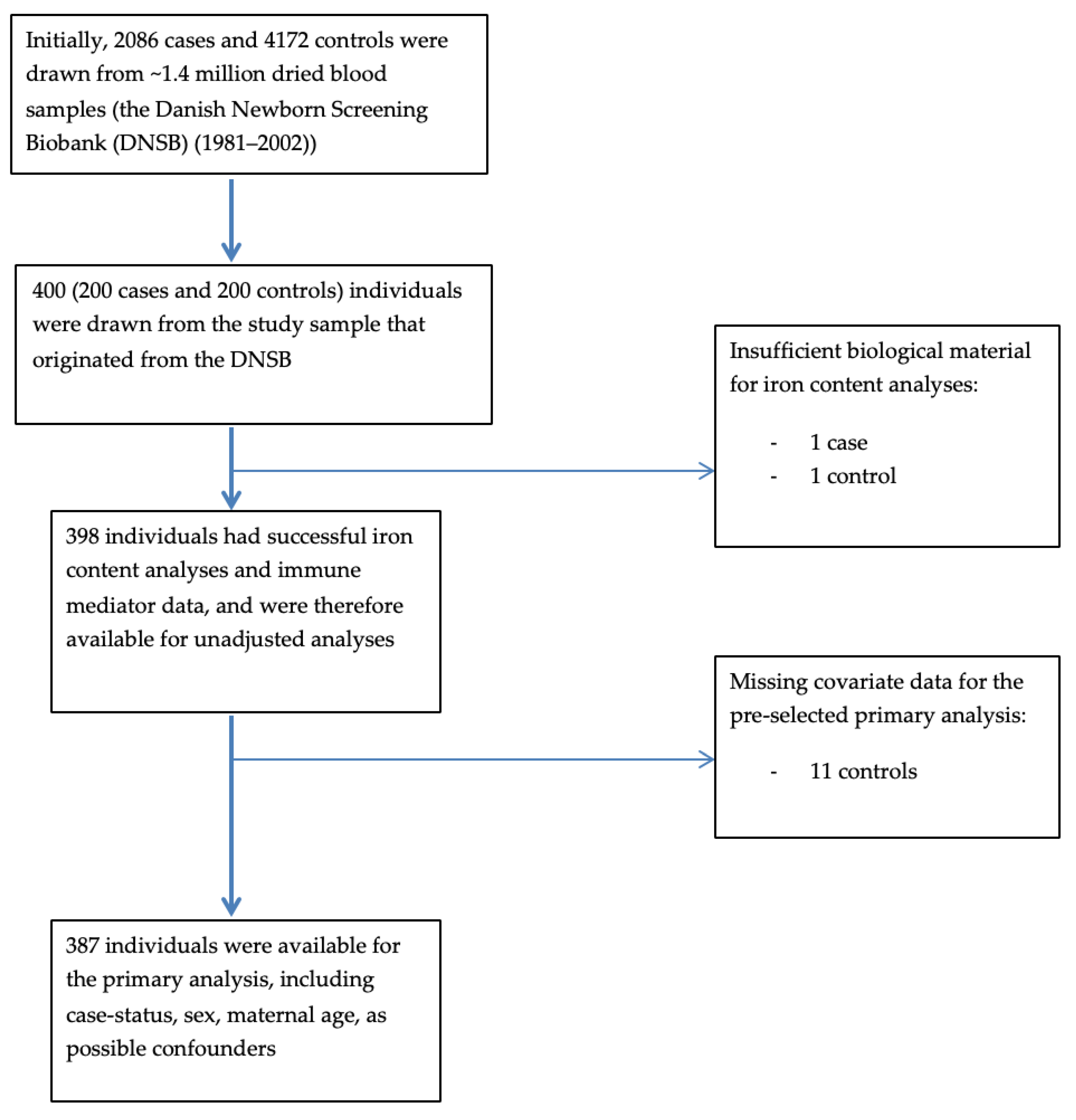
2.1. Overview of Study Design and Sampling
2.2. Exposure Assessment
2.2.1. Assessment of Whole Blood Iron Content
2.2.2. Other Variables
2.3. Outcome Assessment
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Basic Characteristics
3.2. Unadjusted Models
3.3. Adjusted Models
3.4. Sensitivity Analyses
4. Discussion
4.1. Comparison with Other Studies
4.2. Strengths and Limitations
4.3. Future Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Zhivaki, D.; Lo-Man, R. Unique aspects of the perinatal immune system. Nat. Rev. Immunol. 2017, 17, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Brook, B.; Harbeson, D.; Ben-Othman, R.; Viemann, D.; Kollmann, T.R. Newborn susceptibility to infection vs. disease depends on complex in vivo interactions of host and pathogen. Semin. Immunopathol. 2017, 39, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Scorza, M.; Liguori, R.; Elce, A.; Salvatore, F.; Castaldo, G. Biological role of mannose binding lectin: From newborns to centenarians. Clinica. Chimica. Acta. 2015, 451, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Iron and infection. Int. J. Hematol. 2018, 107, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. The role of hepcidin in iron metabolism. Acta. Haematol. 2009, 122, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015, 15, 500–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassat, J.E.; Skaar, E.P. Iron in Infection and Immunity. Cell Host. Microbe. 2013, 13, 509–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moen, I.W.; Bergholdt, H.K.M.; Mandrup-Poulsen, T.; Nordestgaard, B.G.; Ellervik, C. Increased Plasma Ferritin Concentration and Low-Grade Inflammation-A Mendelian Randomization Study. Clin. Chem. 2018, 64, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Cherayil, B.J. Iron and immunity: immunological consequences of iron deficiency and overload. Arch. Immunol. Ther. Exp. (Warsz) 2010, 58, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G. Iron and immunity: a double-edged sword. Eur. J. Clin. Investig. 2002, 32, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Kyvsgaard, J.N.; Overgaard, A.J.; Thorsen, S.U.; Hansen, T.H.; Pipper, C.B.; Mortensen, H.B.; Pociot, F.; Svensson, J. High Neonatal Blood Iron Content Is Associated with the Risk of Childhood Type 1 Diabetes Mellitus. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Ellervik, C.; Mandrup-Poulsen, T.; Andersen, H.U.; Tybjærg-Hansen, A.; Frandsen, M.; Birgens, H.; Nordestgaard, B.G. Elevated Transferrin Saturation and Risk of Diabetes. Diabetes Care 2011, 34, 2256–2258. [Google Scholar] [CrossRef] [PubMed]
