Mediators of Lifestyle Behaviour Changes in Obese Pregnant Women. Secondary Analyses from the DALI Lifestyle Randomised Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Randomisation and Interventions
2.3. Outcomes
2.4. Potential Mediators
2.5. Statistical Analyses
3. Results
3.1. Intervention Effects on Psychological Variables (A-Path)
3.2. Association of Psychological Variables with Lifestyle Behaviours (B-Path)
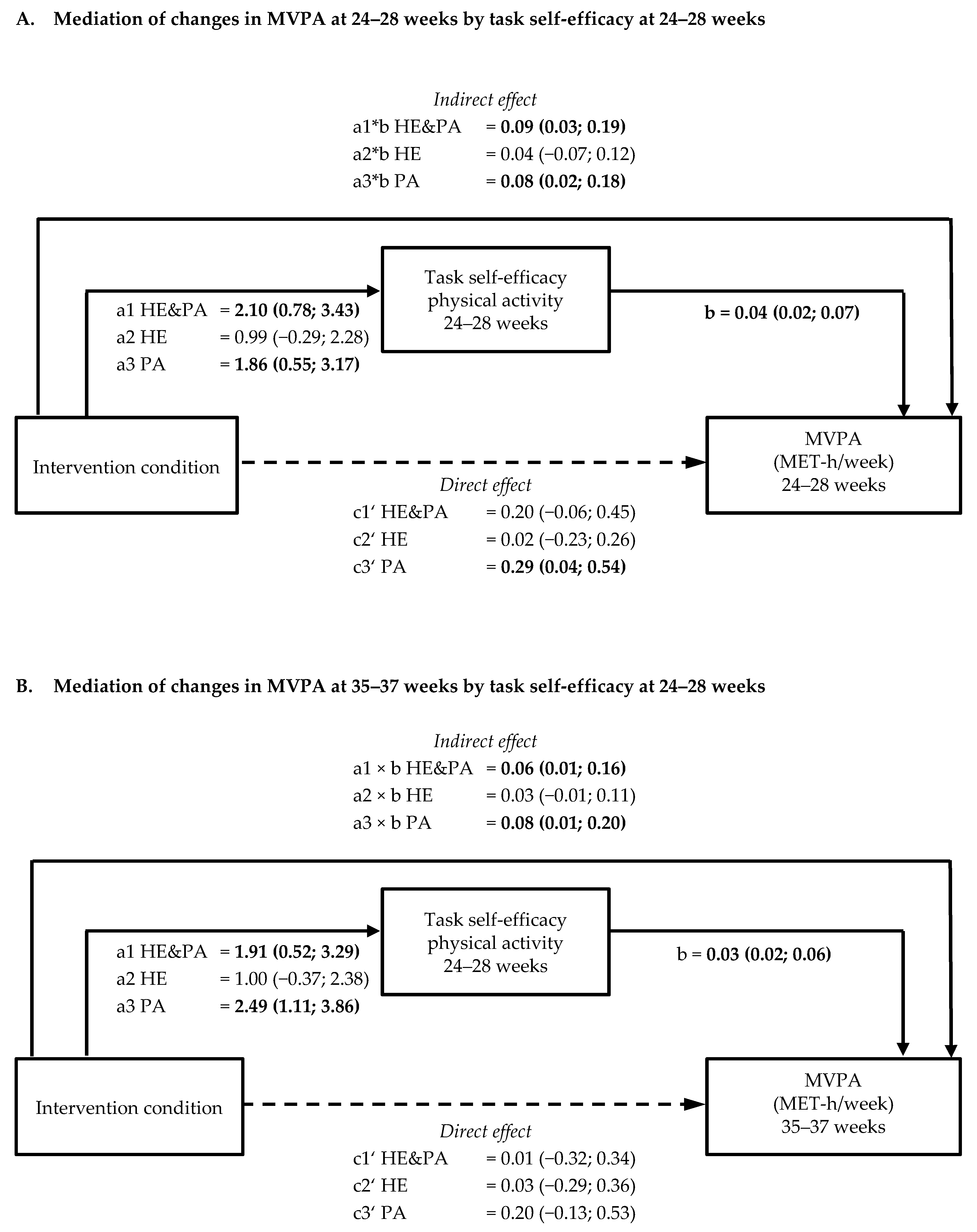
3.3. Mediation by Psychological Variables of Intervention Effects on Physical Activity and Sedentary Behaviour (a × b path)
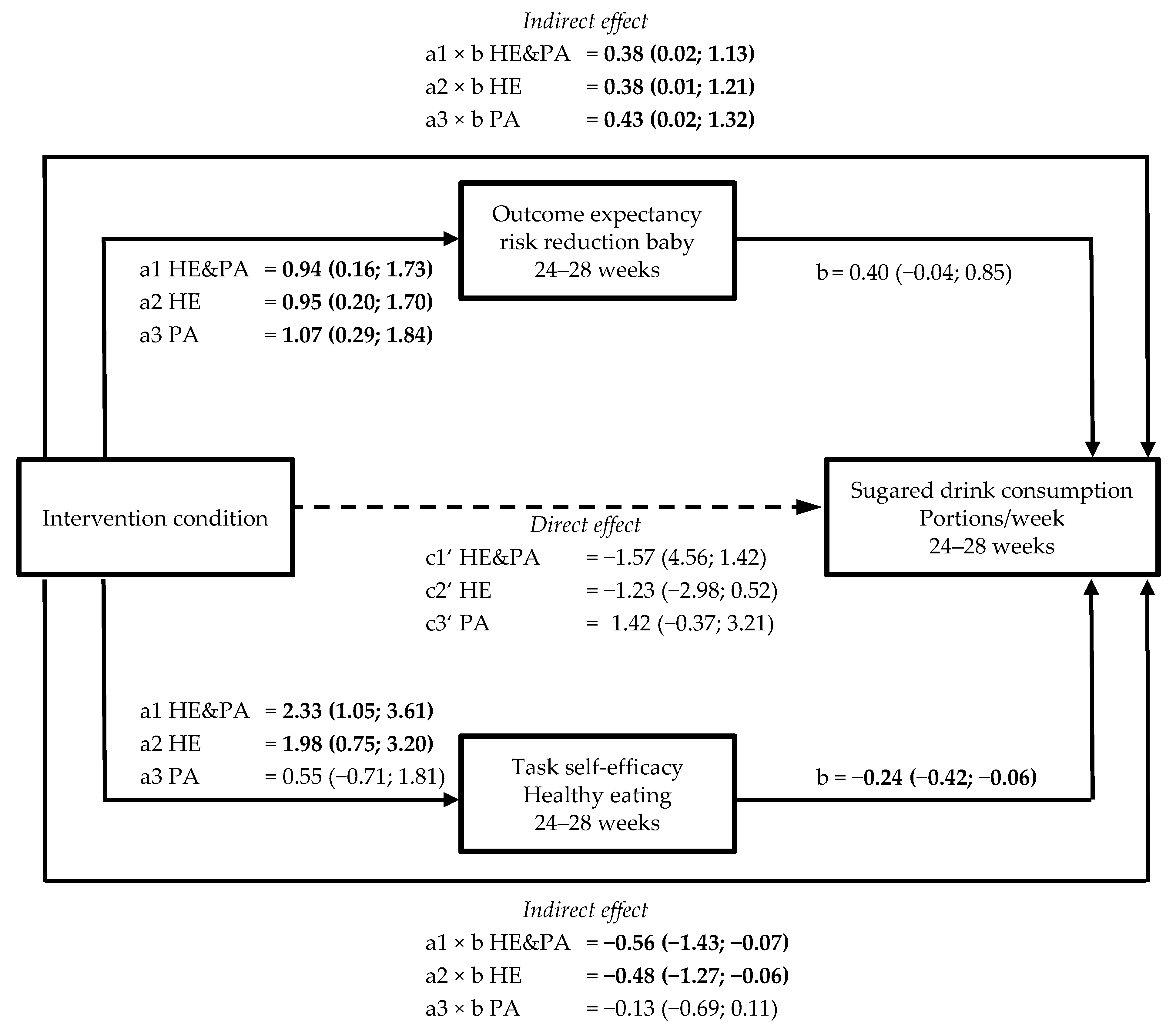
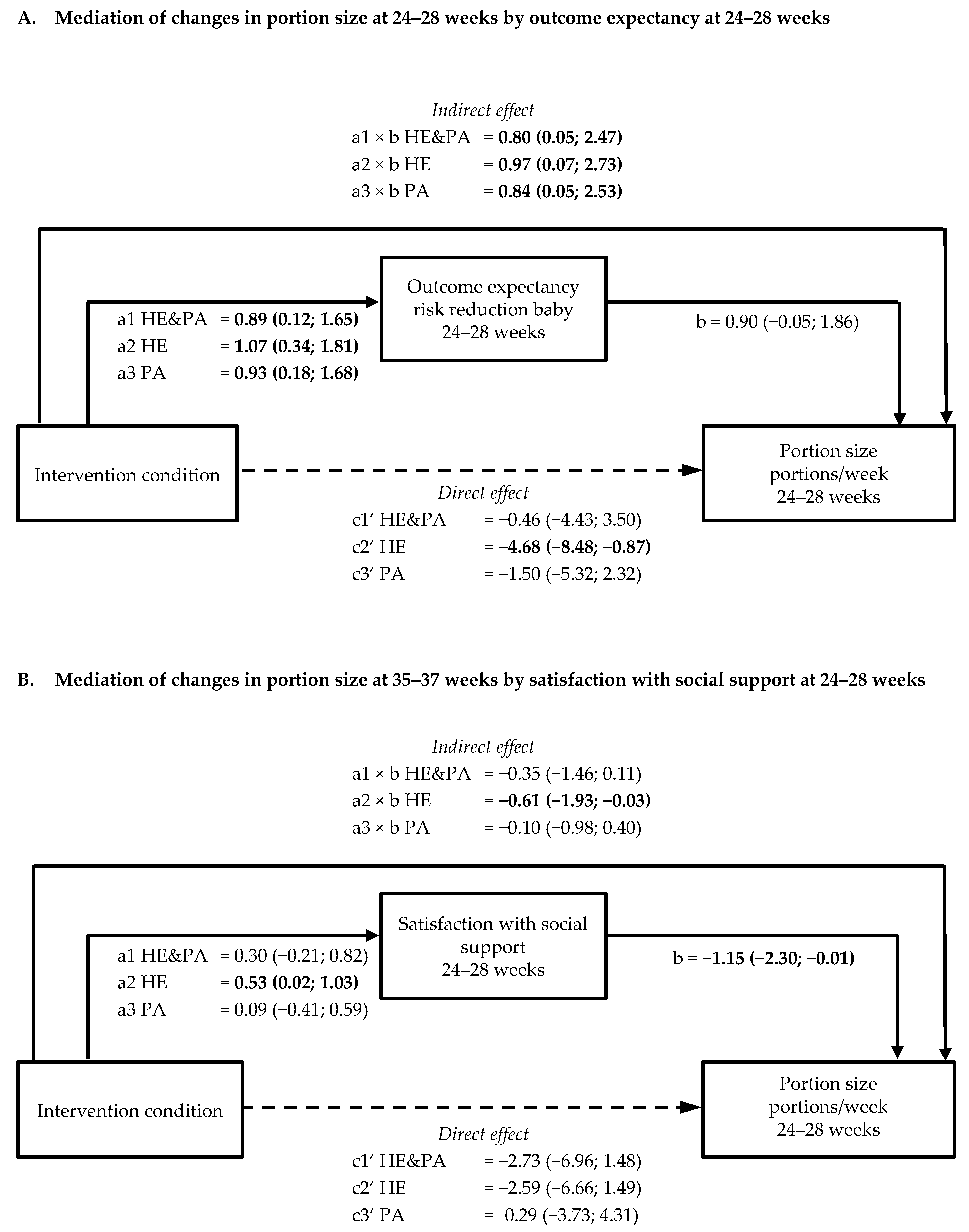
3.4. Mediation by Psychological Variables of Intervention Effects on Healthy Eating Behaviours (a × b path)
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Devlieger, R.; Benhalima, K.; Damm, P.; van Assche, A.; Mathieu, C.; Mahmood, T.; Dunne, F.; Bogaerts, A. Maternal obesity in Europe: Where do we stand and how to move forward?: A scientific paper commissioned by the European Board and College of Obstetrics and Gynaecology (EBCOG). Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 201, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Heslehurst, N.; Ells, L.J.; Simpson, H.; Batterham, A.; Wilkinson, J.; Summerbell, C.D. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36 821 women over a 15-year period. BJOG 2007, 114, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.; Stecher, L.; Ziebarth, S.; Nehring, I.; Rifas-Shiman, S.L.; Sommer, C.; Hauner, H.; von Kries, R. Excessive gestational weight gain prior to glucose screening and the risk of gestational diabetes: A meta-analysis. Diabetologia 2015, 58, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.Y.; Callaghan, W.M.; Kim, S.Y.; Schmid, C.H.; Lau, J.; England, L.J.; Dietz, P.M. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007, 30, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Rogozińska, E.; Marlin, N.; Jackson, L.; Rayanagoudar, G.; Ruifrok, A.E.; Dodds, J.; Molyneaux, E.; van Poppel, M.N.; Poston, L.; Vinter, C.A.; et al. Effects of antenatal diet and physical activity on maternal and fetal outcomes: Individual patient data meta-analysis and health economic evaluation. Health Technol. Assess. 2017, 21, 1–158. [Google Scholar] [CrossRef] [PubMed]
- Bain, E.; Crane, M.; Tieu, J.; Han, S.; Crowther, C.A.; Middleton, P. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Bell, R.; Croker, H.; Flynn, A.C.; Godfrey, K.M.; Goff, L.; Hayes, L.; Khazaezadeh, N.; Nelson, S.M.; Oteng-Ntim, E.; et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Dodd, J.M.; Cramp, C.; Sui, Z.; Yelland, L.N.; Deussen, A.R.; Grivell, R.M.; Moran, L.J.; Crowther, C.A.; Turnbull, D.; McPhee, A.J.; et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on maternal diet and physical activity: The LIMIT randomised trial. BMC 2014, 12, 161. [Google Scholar] [CrossRef]
- Jelsma, J.G.M.; van Poppel, M.N.M.; Galjaard, S.; Desoye, G.; Corcoy, R.; Devlieger, R.; van Assche, A.; Timmerman, D.; Jans, G.; Harreiter, J.; et al. DALI: Vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention: An European multicentre, randomised trial—study protocol. BMC 2013, 13, 142. [Google Scholar] [CrossRef]
- Miller, W.R.; Rollnick, S. Motivational Interviewing: Helping People Change, 3rd ed.; Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Simmons, D.; Devlieger, R.; van Assche, A.; Jans, G.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Desoye, G.; Harreiter, J.; et al. Effect of Physical Activity and/or Healthy Eating on GDM Risk: The DALI Lifestyle Study. J. Clin. Endocrinol. Metab. 2017, 102, 903–913. [Google Scholar] [CrossRef]
