Activation of Lipid Mediator Formation Due to Lipoprotein Apheresis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Blood Sampling
2.2. Fatty Acid Measurements
2.3. Sample Preparation and LC/ESI-MS/MS
2.4. Statistical Analysis
3. Results
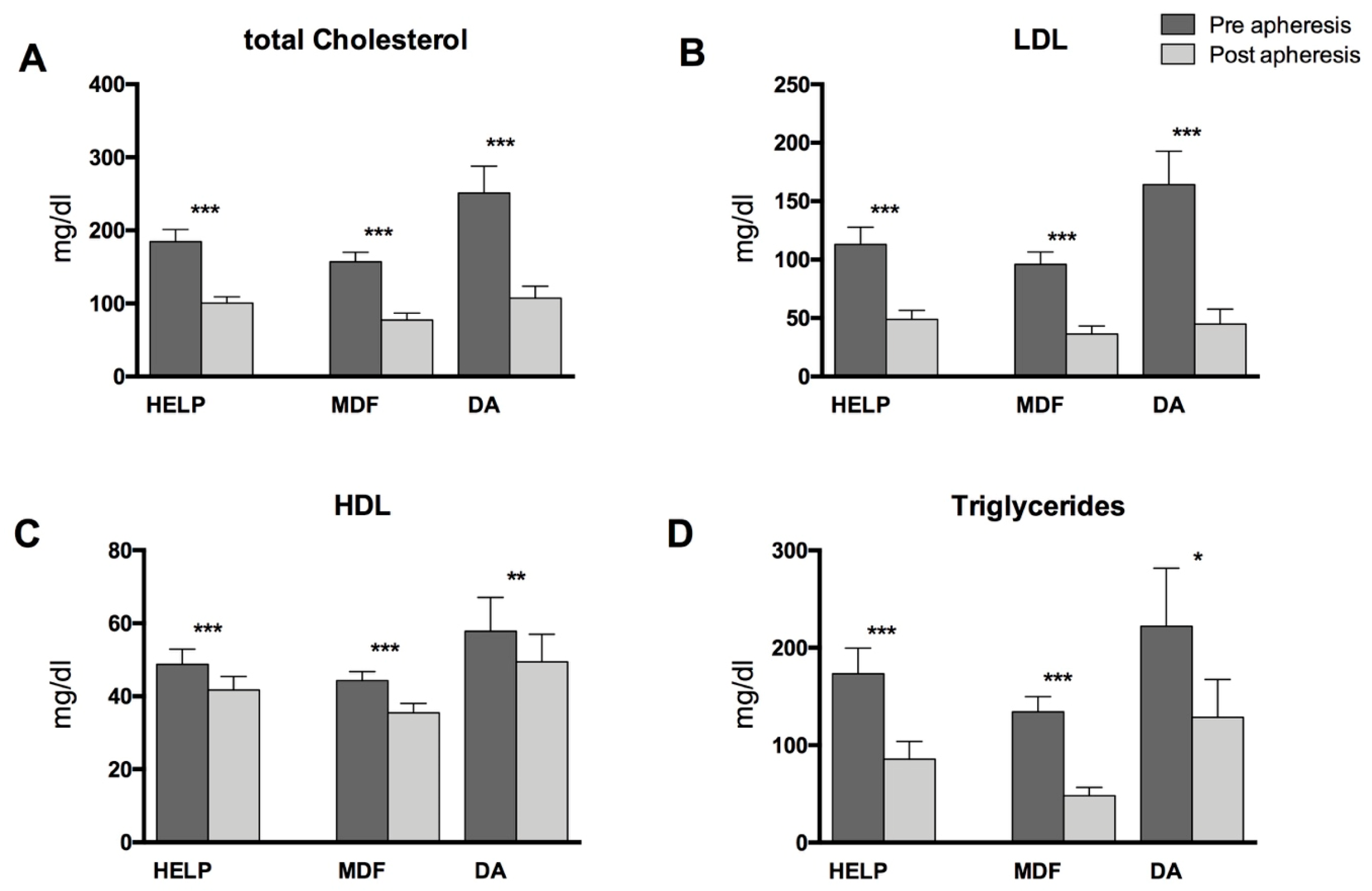
3.1. Patient Characteristics, Standard Lipid Parameters, and PUFA-Levels in Blood Plasma
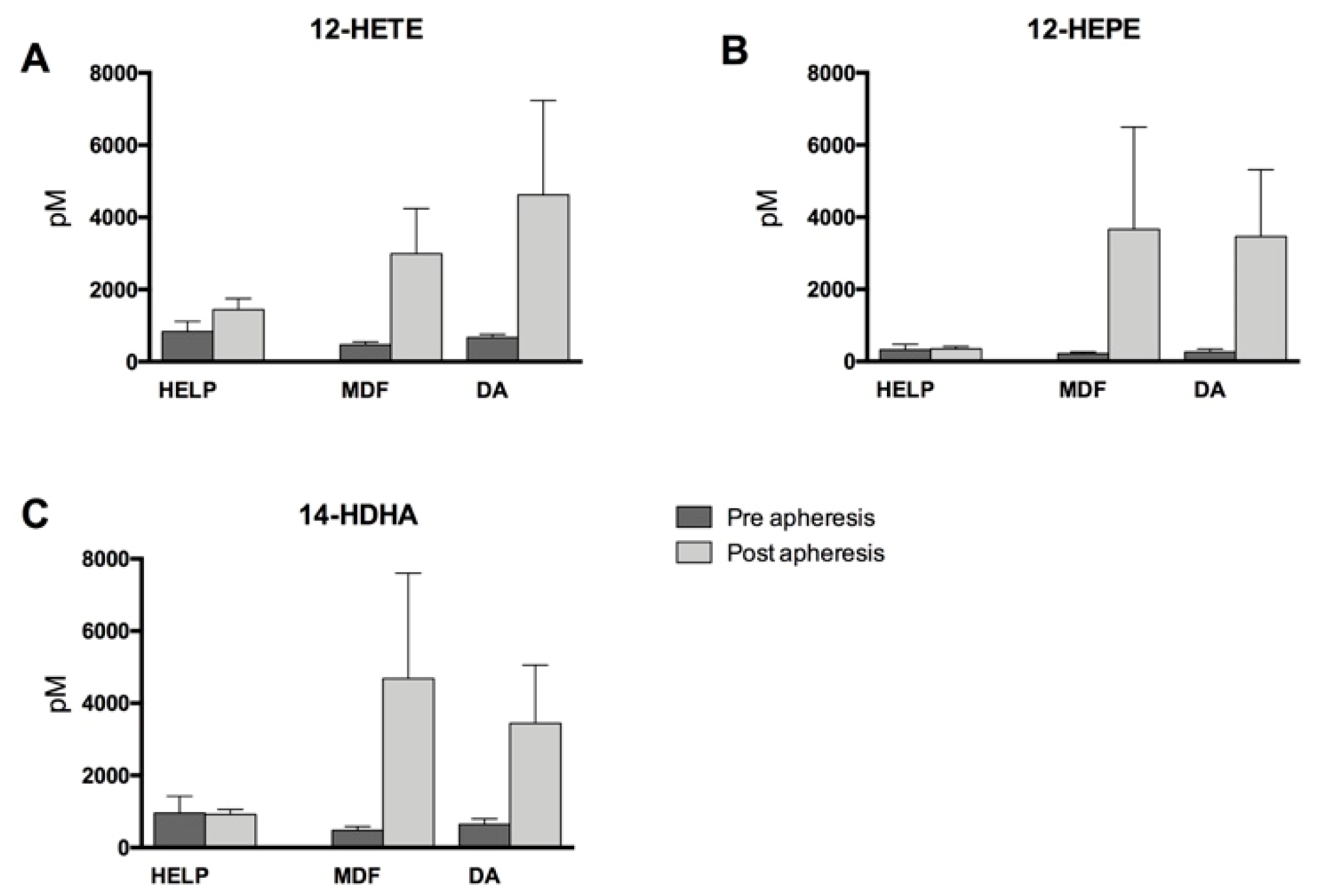
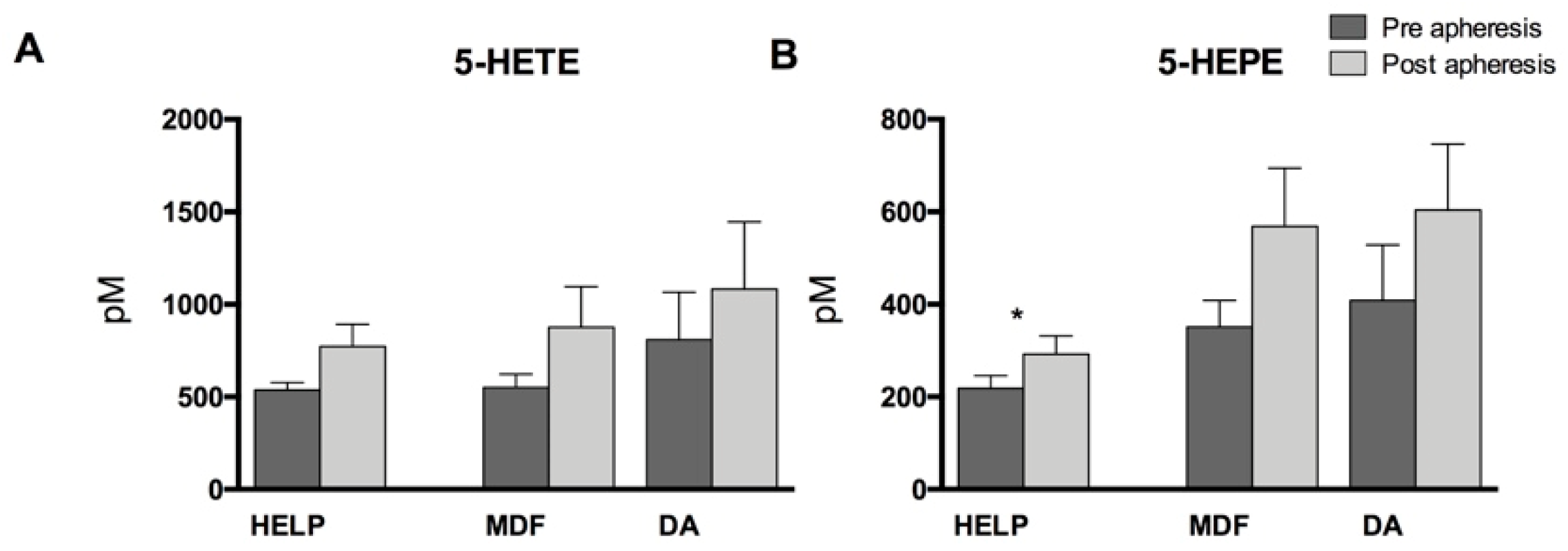
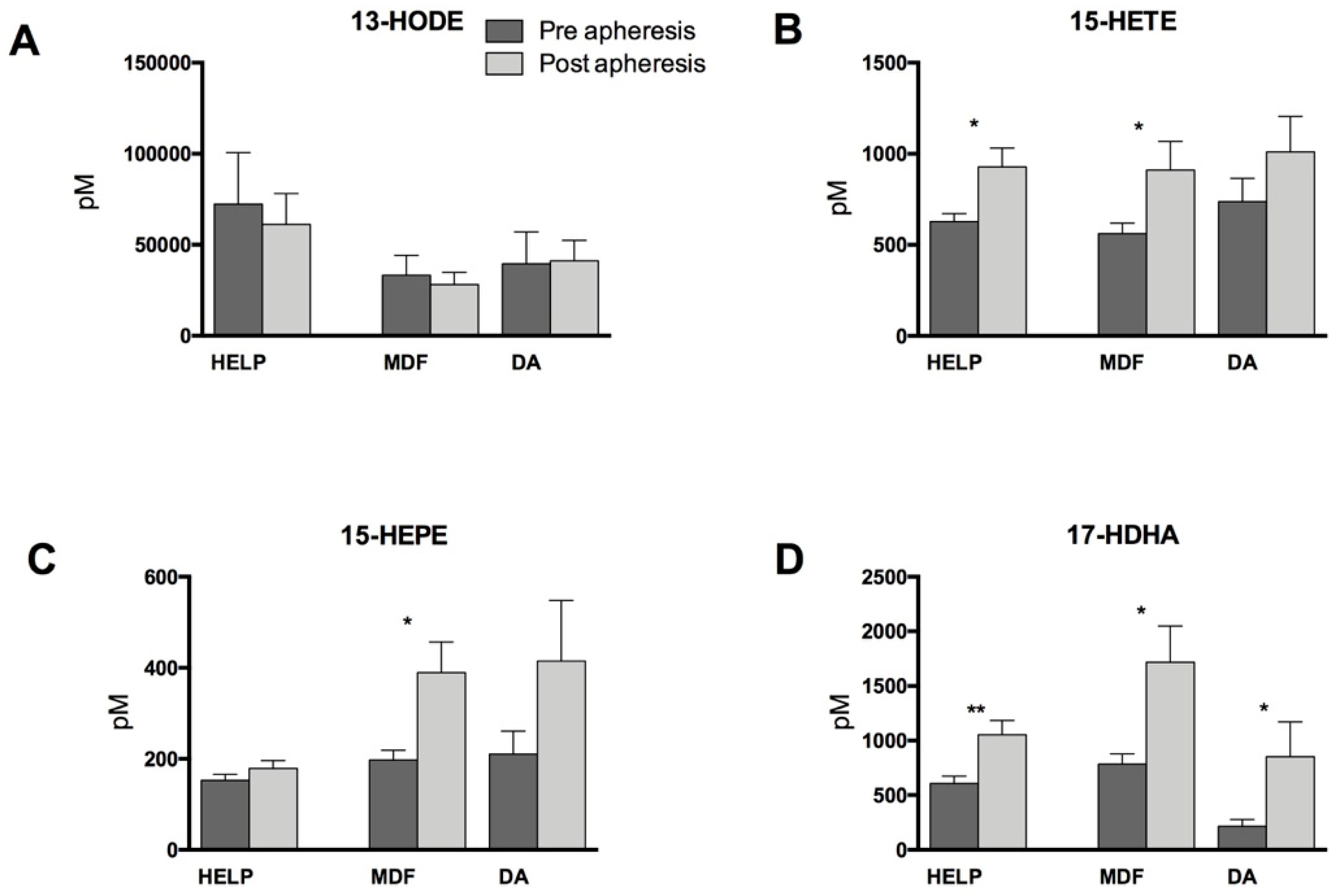
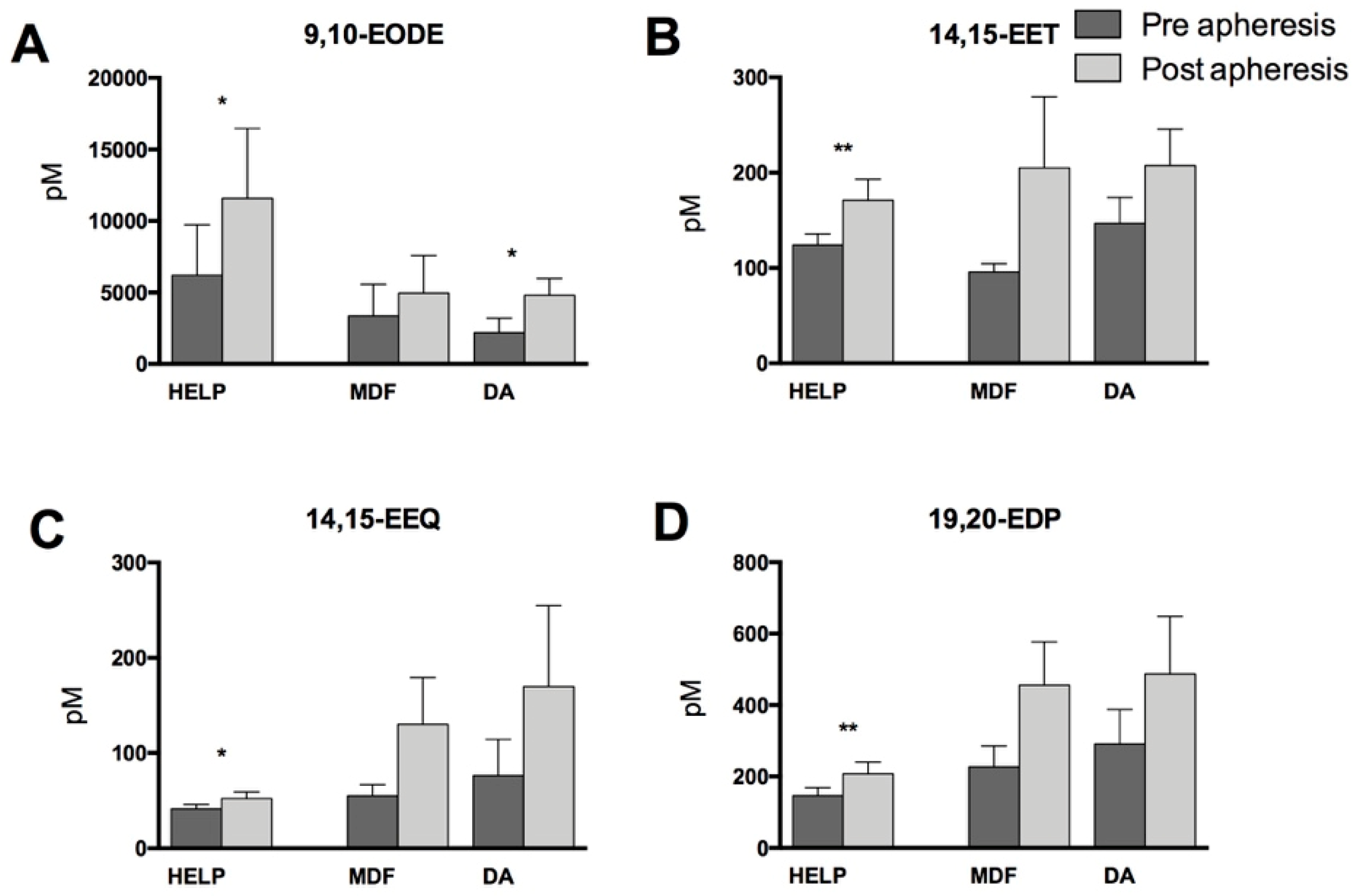
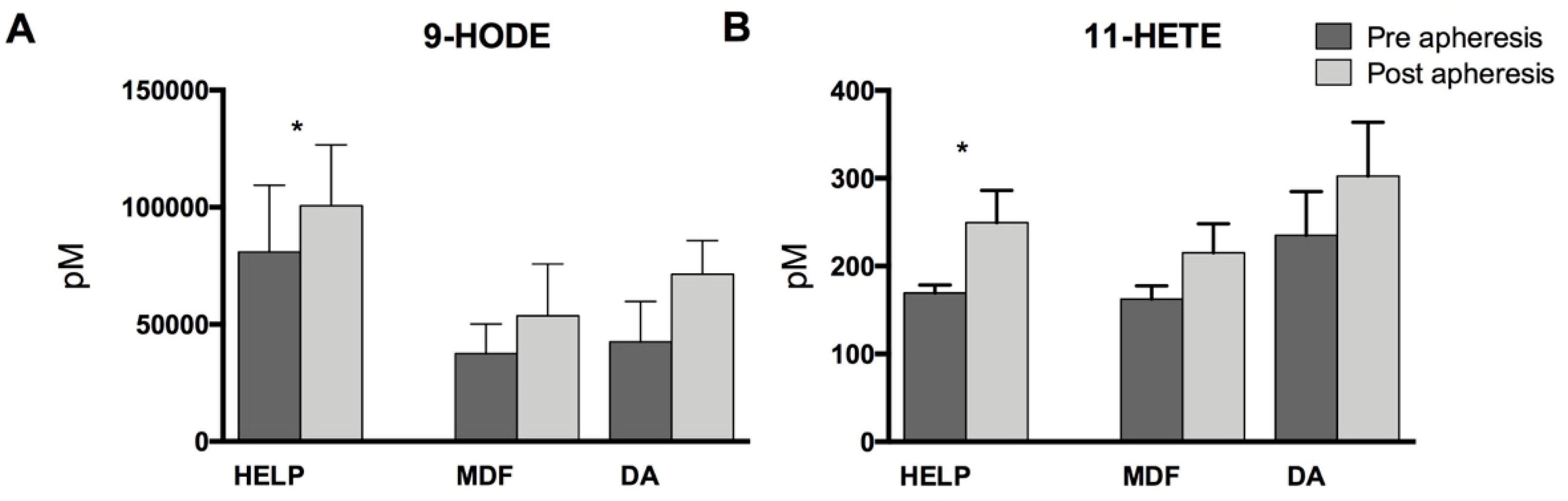
3.2. Lipid Mediators Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grützmacher, P.; Kleinert, C.; Dorbath, C.; Ohm, B. Indications for apheresis as an ultima ratio treatment of refractory hyperlipidemias. Clin. Res. Cardiol. Suppl. 2015, 10, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Julius, U. Lipoprotein apheresis in the management of severe hypercholesterolemia and of elevation of lipoprotein(a): Current perspectives and patient selection. Med. Devices Evid. Res. 2016, 9, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Kamstrup, P.R. Lipoprotein(a) and ischemic heart disease—A causal association? A review. Atherosclerosis 2010, 211, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kamstrup, P.R.; Tybjærg-Hansen, A.; Steffensen, R.; Nordestgaard, B.G. Genetically Elevated Lipoprotein(a) and Increased Risk of Myocardial Infarction. JAMA 2009, 301, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Leebmann, J.; Roeseler, E.; Julius, U.; Heigl, F.; Spitthoever, R.; Heutling, D.; Breitenberger, P.; Maerz, W.; Lehmacher, W.; Heibges, A.; et al. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: Prospective observational multicenter study. Circulation 2013, 128, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Pilcher, G.J.; Panz, V.R.; Van Deventer, H.E.; Brice, B.C.; Blom, D.J.; Marais, A.D. Msc Reduction in Mortality in Subjects With Homozygous Familial Hypercholesterolemia Associated With Advances in Lipid-Lowering Therapy. Circulation 2011, 124, 2202–2207. [Google Scholar] [CrossRef] [PubMed]