- Ellervik, C.; Mandrup-Poulsen, T.; Nordestgaard, B.G.; Larsen, L.E.; Appleyard, M.; Frandsen, M.; Petersen, P.; Schlichting, P.; Saermark, T.; Tybjaerg-Hansen, A.; et al. Prevalence of hereditary haemochromatosis in late-onset type 1 diabetes mellitus: A retrospective study. Lancet 2001, 358, 1405–1409. [Google Scholar] [CrossRef]
- Backe, M.B.; Moen, I.W.; Ellervik, C.; Hansen, J.B.; Mandrup-Poulsen, T. Iron Regulation of Pancreatic Beta-Cell Functions and Oxidative Stress. Annu. Rev. Nutr. 2016, 36, 241–273. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, K.L.; Ellervik, C.; Svensson, J.; Thorsen, S.U. The Role of Iron in Type 1 Diabetes Etiology: A Systematic Review of New Evidence on a Long-Standing Mystery. Rev. Diabet. Stud. 2017, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Dunmore, S.J.; Brown, J.E.P. The role of adipokines in β-cell failure of type 2 diabetes. J. Endocrinol. 2013, 216, T37–T45. [Google Scholar] [CrossRef] [PubMed]
- Wessling-Resnick, M. Iron homeostasis and the inflammatory response. Annu. Rev. Nutr. 2010, 30, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard-Pedersen, B.; Hougaard, D.M. Storage policies and use of the Danish Newborn Screening Biobank. J. Inherit. Metab. Dis. 2007, 30, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Hollegaard, M.V.; Grauholm, J.; Nielsen, R.; Grove, J.; Mandrup, S.; Hougaard, D.M. Archived neonatal dried blood spot samples can be used for accurate whole genome and exome-targeted next-generation sequencing. Mol. Genet. Metab. 2013, 110, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, S.U.; Pipper, C.B.; Eising, S.; Skogstrand, K.; Hougaard, D.M.; Svensson, J.; Pociot, F. Neonatal levels of adiponectin, interleukin-10 and interleukin-12 are associated with the risk of developing type 1 diabetes in childhood and adolescence: A nationwide Danish case-control study. Clin. Immunol. 2017, 174, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Cerqueira, C.; Kjærsgaard, P.; Lyngsøe, L.; Hertel, N.T.; Madsen, M.; Mortensen, H.B.; Johannesen, J. Danish Registry of Childhood and Adolescent Diabetes. Clin. Epidemiol. 2016, 8, 679. [Google Scholar] [CrossRef] [PubMed]
- Capiau, S.; Stove, V.V.; Lambert, W.E.; Stove, C.P. Prediction of the hematocrit of dried blood spots via potassium measurement on a routine clinical chemistry analyzer. Anal. Chem. 2013, 85, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Scharpf, R.B.; Bravo, H.C.; Simcha, D.; Langmead, B.; Johnson, W.E.; Geman, D.; Baggerly, K.; Irizarry, R.A. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat. Rev. Genet. 2010, 11, 733–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, J.S.; Zoriy, M.; Matusch, A.; Wu, B.; Salber, D.; Palm, C.; Becker, J.S. Bioimaging of metals by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Mass Spectrom. Rev. 2010, 29, 156–175. [Google Scholar] [CrossRef] [PubMed]
