Association between Nutritional Status and Mortality after Aortic Valve Replacement Procedure in Elderly with Severe Aortic Stenosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anthropometry
2.2. Nutritional Status
2.3. Comorbidity Index
2.4. Biochemistry
2.5. Postoperative Complications/Adverse Events and Mortality
2.6. Statistics
3. Results
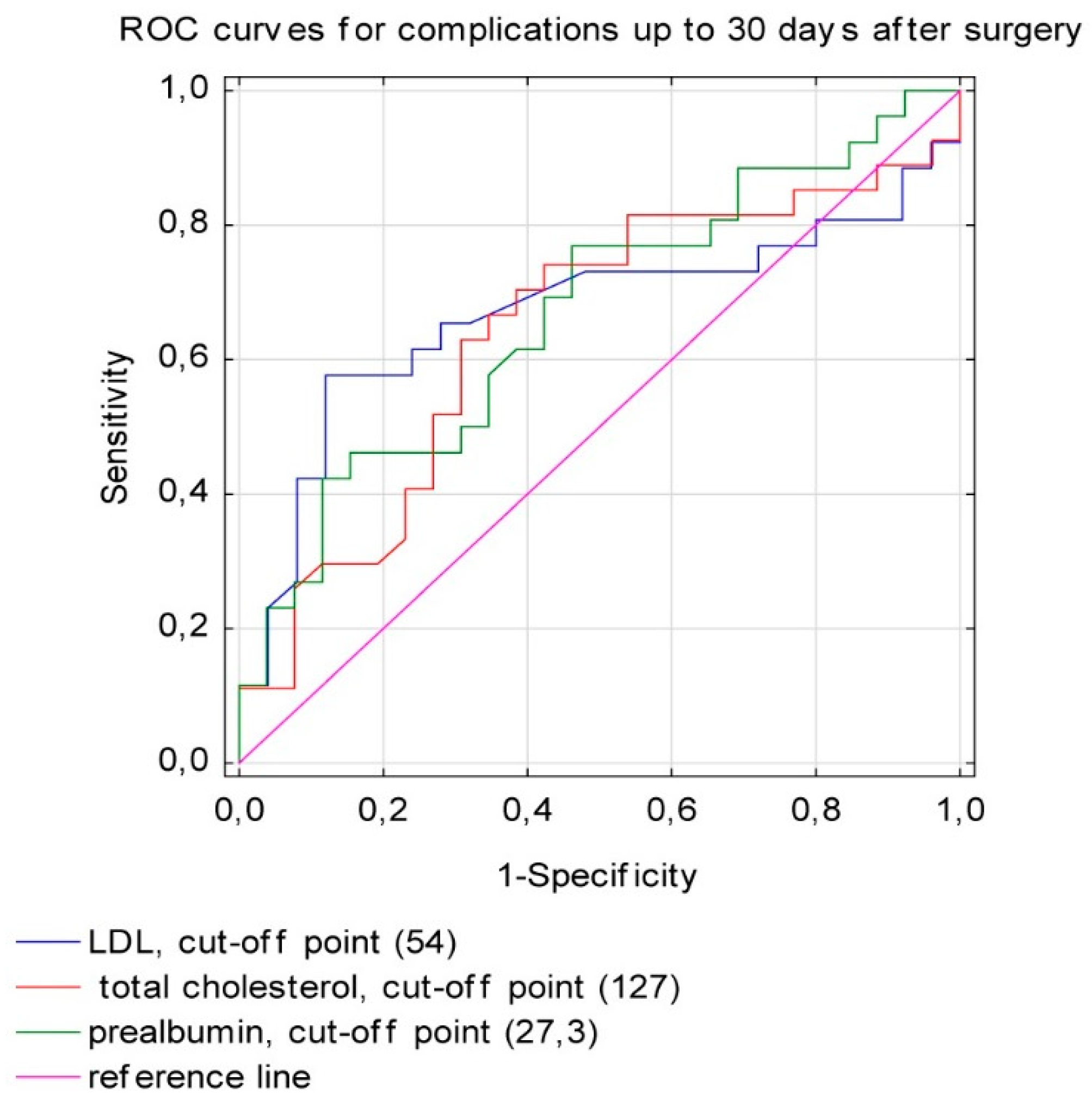
3.1. The Postoperative Complications
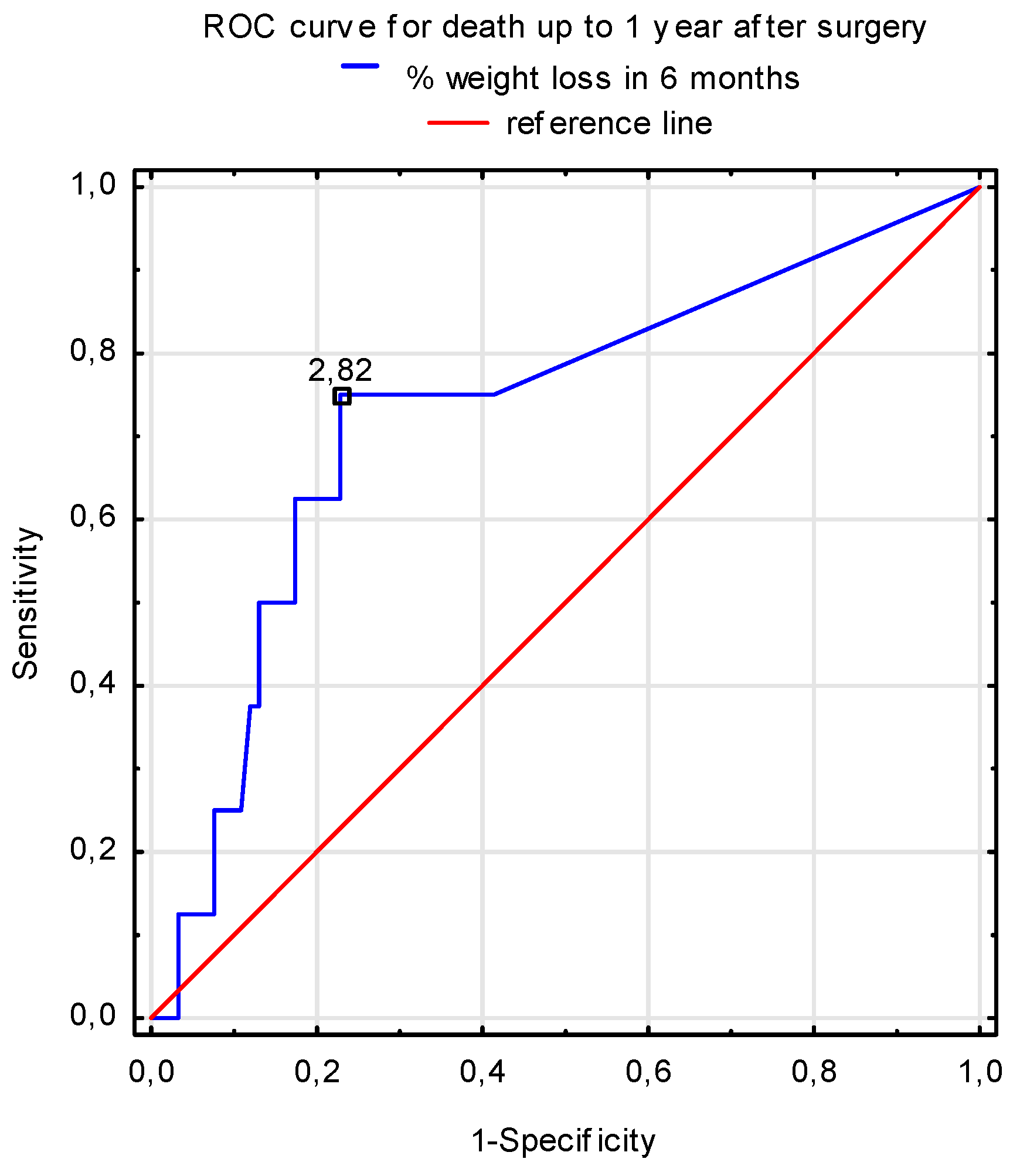
3.2. The Mortality Rate
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wernio, E.; Jagielak, D.; Dardzińska, J.; Aleksandrowicz-Wrona, E.; Rogowski, J.; Gruszecka, A.; Małgorzewicz, S. Analysis of Outcomes of the Nutritional Status in Patients Qualified for Aortic Valve Replacement in Comparison to Healthy Elderly. Nutrients 2018, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.; Hankey, C. Aging, Nutritional Status, and Health. Healthcare 2015, 3, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Amarya, S.; Singh, K.; Sabharwal, M. Changes during aging and their association with malnutrition. J. Clin. Gerontol. Geriatr. 2015, 6, 78–84. [Google Scholar] [CrossRef]
- Evans, C. Malnutrition in the elderly: A multifactorial failure to thrive. Perm. J. 2005, 9, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Fávaro-Moreira, N.C.; Krausch-Hofmann, S.; Matthys, C.; Vereecken, C.; Vanhauwaert, E.; Declercq, A.; Bekkering, G.E.; Duyck, J. Risk Factors for Malnutrition in Older Adults: A Systematic Review of the Literature Based on Longitudinal Data. Adv. Nutr. 2016, 7, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed]
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef]
- Di Eusanio, M.; Fortuna, D.; De Palma, R.; Dell’Amore, A.; Lamarra, M.; Contini, G.A.; Gherli, T.; Gabbieri, D.; Ghidoni, I.; Cristell, D.; et al. Aortic valve replacement: Results and predictors of mortality from a contemporary series of 2256 patients. J. Thorac. Cardiovasc. Surg. 2011, 141, 940–947. [Google Scholar] [CrossRef]