- Connolly, K.D.; Willis, G.R.; Datta, D.B.N.; Ellins, E.; Ladell, K.; Price, D.A.; Guschina, I.A.; Rees, D.A.; James, P.E. Lipoprotein-apheresis reduces circulating microparticles in individuals with familial hypercholesterolemia. J. Lipid Res. 2014, 55, 2064–2072. [Google Scholar] [CrossRef]
- Heigl, F.; Hettich, R.; Lotz, N.; Reeg, H.; Pflederer, T.; Osterkorn, D.; Osterkorn, K.; Klingel, R. Clinical benefit of long-term lipoprotein apheresis in patients with severe hypercholesterolemia or Lp(a)-hyperlipoproteinemia with progressive cardiovascular disease. Clin. Res. Cardiol. Suppl. 2015, 10, 8–13. [Google Scholar] [CrossRef]
- Tselmin, S.; Julius, U.; Müller, G.; Fischer, S.; Bornstein, S. Cardiovascular Events in Patients with Increased Lipoprotein (a)—Retrospective Data Analysis in an Outpatient Department of Lipid Disorders. Atheroscler. Suppl. 2009, 10, 79–84. [Google Scholar] [CrossRef]
- Morawietz, H.; Goettsch, W.; Brux, M.; Reimann, M.; Bornstein, S.R.; Julius, U.; Ziemssen, T. Lipoprotein apheresis of hypercholesterolemic patients mediates vasoprotective gene expression in human endothelial cells. Atheroscler. Suppl. 2013, 14, 107–113. [Google Scholar] [CrossRef]
- Tsurumi-Ikeya, Y.; Tamura, K.; Azuma, K.; Mitsuhashi, H.; Wakui, H.; Nakazawa, I.; Sugano, T.; Mochida, Y.; Ebina, T.; Hirawa, N.; et al. Sustained Inhibition of Oxidized Low-Density Lipoprotein Is Involved in the Long-Term Therapeutic Effects of Apheresis in Dialysis Patients. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Stefanutti, C.; Vivenzio, A.; Di Giacomo, S.; Ferraro, P.; Ferraro, P.; et al. Cytokines profile in serum of homozygous familial hypercholesterolemia is changed by LDL-apheresis. Cytokine 2011, 55, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Ba, G.N.; Couvreur, P.; Tew, K.; et al. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef]