- Konz, I.; Fernández, B.; Fernández, M.L.; Pereiro, R.; Sanz-Medel, A. Laser ablation ICP-MS for quantitative biomedical applications. Anal. Bioanal. Chem. 2012, 403, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Skogstrand, K.; Thorsen, P.; Nørgaard-Pedersen, B.; Schendel, D.E.; Sørensen, L.C.; Hougaard, D.M. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin. Chem. 2005, 51, 1854–1866. [Google Scholar] [CrossRef] [PubMed]
- Skogstrand, K. Multiplex assays of inflammatory markers, a description of methods and discussion of precautions—Our experience through the last ten years. Methods (San Diego Calif.) 2012, 56, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Pipper, C.B.; Ritz, C.; Bisgaard, H. A versatile method for confirmatory evaluation of the effects of a covariate in multiple models. J. R. Stat. Soc. Ser. C (Appl. Stat.) 2012, 61, 315–326. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Interaction revisited: The difference between two estimates. BMJ 2003, 326, 219. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Riejos, P.; Najib, S.; Santos-Alvarez, J.; Martín-Romero, C.; Pérez-Pérez, A.; González-Yanes, C.; Sánchez-Margalet, V. Role of Leptin in the Activation of Immune Cells. Mediators Inflamm. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- La Cava, A. Leptin in inflammation and autoimmunity. Cytokine 2017, 98, 51–58. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, I.; Zhang, T.Y.; Koening, C.L.; Branch, R.W.; London, N.; Lo, E.; Daynes, R.A.; Kushner, J.P.; Li, D.; Ward, D.M.; et al. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J. Clin. Investig. 2010, 120, 2395–2405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maródi, L. Neonatal Innate Immunity to Infectious Agents. Infect. Immun. 2006, 74, 1999–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, J.H.; Bradbury, R.S.; Fulford, A.J.; Jallow, A.T.; Wegmüller, R.; Prentice, A.M.; Cerami, C. Oral iron acutely elevates bacterial growth in human serum. Sci. Rep. 2015, 5, 16670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Harrington, L.; Trebicka, E.; Shi, H.N.; Kagan, J.C.; Hong, C.C.; Lin, H.Y.; Babitt, J.L.; Cherayil, B.J. Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J. Clin. Investig. 2009, 119, 3322–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jason, J.; Archibald, L.K.; Nwanyanwu, O.C.; Bell, M.; Jensen, R.J.; Gunter, E.; Buchanan, I.; Larned, J.; Kazembe, P.N.; Dobbie, H.; et al. The effects of iron deficiency on lymphocyte cytokine production and activation: Preservation of hepatic iron but not at all cost. Clin. Exp. Immunol. 