- Babiarczyk, B.; Turbiarz, A. Body Mass Index in elderly people—Do the reference ranges matter? Prog. Health Sci. 2012, 2, 58–67. [Google Scholar]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Nestle Nutrition Institute. A Guide to Completing the Mini Nutritional Assessment Shor-Short: Nestle Nutrition Institute. 2018. Available online: https://www.mna-elderly.com/forms/mna_guide_english_sf.pdf (accessed on 12 December 2018).
- Visser, R.; Dekker, F.W.; Boeschoten, E.W.; Stevens, P.; Krediet, R.T. Reliability of the 7-point subjective global assessment scale in assessing nutritional status of dialysis patients. Adv. Perit. Dial. Conf. Perit. Dial. 1999, 15, 222–225. [Google Scholar]
- Wilson, M.M.; Thomas, D.R.; Rubenstein, L.Z.; Chibnall, J.T.; Anderson, S.; Baxi, A.; Diebold, M.R.; Morley, J.E. Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am. J. Clin. Nutr. 2005, 82, 1074–1081. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Iung, B.; Vahanian, A. Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 2011, 8, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Prendergast, B. Aortic-valve stenosis–from patients at risk to severe valve obstruction. N. Engl. J. Med. 2014, 371, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Basra, S.S.; Skolnick, A.H.; Wenger, N.K. Aortic valve disease in the older adult. J. Geriatr. Cardiol. 2016, 13, 941–944. [Google Scholar] [PubMed]
- Chermesh, I.; Hajos, J.; Mashiach, T.; Bozhko, M.; Shani, L.; Nir, R.R.; Bolotin, G. Malnutrition in cardiac surgery: Food for thought. Eur. J. Prev. Cardiol. 2014, 21, 475–483. [Google Scholar] [CrossRef]
- Eichler, S.; Salzwedel, A.; Harnath, A.; Butter, C.; Wegscheider, K.; Chiorean, M.; Völler, H.; Reibis, R. Nutrition and mobility predict all-cause mortality in patients 12 months after transcatheter aortic valve implantation. Clin. Res. Cardiol. 2017, 107, 304–311. [Google Scholar] [CrossRef]