- Buczynski, M.W.; Dumlao, D.S.; Dennis, E.A. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 2009, 50, 1015–1038. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Schunck, W.-H.; Konkel, A.; Fischer, R.; Weylandt, K.-H.; et al. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol. Ther. 2018, 183, 177–204. [Google Scholar] [CrossRef] [PubMed]
- Schmocker, C.; Kassner, U.; Kiesler, S.; Bismarck, M.; Rothe, M.; Steinhagen-Thiessen, E.; Weylandt, K.H. A lipidomic analysis approach in patients undergoing lipoprotein apheresis. Atherosclerosis 2016, 249, 30–35. [Google Scholar] [CrossRef]
- Bolick, D.T.; Orr, A.W.; Whetzel, A.; Srinivasan, S.; Hatley, M.E.; Schwartz, M.A.; Hedrick, C.C. 12/15-lipoxygenase regulates intercellular adhesion molecule-1 expression and monocyte adhesion to endothelium through activation of RhoA and nuclear factor-kappaB. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2301–2307. [Google Scholar] [CrossRef]
- Schmöcker, C.; Kassner, U.; Ostermann, A.; Kiesler, S.; Steinhagen-Thiessen, E.; Schebb, N.; Weylandt, K.; et al. Effect of different lipid apheresis methods on plasma polyunsaturated fatty acids. Atheroscler. Suppl. 2017, 30, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, A.I.; Müller, M.; Willenberg, I.; Schebb, N.H.; et al. Determining the fatty acid composition in plasma and tissues as fatty acid methyl esters using gas chromatography—A comparison of different derivatization and extraction procedures. Prostaglandins Leukot. Essent. Fatty Acids 2014, 91, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, A.I.; Willenberg, I.; Schebb, N.H.; et al. Comparison of sample preparation methods for the quantitative analysis of eicosanoids and other oxylipins in plasma by means of LC-MS/MS. Anal. Bioanal. Chem. 2014, 407, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Schmöcker, C.; Zhang, I.; Kiesler, S.; Kassner, U.; Ostermann, A.; Steinhagen-Thiessen, E.; Schebb, N.; Weylandt, K.