- Broekhuizen, K.; Simmons, D.; Devlieger, R.; van Assche, A.; Jans, G.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Desoye, G.; et al. Cost-effectiveness of healthy eating and/or physical activity promotion in pregnant women at increased risk of gestational diabetes mellitus: Economic evaluation alongside the DALI study, a European multicenter randomized controlled trial. Int. J. Behav. Med. 2018, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Van Poppel, M.N.M.; Simmons, D.; Desoye, G. DALI Core Investigator Group. A Reduction in Sedentary Behavior in Obese Women Reduces Neonatal Adiposity—The DALI Randomized Controlled Trial. Diabetes 2018, 67, 1416. [Google Scholar] [CrossRef]
- Schwarzer, R. Modeling health behavior change: How to predict and modify the adoption and maintenance of health behaviors. J. Appl. Psychol. 2008, 57, 1–29. [Google Scholar] [CrossRef]
- Lindström, J.; Neumann, A.; Sheppard, K.E.; Gilis-Januszewska, A.; Greaves, C.J.; Handke, U.; Pajunen, P.; Puhl, S.; Pölönen, A.; Rissanen, A.; et al. Take action to prevent diabetes—the IMAGE toolkit for the prevention of type 2 diabetes in Europe. Horm. Metab. Res. 2010, 42, S37–S55. [Google Scholar] [CrossRef] [PubMed]
- Pinidiyapathirage, J.; Jayasuriya, R.; Cheung, N.W.; Schwarzer, R. Self-efficacy and planning strategies can improve physical activity levels in women with a recent history of gestational diabetes mellitus. Health Psychol. 2018, 33, 1062–1077. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, R.; Luszczynska, A. How to overcome health-compromising behaviors: The health action process approach. Eur. J. Psychol. Assess. 2008, 13, 141–151. [Google Scholar] [CrossRef]