2001, 126, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, F.T.; Dijkhuizen, M.A.; West, C.E.; Ven-Jongekrijg, J.; van der Meer, J.W.M. Reduced production of immunoregulatory cytokines in vitamin A- and zinc-deficient Indonesian infants. Eur. J. Clin. Nutr. 2004, 58, 1498–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sildorf, S.M.; Eising, S.; Hougaard, D.M.; Mortensen, H.B.; Skogstrand, K.; Pociot, F.; Johannesen, J.; Svensson, J. Differences in MBL levels between juvenile patients newly diagnosed with type 1 diabetes and their healthy siblings. Mol. Immunol. 2014, 62, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Hempel, J.C.; Poppelaars, F.; da Costa, M.G.; Franssen, C.F.M.; de Vlaam, T.P.G.; Daha, M.R.; Berger, S.P.; Seelen, M.A.J.; Gaillard, C.A.J.M. Distinct in vitro Complement Activation by Various Intravenous Iron Preparations. AJN 2017, 45, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Safai, N.; Eising, S.; Hougaard, D.M.; Mortensen, H.B.; Skogstrand, K.; Pociot, F.; Johannesen, J.; Svensson, J. Levels of adiponectin and leptin at onset of type 1 diabetes have changed over time in children and adolescents. Acta. Diabetol. 2015, 52, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Z.; Gabrielsen, J.S.; Simcox, J.A.; Lee, S.; Jones, D.; Cooksey, B.; Stoddard, G.; Cefalu, W.T.; McClain, D.A. Adipocyte iron regulates leptin and food intake. J. Clin. Investig. 2015, 125, 3681–3691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, T.H.S.; Britton, G.J.; Hill, E.V.; Verhagen, J.; Burton, B.R.; Wraith, D.C. Regulation of adaptive immunity; the role of interleukin-10. Front. Immunol. 2013, 4, 129. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, J.; Lin, X.; Yang, F.-F.; Tan, J.-H. Effects of IL-10 on iron metabolism in LPS-induced inflammatory mice via modulating hepcidin expression. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3469–3475. [Google Scholar] [PubMed]
- Barbosa, M.C.; Dos Santos, T.E.J.; de Souza, G.F.; de Assis, L.C.; Freitas, M.V.C.; Gonçalves, R.P. Impact of iron overload on interleukin-10 levels, biochemical parameters and oxidative stress in patients with sickle cell anemia. Rev. Bras. Hematol. Hemoter. 2013, 35, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Vosters, O.; Lombard, C.; André, F.; Sana, G.; Sokal, E.M.; Smets, F. The interferon-alpha and interleukin-10 responses in neonates differ from adults, and their production remains partial throughout the first 18 months of life. Clin. Exp. Immunol. 