-H.; et al. Effect of Omega-3 Fatty Acid Supplementation on Oxylipins in a Routine Clinical Setting. Int. J. Mol. Sci. 2018, 19, 180. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R. n-3 fatty acids in cardiovascular disease. N. Engl. J. Med. 2011, 364, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Leaf, A.; Kang, J.X.; Xiao, Y.-F.; Billman, G.E.; et al. Clinical Prevention of Sudden Cardiac Death by n-3 Polyunsaturated Fatty Acids and Mechanism of Prevention of Arrhythmias by n-3 Fish Oils. Circulation 2003, 107, 2646–2652. [Google Scholar] [CrossRef] [PubMed]
- Weylandt, K.H.; Chiu, C.-Y.; Gomolka, B.; Waechter, S.F.; Wiedenmann, B.; et al. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012, 97, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A. Plasma Free Fatty Acid and Lipoproteins as Sources of Polyunsaturated Fatty Acid for the Brain. J. Mol. Neurosci. 2001, 16, 159–166. [Google Scholar] [CrossRef]
- Goodman, D.S.; Shiratori, T. Fatty acid composition of human plasma lipoprotein fractions. J. Lipid. Res. 1964, 5, 307–313. [Google Scholar] [PubMed]
- Chiang, N.; Arita, M.; Serhan, C.N.; et al. Anti-inflammatory circuitry: Lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot. Essent. Fatty Acids 2005, 73, 163–177. [Google Scholar] [CrossRef]
- McMahon, G.S.; Jones, C.I.; Hayes, P.D.; Naylor, A.R.; Goodall, A.H.; et al. Transient heparin-induced platelet activation linked to generation of platelet 12-lipoxygenase. Thromb. Haemost. 2013, 109, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Bambauer, C.; Lehmann, B.; Latza, R.; Schiel, R.; Bambauer, R.; et al. LDL-Apheresis: Technical and Clinical Aspects. Sci. World J. 2012, 2012, 1–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weiss, R.; Fischer, M.B.; Weber, V.; Spittler, A.; Schmitz, G.; et al. Thrombocyte Adhesion and Release of Extracellular Microvesicles Correlate with Surface Morphology of Adsorbent Polymers for Lipid Apheresis. Biomacromolecules 2014, 15, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Gerrity, R.G.; Gu, J.