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy; WHO/NMH/MND/13.2.; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Rasmussen, K.M.; Yaktine, A.L. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Jensen, D.M.; Ovesen, P.; Beck-Nielsen, H.; Molsted-Pedersen, L.; Sorensen, B.; Vinter, C.; Damm, P. Gestational weight gain and pregnancy outcomes in 481 obese glucose-tolerant women. Diabetes Care 2005, 28, 2118–2122. [Google Scholar] [CrossRef]
- Asbjörnsdóttir, B.; Rasmussen, S.S.; Kelstrup, L.; Damm, P.; Mathiesen, E.R. Impact of restricted maternal weight gain on fetal growth and perinatal morbidity in obese women with type 2 diabetes. Diabetes Care 2013, 36, 1102–1106. [Google Scholar] [CrossRef]
- Chasan-Taber, L.; Schmidt, M.D.; Roberts, D.E.; Hosmer, D.; Markenson, G.; Freedson, P.S. Development and Validation of a Pregnancy Physical Activity Questionnaire. Med. Sci. Sports Exerc. 2004, 36, 1750–1760. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Simmons, D.; Mandell, C.; Fleming, C.; Gatland, B.; Leakehe, L. Evaluation of a diabetes knowledge and behaviour (DKB) questionnaire. Asia Pac. J. Clin. Nutr. 1994, 3, 193–200. [Google Scholar]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford Publications: New York, NY, USA, 2013. [Google Scholar]
- Hayes, A.F.; Preacher, K.J. Statistical mediation analysis with a multicategorical independent variable. Br. J. Math. Stat. Psychol. 2014, 67, 451–470. [Google Scholar] [CrossRef] [PubMed]
- Copeland, L.; McNamara, R.; Kelson, M.; Simpson, S. Mechanisms of change within motivational interviewing in relation to health behaviors outcomes: A systematic review. Patient Educ. Couns. 2015, 98, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Ainscough, K.M.; Lindsay, K.L.; O’Sullivan, E.J.; Gibney, E.R.; McAuliffe, F.M. Behaviour change in overweight and obese pregnancy: A decision tree to support the development of antenatal lifestyle interventions. Public Health Nutri. 2017, 20, 2642–2648. [Google Scholar] [CrossRef]