- Engelman, D.T.; Adams, D.H.; Byrne, J.G.; Aranki, S.F.; Collins, J.J., Jr.; Couper, G.S.; Allred, E.N.; Cohn, L.H.; Rizzo, R.J. Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J. Thorac. Cardiovasc. Surg. 1999, 118, 866–873. [Google Scholar] [CrossRef]
- Nochioka, K.; Sakata, Y.; Takahashi, J.; Miyata, S.; Miura, M.; Takada, T.; Fukumoto, Y.; Shiba, N.; Shimokawa, H. CHART-2 Investigators Prognostic Impact of Nutritional Status in Asymptomatic Patients with Cardiac Diseases. Circ. J. 2013, 77, 2318–2326. [Google Scholar] [CrossRef]
- Shenkin, A. Serum prealbumin: Is it a marker of nutritional status or of risk of malnutrition? Clin. Chem. 2006, 52, 2177–2179. [Google Scholar] [CrossRef] [PubMed]
- Delliere, S.; Cynober, L. Is transthyretin a good marker of nutritional status? Clin. Nutr. 2017, 36, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y.; Young, V.R. Significance of transthyretin in protein metabolism. Clin. Chem. Lab. Med. 2002, 40, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Critsinelis, A.C.; Kurihara, C.; Kawabori, M.; Sugiura, T.; Civitello, A.B.; Morgan, J.A. Preoperative Prealbumin Level as a Predictor of Outcomes in Patients Who Underwent Left Ventricular Assist Device Implantation. Am. J. Cardiol. 2017, 120, 1998–2002. [Google Scholar] [CrossRef]
- Yu, P.J.; Cassiere, H.A.; Dellis, S.L.; Manetta, F.; Kohn, N.; Hartman, A.R. Impact of Preoperative Prealbumin on Outcomes After Cardiac Surgery. JPEN J. Parent. Enter. Nutr. 2015, 39, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.P.; Friões, F.; Azevedo, A.; Lourenço, P.; Rocha-Gonçalves, F.; Ferreira, A.; Bettencourt, P. Cholesterol—A marker of nutritional status in mild to moderate heart failure. Int. J. Cardiol. 2008, 129, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, H.; Raman, N.; Täger, T.; Schellberg, D.; Goode, K.M.; Kazmi, S.; Grundtvig, M.; Hole, T.; Cleland, J.G.; Katus, H.A.; Agewall, S. Statins attenuate but do not eliminate the reverse epidemiology of total serum cholesterol in patients with non-ischemic chronic heart failure. Int. J. Cardiol. 2017, 238, 97–104. [Google Scholar] [CrossRef]