-L.; Lanting, L.; Thomas, L.; Nadler, J.L.; et al. Role of 12-lipoxygenase and oxidant stress in hyperglycaemia-induced acceleration of atherosclerosis in a diabetic pig model. Diabetologia 2002, 45. [Google Scholar] [CrossRef]
- Chatterjee, A.; Toy, R.; Mottola, G.; Sharma, A.; Chen, M.; Conte, M.S.; et al. The Pro-Resolving Lipid Mediator Maresin 1 (MaR1) Attenuates Inflammatory Signaling Pathways in Vascular Smooth Muscle and Endothelial Cells. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Dalli, J.; Karamnov, S.; Choi, A.; Park, C.-K.; Xu, Z.-Z.; Ji, R.-R.; Zhu, M.; Petasis, N.A.; et al. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012, 26, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.; Snyder, G.D.; Weintraub, N.L.; Fang, X.; et al. Epoxyeicosatrienoic acids (EETs): Metabolism and biochemical function. Prog. Lipid Res. 2004, 43, 55–90. [Google Scholar] [CrossRef]
- Roman, R.J. P-450 Metabolites of Arachidonic Acid in the Control of Cardiovascular Function. Physiol. Rev. 2002, 82, 131–185. [Google Scholar] [CrossRef] [PubMed]
- Schuck, R.N.; Theken, K.N.; Edin, M.L.; Caughey, M.; Bass, A.; Ellis, K.; Tran, B.; Steele, S.; Simmons, B.P.; Lih, F.B.; et al. Cytochrome P450-derived eicosanoids and vascular dysfunction in coronary artery disease patients. Atherosclerosis 2013, 227, 442–448. [Google Scholar] [CrossRef]
- Shen, L.; Peng, H.; Peng, R.; Fan, Q.; Zhao, S.; Xu, D.; Morisseau, C.; Chiamvimonvat, N.; Hammock, B.D.; et al. Inhibition of soluble epoxide hydrolase in mice promotes reverse cholesterol transport and regression of atherosclerosis. Atherosclerosis 2015, 239, 557–565. [Google Scholar] [CrossRef]
| Summary/Average | HELP | MDF | DA | |
|---|---|---|---|---|
| n | 33 | 17 | 9 | 7 |
| Age (years) | 58.3 | 57.6 | 58.4 | 63.6 |