- Allen, N.A. Social cognitive theory in diabetes exercise research: An integrative literature review. Diabetes Educ. 2004, 30, 805–819. [Google Scholar] [CrossRef]
- Perrier, M.-J.; Sweet, S.N.; Strachan, S.M.; Latimer-Cheung, A.E. I act, therefore I am: Athletic identity and the health action process approach predict sport participation among individuals with acquired physical disabilities. J. Sport Exerc. Psychol. 2012, 13, 713–720. [Google Scholar] [CrossRef]
- Barg, C.J.; Latimer, A.E.; Pomery, E.A.; Rivers, S.E.; Rench, T.A.; Prapavessis, H.; Salovey, P. Examining predictors of physical activity among inactive middle-aged women: An application of the health action process approach. Health Psychol. 2012, 27, 829–845. [Google Scholar] [CrossRef]
| Variable | UC n = 105 | HE&PA n = 108 | HE n = 113 | PA n = 110 | Total n = 436 | p |
|---|---|---|---|---|---|---|
| Age, year, mean ± SD | 31.8 ± 5.6 | 31.9 ± 5.3 | 32.5 ± 5.5 | 31.7 ± 5.1 | 32.0 ± 5.4 | 0.68 |
| Multiparous, n (%) | 50 (48%) | 56 (52%) | 64 (57%) | 51 (46%) | 221 (51%) | 0.41 |
| European descent, n (%) | 94 (90%) | 95 (88%) | 95 (84%) | 94 (86%) | 378 (87%) | 0.65 |
| Lives with partner, n (%) | 100 (95%) | 99 (92%) | 108 (96%) | 103 (94%) | 410 (94%) | 0.60 |
| Higher education, n (%) | 55 (52%) | 59 (55%) | 65 (58%) | 60 (55%) | 239 (55%) | 0.90 |
| Maternal smoking, n (%) | 18 (17%) | 11 (10%) | 20 (18%) | 18 (17%) | 67 (15%) | 0.38 |
| History of GDM, n (%) | 3 (5%) | 4 (6%) | 6 (7%) | 4 (6%) | 17 (6%) | 0.95 |
| 1st degree FH DM, n (%) | 28 (27%) | 18 (17%) | 28 (25%) | 27 (25%) | 101 (23%) | 0.31 |
| Chronic hypertension, n (%) | 9 (9%) | 12 (11%) | 17 (15%) | 17 (16%) | 55 (13%) | 0.36 |
| Gestation on entry, weeks, mean ± SD | 15.2 ± 2.4 | 15.2 ± 2.2 | 15.3 ± 2.5 | 15.5 ± 2.3 | 15.3 ± 2.3 | 0.58 |
| Weight at entry, kg, mean ± SD | 94.2 ± 12.6 | 95.2 ± 13.8 | 94.9 ± 13.2 | 94.6 ± 12.8 | 94.7 ± 13.1 | 0.89 |