- Wang, J.; Hong, Z. Low Plasma Total Cholesterol Concentration: A Sensitive Evaluation Marker in Hospitalized Patients with Nutritional Deficiency Malnutrition. J. Food Nutr. Res. 2014, 2, 551–555. [Google Scholar] [CrossRef]
- Pocock, S.J.; McMurray, J.J.; Dobson, J.; Yusuf, S.; Granger, C.B.; Michelson, E.L.; Östergren, J.; Pfeffer, M.A.; Solomon, S.D.; Anker, S.D.; Swedberg, K.B. Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: Assessment of reduction in mortality and morbidity (CHARM) programme. Eur. Heart J. 2008, 29, 2641–2650. [Google Scholar] [CrossRef]
- Fleming, I.O.; Garratt, C.; Guha, R.; Desai, J.; Chaubey, S.; Wang, Y.; Leonard, S.; Kunst, G. Aggregation of Marginal Gains in Cardiac Surgery: Feasibility of a Perioperative Care Bundle for Enhanced Recovery in Cardiac Surgical Patients. J. Cardiothorac. Vasc. Anesth. 2016, 30, 665–670. [Google Scholar] [CrossRef]
- Krzych, L.; Kucewicz-Czech, E. It is time for enhanced recovery after surgery in cardiac surgery. Kardiologia Polska 2017, 75, 415–420. [Google Scholar] [CrossRef] [PubMed]

| Parameters (Mean ± SD or Median and Range) | Patients with AS (n = 101) |
|---|---|
| Age (years) | 74.6 ± 5.2 |
| Age-adjusted CCI | 4 (2–9) |
| Length of hospital stay (days) | 9.96 ± 5 |
| Number of medications taken | 5 (2–11) |
| Female/male (n) | 48/53 |
| AVA (cm2) | 0.73 ± 0.2 |
| Mean gradient (mm Hg) | 47.4 ± 12.9 |
| Peak aortic velocity (m/s) | 4.4 ± 0.6 |
| LVEF (%) | 50 (15–80) |
| EUROScore II | 1.98 (0.7–72) |
| Surgical procedure (n, %) | |
| AVR | 54 (54) |
| AVR + CABG | 32 (32) |
| AVR + MRV | 1 (1) |
| AVR + MRV + CABG | 2 (2) |
| TAVI | 12 (12) |
| Comorbid diseases (%) | |
| Diabetes | 30 |
| Hypertension | 93 |
| Hypercholesterolemia | 24 |
| Chronic renal disease | 13 |
| Chronic obstructive pulmonary disease | 9 |
| Coronary artery disease | 70 |
| Chronic heart failure | 10 |
| Peripheral vascular diseases | 3 |
| History of myocardial infarction | 3 |