| Male:Female | 30:3 | 15:2 | 9:0 | 6:1 |
| BMI (kg/m2) | 29.2 | 27.82 | 30.5 | 29.9 |
| Apheresis indication | ||||
| Hypercholesterolemia | 25 | 12/17 | 7/9 | 6/7 |
| Hyperlipoproteinemia (a) | 23 | 14/17 | 7/9 | 2/7 |
| Cardiovascular disease | ||||
| Arterial hypertension | 25 | 13/17 | 7/9 | 5/7 |
| CHD | 31 | 17/17 | 9/9 | 6/7 |
| condition after myocardial infarction | 18 | 9/17 | 6/9 | 3/7 |
| PTCA with stenting | 25 | 15/17 | 8/9 | 2/7 |
| CABG | 10 | 4/17 | 2/9 | 4/7 |
| Diabetes mellitus type II | 3 | 2/17 | - | 1/7 |
| Medication | ||||
| ASS 100 | 32 | 17/17 | 8/9 | 7/7 |
| Thienopyridine | 11 | 5/17 | 5/9 | 1/7 |
| Omega-3 ethylester | 18 | 6/17 | 8/9 | 4/7 |
| Statins | 27 | 15/17 | 8/9 | 4/7 |
| Ezetimibe | 13 | 6/17 | 2/9 | 5/7 |
| ACE inhibitor | 12 | 6/17 | 5/9 | 1/7 |
| AT1-blocker | 10 | 6/17 | 3/9 | 1/7 |
| ß-blocker | 24 | 12/17 | 6/9 | 6/7 |
| Calcium blocker | 10 | 5/17 | 2/9 | 3/7 |
| Diuretic | 8 | 3/17 | 2/9 | 3/7 |
| FA Group | HELP | MDF | DA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| pre | post | pre | post | pre | post | ||||
| conc. mg/mL | conc. mg/mL | % change | conc. mg/mL | conc. mg/mL | % change | conc. mg/mL | conc. mg/mL | % change | |
| n-3 FA | |||||||||
| C18:3 n-3 | 26.04 ± 13.43 | 16.74 ** ± 7.13 | −35.71 | 23.03 ± 12.28 | 14.53 ± 5.11 | −36.9 | 41.01 ± 29.30 | 24.57 ± 15.96 | −40.1 |
| C20:5 n-3 | 39.99 ± 16.96 | 28.79 ** ± 16.80 | −28.01 | 57.21 ± 35.47 | 48.98 ± 30.00 | −14.4 | 66.12 ± 41.47 | 37.87 * ± 23.15 | 42.7 |
| C22:5 n-3 | 18.54 ± 7.22 | 13.09 *** ± 5.12 | −29.38 | 17.27 ± 7.18 | 15.59 ± 6.63 | −9.8 | 23.94 ± 8.34 | 14.98 ± 4.04 | −37.4 |
| C22:6 n-3 | 61.26 ± 15.25 | 45.93 *** ± 18.20 | −25.02 | 60.49 ± 27.80 | 56.93 ± 27.76 | −5.9 | 92.14 ± 41.66 | 60.86 ** ± 26.71 | −33.9 |
| n-6 FA | |||||||||
| C18:2 n-6 | 713.11 ± 209.94 | 488.11 *** ± 150.23 | −31.55 | 515.50 ± 195.36 | 430.18 ± 183.21 | −16.6 | 903.77 ± 338.23 | 508.98 ** ± 189.83 | −43.7 |