| Height, cm, mean ± SD | 165.9 ± 6.7 | 166.0 ± 6.6 | 165.2 ± 6.6 | 165.6 ± 7.2 | 165.7 ± 6.8 | 0.81 |
| BMI at entry, kg/m2, mean ± SD | 34.2 ± 3.9 | 34.5 ± 4.0 | 34.7 ± 4.2 | 34.4 ± 3.8 | 34.5 ± 4.0 | 0.83 |
| Psychological Variables | UC | HE&PA | HE | PA | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline <20 Weeks | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | p |
| Attitude towards weight (range 2–20) | 103 | 15.7 ± 3.4 | 108 | 15.2 ± 4.1 | 112 | 15.3 ± 3.5 | 107 | 15.9 ± 3.6 | 0.41 |
| Perceived risk for GDM (range 1–5) | 105 | 2.8 ± 0.8 | 107 | 2.8 ± 0.9 | 113 | 2.9 ± 0.8 | 110 | 2.9 ± 0.9 | 0.75 |
| Outcome expectancy GDM risk reduction (range 2–20) | |||||||||
| Healthy eating | 104 | 16.5 ± 3.1 | 106 | 16.6 ± 3.0 | 112 | 16.8 ± 2.9 | 108 | 17.0 ± 3.0 | 0.60 |
| Physical activity | 103 | 16.3 ± 3.0 | 106 | 16.3 ± 3.2 | 112 | 16.5 ± 3.0 | 106 | 16.8 ± 3.2 | 0.64 |
| Outcome expectancy risk reduction baby (range 2–20) | |||||||||
| Healthy eating | 104 | 17.3 ± 2.5 | 106 | 16.8 ± 3.0 | 112 | 17.5 ± 2.7 | 109 | 17.7 ± 2.6 | 0.11 |
| Physical activity | 104 | 16.8 ± 2.7 | 107 | 16.6 ± 3.2 | 112 | 17.3 ± 2.8 | 110 | 17.4 ± 2.8 | 0.09 |
| Task self-efficacy (range 3–30) | |||||||||
| Healthy eating | 104 | 21.2 ± 5.2 | 108 | 22.0 ± 5.1 | 112 | 21.3 ± 5.2 | 110 | 21.8 ± 5.0 | 0.63 |
| Physical activity | 104 | 20.7 ± 5.2 | 107 | 20.8 ± 5.1 | 112 | 20.7 ± 5.1 | 109 | 21.4 ± 5.5 | 0.67 |
| Satisfaction with social support (range 1–10) | |||||||||
| Healthy eating | 104 | 7.6 ± 2.0 | 108 | 7.6 ± 1.9 | 112 | 7.4 ± 2.1 | 109 | 7.8 ± 2.2 | 0.61 |
| Physical activity | 104 | 7.7 ± 2.0 | 108 | 7.5 ± 2.1 | 112 | 7.4 ± 2.3 | 108 | 7.8 ± 2.2 | 0.39 |
| 24–28 weeks | |||||||||
| Attitude towards weight (range 2–20) | 99 | 13.9 ± 3.7 | 95 | 14.8 ± 3.5 | 104 | 14.7 ± 3.8 | 101 | 14.7 ± 3.5 | 0.30 |