| History of stroke | 2 |
| Patients with AS (n = 101) | Mean ± SD or Median and Range |
|---|---|
| f-MNA | 24.3 ± 2.55 |
| 7-SGA | 6 (2-6) |
| SNAQ | 15.76 ± 1.8 |
| BMI (kg/m2) | 28.9 ± 5.7 |
| HGS (kg) | 26.5 ± 9.6 |
| Weight loss (%) | 0 (0–11) |
| Phase angle (50 kHz) | 8.7 ± 1.8 |
| Biochemical parameters | |
| Albumin (g/L) | 36.7 ± 6.7 |
| Prealbumin (mg/dL) | 31.83 ± 7.03 |
| White blood cells (×109/L) | 7.6 ± 2.1 |
| Total number of lymphocytes (/mm3) | 1.8 ± 0.6 |
| Red blood cells (×109/L) | 4.4 ± 0.5 |
| Hemoglobin (mg/dL) | 13.2 ± 1.4 |
| CRP (mg/dL) | 1.73 (0.01–18.4) |
| HDL-cholesterol (mg/dL) | 47.9 ± 14.4 |
| LDL-cholesterol (mg/dL) | 67.5 (23–177) |
| Total cholesterol (mg/dL) | 144.1 ± 42.1 |
| n = 101 | AUC | SE | AUC Lower 95% | AUC Upper 95% | p-Value |
|---|---|---|---|---|---|
| f-MNA | 0.624 | 0.056 | 0.514 | 0.734 | 0.027 |
| 7-SGA | 0.374 | 0.053 | 0.269 | 0.479 | 0.023 |
| SNAQ | 0.428 | 0.055 | 0.320 | 0.537 | 0.195 |
| n = 101 | AUC | SE | AUC Lower 95% | AUC Upper 95% | p-Value |
|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | 0.642 | 0.078 | 0.49 | 0.795 | 0.007 |
| LDL-cholesterol (mg/dL) | 0.668 | 0.08 | 0.511 | 0.826 | 0.036 |
| Prealbumin (mg/dL) | 0.668 | 0.075 | 0.52 | 0.816 | 0.025 |
| Data Presented as mean ± SD or Median and Range | Complicated (n = 50) | Uncomplicated (n = 51) | p-Value |
|---|---|---|---|
| Female/male (n) | 22/28 | 26/25 | 0.434 |
| Age (years) | 75.5 ± 5.4 | 73.9 ± 5 | 0.062 |
| Age adjusted CCI | 5 (2–9) | 4 (3–8) | 0.308 |
| Hospital length of stay (days) | 11.1 ± 5.8 | 8.8 ± 3.8 | 0.038 |
| LVEF (%) | 50 ± 9 | 53.4 ± 8 | 0.079 |
| AVA (cm2) | 0.735 ± 0.18 | 0.889 ± 0.9 | 0.659 |
| Flow speed (m/s) | 4.4 ± 0.7 | 4.4 ± 0.5 | 0.907 |
| Mean gradient (mm Hg) | 47.5 ± 12.9 | 46.8 ± 13 | 0.928 |
| EUROScore II | 2.3 (0.8–7.2) | 1.9 (0.7–6.2) | 0.149 |
| 6-month weight loss (%) | 1.9 (0–9) | 1.5 (0–11.3) | 0.459 |
| BMI (kg/m2) | 28.7 ± 5.9 | 28.9 ± 5.5 | 0.975 |
| f-MNA | 23.7 ± 2.7 | 25 ± 2.3 | 0.033 |