| C18:3 n-6 | 12.29 ± 7.17 | 7.25 *** ± 4.22 | −41.01 | 7.84 ± 4.23 | 7.03 ± 4.66 | −10.4 | 15.76 ± 7.64 | 8.15 ** ± 4.32 | −48.3 |
| C20:2 n-6 | 6.18 ± 2.62 | 4.53 ** ± 2.50 | −26.74 | 4.20 ± 1.40 | 3.87 ± 2.30 | −8.0 | 7.17 ± 2.54 | 4.70 ** ± 1.67 | −34.4 |
| C20:3 n-6 | 40.57 ± 10.76 | 30.03 *** ± 9.75 | −25.98 | 28.35 ± 9.57 | 29.69 ± 17.12 | −4.7 | 52.93 ± 23.88 | 33.56 *** ± 16.64 | −36.6 |
| C20:4 n-6 | 205.57 ± 78.59 | 151.68 *** ± 64.79 | −26.22 | 143.67 ± 42.91 | 154.43 ± 91.39 | −7.5 | 259.19 ± 51.58 | 158.45 *** ± 33.10 | −38.9 |
| C22:4 n-6 | 5.31 ± 3.02 | 3.52 *** ± 2.23 | −33.66 | 2.98 ± 1.28 | 3.21 ± 2.03 | −7.7 | 5.77 ± 1.75 | 3.69 ** ± 1.17 | −36.7 |
| MU-FA | |||||||||
| C14:1 n-5 | 2.83 ± 2.39 | 1.54 * ± 1.35 | −45.63 | 2.79 ± 1.68 | 1.90 ± 1.19 | −31.88 | 3.04 ± 2.39 | 1.68 ** ± 1.87 | −44.65 |
| C16:1 n-7 | 76.31 ± 66.30 | 48.31 ** ± 45.88 | −36.69 | 54.16 ± 27.30 | 51.94 ± 38.10 | −4.09 | 98.97 ± 70.18 | 55.16 * ± 40.86 | −44.26 |
| C18:1 n-9c | 822.93 ± 436.22 | 545.52 ** ± 326.87 | −33.71 | 546.09 ± 192.72 | 481.01 ± 288.90 | −11.92 | 1077.42 ± 512.09 | 665.84 * ± 311.13 | −38.2 |
| C18:1 n-7c | 71.11 ± 35.72 | 47.87 *** ± 28.26 | −32.86 | 48.86 ± 15.11 | 47.01 ± 27.14 | −3.78 | 90.70 ± 38.51 | 90.70 ** ± 38.51 | −38.29 |
| C20:1 n-9 | 7.63 ± 4.40 | 5.73 * ± 3.70 | −24.94 | 4.33 ± 1.53 | 3.89 ± 2.36 | −10.04 | 10.07 ± 4.87 | 6.74 * ± 3.17 | −33.03 |
| C22:1 n-9 | 1.54 ± 1.36 | 1.65 ± 1.35 | 6.87 | 1.44 ± 0.41 | 1.10 ± 0.70 | −23.38 | 2.38 ± 1.78 | 2.25 ± 1.48 | −5.56 |
| C24:1 n-9 | 27.66 ± 6.11 | 18.61 *** ± 5.99 | −32.71 | 20.99 ± 5.89 | 20.28 ± 10.83 | −3.41 | 40.38 ± 10.59 | 20.60 *** ± 7.23 | −48.98 |
| S-FA | |||||||||
| C12:0 | 5.78 ± 5.24 | 5.49 ± 6.78 | −5.05 | 5.01 ± 3.66 | 3.93 ± 3.04 | −21.51 | 6.46 ± 2.03 | 3.23 ** ± 2.95 | −50.03 |
| C14:0 | 43.39 ± 32.30 | 25.70 * ± 19.34 | −40.76 | 38.11 ± 19.63 | 27.90 ± 17.41 | −26.80 | 49.70 ± 30.96 | 27.72 * ± 24.01 | −44.22 |