2010, 162, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Balesaria, S.; Hanif, R.; Salama, M.F.; Raja, K.; Bayele, H.K.; McArdle, H.; Srai, S.K.S. Fetal iron levels are regulated by maternal and fetal Hfe genotype and dietary iron. Haematologica 2012, 97, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Størdal, K.; McArdle, H.J.; Hayes, H.; Tapia, G.; Viken, M.K.; Lund-Blix, N.A.; Haugen, M.; Joner, G.; Skrivarhaug, T.; Mårild, K.; et al. Prenatal iron exposure and childhood type 1 diabetes. Sci. Rep. 2018, 8, 9067. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, S.F.; Carvalho, I.F.; Cardoso, C.S.; Cordeiro, J.V.; Azevedo, J.E.; Neefjes, J.; de Sousa, M. HFE cross-talks with the MHC class I antigen presentation pathway. Blood 2005, 106, 971–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munafò, M.R.; Smith, G.D. Robust research needs many lines of evidence. Nature 2018, 553, 399. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, B.; Esko, T.; Ried, J.S.; Radhakrishnan, A.; Vermeulen, S.H.; Traglia, M.; Gögele, M.; Anderson, D.; Broer, L.; Podmore, C.; et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat. Commun. 2014, 5, 4926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | Cases (n = 199) | Controls (n = 199) |
|---|---|---|
| Sex 1 | ||
| Female, n/% of total | 103/51.8 | 95/47.7 |
| Gestational age 2, | ||
| Median/interquartile range (IQR), weeks | 40.0/1.0 | 40.0/2.0 |
| Birth weight 3, | ||
| Median/IQR, grams | 3500/630 | 3500/744 |
| Maternal age 4, | ||
| Median/IQR, years | 29.0/6.0 | 28.0/8.0 |
| Season of blood sampling, n/% from total | ||
| Winter Spring Summer Autumn | 41/20.6 49/24.6 59/29.6 50/25.1 | 40/20.1 49/24.6 60/30.2 50/25.1 |
| Period of blood sampling, n/% from total | ||
| 1991–1993 1994–1998 | 109/54.8 90/45.2 | 109/54.8 90/45.2 |
| Human leukocyte antigen (HLA)-risk groups 5, n/% from total | ||
| High/moderate 6 Low/protective 7 | 151/82.5 32/17.5 | 68/40.0 102/60.0 |
| Outcome | Variable | Univariate Model | p-value | Multivariate Model | p-value |
|---|---|---|---|---|---|
| IL-1β | WB-Iron content | 1.42 (0.97; 2.07) | 0.07 | 1.37 (0.93; 2.03) | 0.11 |
| IL-4 | WB-Iron content | 1.03 (0.82; 1.30) | 0.78 | 1.05 (0.83; 1.33) | 0.69 |
| IL-6 | WB-Iron content | 0.62 (0.40; 0.95) | 0.03 | 0.62 (0.40; 0.95) | 0.03 |
| IL-8 | WB-Iron content | 1.09 (0.89; 1.32) | 0.41 | 1.13 (0.92; 1.39) | 0.25 |
| IL-10 | WB-Iron content | 0.78 (0.43; 1.42) | 0.41 | 0.69 (0.37; 1.27) | 0.23 |
| IL-12 | WB-Iron content | 1.25 (0.90; 1.75) | 0.19 | 1.25 (0.90; 1.75) | 0.18 |
| IFNγ | WB-Iron content | 1.10 (0.84; 1.45) | 0.47 | 1.09 (0.83; 1.44) | 0.53 |
| TNFα | WB-Iron content | 0.94 (0.67; 1.32) | 0.70 | 0.99 (0.70; 1.40) | 0.95 |
| TGFβ | WB-Iron content | 0.92 (0.72; 1.18) | 0.52 | 0.96 (0.76; 1.21) | 0.73 |
| Adiponectin | WB-Iron content | 1.09 (0.89; 1.34) | 0.39 | 1.12 (0.91; 1.37) | 0.28 |