| 7-SGA | 5 (2–6) | 6 (3–6) | 0.034 |
| SNAQ | 15.7 ± 2 | 15.9 ± 1.6 | 0.487 |
| HGS (kg) | 25.3 ± 9.0 | 27.9 ± 9.9 | 0.170 |
| Phase angle (50kHZ) | 8.5 ± 1.32 | 8.9 ± 2.1 | 0.696 |
| Albumin (g/L) | 35.7 ± 6.5 | 38.3 ± 7.4 | 0.230 |
| Prealbumin (mg/dL) | 29.73 ± 8.23 | 34.4 ± 7.7 | 0.038 |
| CRP (mg/dL) | 1.29 (0.01– 12.49) | 2.3 (0.01–18.4) | 0.364 |
| LDL-cholesterol (mg/dL) | 53.5 (23–177) | 71 (43–112) | 0.039 |
| Total cholesterol (mg/dL) | 134.03 ± 47.8 | 148.4 ± 35.8 | 0.076 |
| Total number of lymphocytes (/mm3) | 1.7 ± 0.6 | 1.9 ± 0.6 | 0.054 |
| Haemoglobin (g/dL) | 13.15 ± 1.54 | 13.3 ± 1.4 | 0.495 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wernio, E.; Małgorzewicz, S.; Dardzińska, J.A.; Jagielak, D.; Rogowski, J.; Gruszecka, A.; Klapkowski, A.; Bramlage, P. Association between Nutritional Status and Mortality after Aortic Valve Replacement Procedure in Elderly with Severe Aortic Stenosis. Nutrients 2019, 11, 446. https://doi.org/10.3390/nu11020446
Wernio E, Małgorzewicz S, Dardzińska JA, Jagielak D, Rogowski J, Gruszecka A, Klapkowski A, Bramlage P. Association between Nutritional Status and Mortality after Aortic Valve Replacement Procedure in Elderly with Severe Aortic Stenosis. Nutrients. 2019; 11(2):446. https://doi.org/10.3390/nu11020446
Chicago/Turabian StyleWernio, Edyta, Sylwia Małgorzewicz, Jolanta Anna Dardzińska, Dariusz Jagielak, Jan Rogowski, Agnieszka Gruszecka, Andrzej Klapkowski, and Peter Bramlage. 2019. "Association between Nutritional Status and Mortality after Aortic Valve Replacement Procedure in Elderly with Severe Aortic Stenosis" Nutrients 11, no. 2: 446. https://doi.org/10.3390/nu11020446
APA StyleWernio, E., Małgorzewicz, S., Dardzińska, J. A., Jagielak, D., Rogowski, J., Gruszecka, A., Klapkowski, A., & Bramlage, P. (2019). Association between Nutritional Status and Mortality after Aortic Valve Replacement Procedure in Elderly with Severe Aortic Stenosis. Nutrients, 11(2), 446. https://doi.org/10.3390/nu11020446