| C16:0 | 631.74 ± 183.78 | 446.20 * ± 152.09 | −29.37 | 594.37 ± 223.44 | 448.72 ± 186.17 | −24.51 | 782.43 ± 115.50 | 532.66 * ± 92.20 | −31.92 |
| C17:0 | 9.44 ± 3.73 | 6.27 *** ± 2.42 | 33.60 | 7.18 ± 2.69 | 6.27 ± 2.74 | −12.63 | 10.26 ± 3.64 | 6.38 ** ± 2.25 | 37.81 |
| C18:0 | 245.11 ± 89.13 | 180.60 *** ± 79.90 | −33.71 | 175.23 ± 64.06 | 164.04 ± 84.84 | −6.39 | 285.69 ± 88.19 | 192.91 ** ± 57.01 | −32.48 |
| C20:0 | 6.44 ± 2.13 | 4.48 *** ± 2.01 | −30.40 | 4.73 ± 1.67 | 4.35 ± 2.07 | −8.01 | 8.86 ± 3.05 | 4.97 ** ± 1.43 | −43.89 |
| C22:0 | 15.44 ± 5.14 | 10.38 *** ± 4.55 | −32.76 | 11.43 ± 4.19 | 9.69 ± 4.95 | −15.21 | 23.56 ± 5.92 | 12.37 *** ± 5.34 | −47.51 |
| C24:0 | 11.13 ± 3.75 | 7.29 *** ± 2.76 | −34.49 | 8.41 ± 3.45 | 7.66 ± 3.62 | −8.94 | 16.34 ± 3.81 | 7.89 *** ± 2.39 | −51.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weylandt, K.-H.; Schmöcker, C.; Ostermann, A.I.; Kutzner, L.; Willenberg, I.; Kiesler, S.; Steinhagen-Thiessen, E.; Schebb, N.H.; Kassner, U. Activation of Lipid Mediator Formation Due to Lipoprotein Apheresis. Nutrients 2019, 11, 363. https://doi.org/10.3390/nu11020363
Weylandt K-H, Schmöcker C, Ostermann AI, Kutzner L, Willenberg I, Kiesler S, Steinhagen-Thiessen E, Schebb NH, Kassner U. Activation of Lipid Mediator Formation Due to Lipoprotein Apheresis. Nutrients. 2019; 11(2):363. https://doi.org/10.3390/nu11020363
Chicago/Turabian StyleWeylandt, Karsten-H., Christoph Schmöcker, Annika I. Ostermann, Laura Kutzner, Ina Willenberg, Stefanie Kiesler, Elisabeth Steinhagen-Thiessen, Nils Helge Schebb, and Ursula Kassner. 2019. "Activation of Lipid Mediator Formation Due to Lipoprotein Apheresis" Nutrients 11, no. 2: 363. https://doi.org/10.3390/nu11020363
APA StyleWeylandt, K.-H., Schmöcker, C., Ostermann, A. I., Kutzner, L., Willenberg, I., Kiesler, S., Steinhagen-Thiessen, E., Schebb, N. H., & Kassner, U. (2019). Activation of Lipid Mediator Formation Due to Lipoprotein Apheresis. Nutrients, 11(2), 363. https://doi.org/10.3390/nu11020363