| Leptin | WB-Iron content | 0.79 (0.59; 1.05) | 0.11 | 0.85 (0.65; 1.12) | 0.26 |
| CRP | WB-Iron content | 0.81 (0.56; 1.17) | 0.26 | 0.76 (0.52; 1.12) | 0.17 |
| MBL | WB-Iron content | 0.62 (0.41; 0.95) | 0.03 | 0.63 (0.41; 0.95) | 0.03 |
| sTREM-1 | WB-Iron content | 0.97 (0.69; 1.37) | 0.88 | 0.98 (0.69; 1.39) | 0.90 |
| Outcome | Variable | Univariate Model | p-value | Multivariate Model | p-value |
|---|---|---|---|---|---|
| IL-1β | WB-Iron content | 1.42 (0.81; 2.48) | 0.59 | 1.37 (0.77; 2.44) | 0.74 |
| IL-4 | WB-Iron content | 1.03 (0.74; 1.45) | 1.00 | 1.05 (0.74; 1.49) | 1.00 |
| IL-6 | WB-Iron content | 0.62 (0.33; 1.16) | 0.28 | 0.62 (0.33; 1.16) | 0.29 |
| IL-8 | WB-Iron content | 1.09 (0.81; 1.45) | 1.00 | 1.13 (0.83; 1.53) | 0.97 |
| IL-10 | WB-Iron content | 0.78 (0.32; 1.89) | 1.00 | 0.69 (0.28; 1.70) | 0.95 |
| IL-12 | WB-Iron content | 1.25 (0.77; 2.04) | 0.91 | 1.25 (0.77; 2.04) | 0.90 |
| IFNγ | WB-Iron content | 1.10 (0.74; 1.65) | 1.00 | 1.09 (0.73; 1.64) | 1.00 |
| TNFα | WB-Iron content | 0.94 (0.57; 1.55) | 1.00 | 0.99 (0.59; 1.64) | 1.00 |
| TGFβ | WB-Iron content | 0.92 (0.64; 1.32) | 1.00 | 0.96 (0.68; 1.36) | 1.00 |
| Adiponectin | WB-Iron content | 1.09 (0.81; 1.48) | 1.00 | 1.12 (0.83; 1.51) | 0.98 |
| Leptin | WB-Iron content | 0.79 (0.51; 1.21) | 0.73 | 0.85 (0.57; 1.28) | 0.97 |
| CRP | WB-Iron content | 0.81 (0.47; 1.40) | 0.97 | 0.76 (0.43; 1.35) | 0.89 |
| MBL | WB-Iron content | 0.62 (0.33; 1.15) | 0.28 | 0.63 (0.34; 1.16) | 0.29 |
| sTREM-1 | WB-Iron content | 0.97 (0.59; 1.61) | 1.00 | 0.98 (0.58; 1.65) | 1.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorsen, S.U.; Pipper, C.B.; Ellervik, C.; Pociot, F.; Kyvsgaard, J.N.; Svensson, J. Association between Neonatal Whole Blood Iron Content and Cytokines, Adipokines, and Other Immune Response Proteins. Nutrients 2019, 11, 543. https://doi.org/10.3390/nu11030543
Thorsen SU, Pipper CB, Ellervik C, Pociot F, Kyvsgaard JN, Svensson J. Association between Neonatal Whole Blood Iron Content and Cytokines, Adipokines, and Other Immune Response Proteins. Nutrients. 2019; 11(3):543. https://doi.org/10.3390/nu11030543
Chicago/Turabian StyleThorsen, Steffen U., Christian B. Pipper, Christina Ellervik, Flemming Pociot, Julie N. Kyvsgaard, and Jannet Svensson. 2019. "Association between Neonatal Whole Blood Iron Content and Cytokines, Adipokines, and Other Immune Response Proteins" Nutrients 11, no. 3: 543. https://doi.org/10.3390/nu11030543
APA StyleThorsen, S. U., Pipper, C. B., Ellervik, C., Pociot, F., Kyvsgaard, J. N., & Svensson, J. (2019). Association between Neonatal Whole Blood Iron Content and Cytokines, Adipokines, and Other Immune Response Proteins. Nutrients, 11(3), 543. https://doi.org/10.3390/nu11030543