| Perceived risk for GDM (range 1–5) | 100 | 2.6 ± 0.9 | 95 | 2.7 ± 1.0 | 104 | 2.7 ± 0.9 | 100 | 2.6 ± 1.0 | 0.87 |
| Outcome expectancy GDM risk reduction (range 2–20) | |||||||||
| Healthy eating | 100 | 15.5 ± 3.3 | 95 | 16.5 ± 3.6 | 105 | 16.9 ± 3.1 | 101 | 16.6 ± 3.2 | 0.01 |
| Physical activity | 100 | 15.1 ± 3.5 | 94 | 16.3 ± 3.7 | 105 | 16.5 ± 3.3 | 101 | 16.6 ± 3.3 | 0.005 |
| Outcome expectancy risk reduction baby (range 2–20) | |||||||||
| Healthy eating | 101 | 16.2 ± 3.3 | 94 | 17.0 ± 3.4 | 104 | 17.5 ± 3.0 | 101 | 17.5 ± 2.5 | 0.01 |
| Physical activity | 101 | 16.0 ± 3.5 | 95 | 16.8 ± 3.5 | 104 | 17.2 ± 3.0 | 101 | 17.4 ± 2.6 | 0.01 |
| Task self-efficacy (range 3–30) | |||||||||
| Healthy eating | 101 | 19.4 ± 4.7 | 96 | 21.8 ± 4.5 | 104 | 21.5 ± 4.7 | 100 | 20.5 ± 5.3 | 0.002 |
| Physical activity | 100 | 18.6 ± 5.0 | 96 | 20.6 ± 5.0 | 105 | 19.6 ± 5.3 | 101 | 20.9 ± 5.6 | 0.01 |
| Satisfaction with social support (range 1–10) | |||||||||
| Healthy eating | 101 | 7.5 ± 1.8 | 96 | 7.8 ± 2.0 | 105 | 7.8 ± 2.0 | 101 | 7.8 ± 2.0 | 0.58 |
| Physical activity | 100 | 7.2 ± 2.1 | 96 | 7.8 ± 2.0 | 105 | 7.6 ± 2.3 | 101 | 8.0 ± 2.2 | 0.047 |
| 35–37 weeks | |||||||||
| Attitude towards weight (range 2–20) | 89 | 13.0 ± 4.3 | 86 | 14.1 ± 4.0 | 88 | 14.0 ± 4.1 | 89 | 14.4 ±3.9 | 0.14 |
| Perceived risk for GDM (range 1–5) | 86 | 2.3 ± 1.1 | 86 | 2.1 ± 1.2 | 85 | 2.3 ± 1.1 | 87 | 2.1 ± 1.2 | 0.52 |
| Outcome expectancy GDM risk reduction (range 2–20) | |||||||||
| Healthy eating | 88 | 14.2 ± 4.6 | 85 | 15.5 ± 5.3 | 88 | 15.3 ± 5.0 | 88 | 15.7 ± 4.5 | 0.20 |
| Physical activity | 88 | 13.9 ± 4.7 | 85 | 15.3 ± 5.3 | 87 | 14.9 ± 5.1 | 88 | 15.8 ± 4.6 | 0.09 |
| Outcome expectancy risk reduction baby (range 2–20) | |||||||||
| Healthy eating | 89 | 15.5 ± 3.6 | 85 | 16.8 ± 3.5 | 87 | 16.7 ± 3.0 | 89 | 16.7 ± 3.0 | 0.03 |
| Physical activity | 89 | 15.2 ± 3.9 | 85 | 16.5 ± 3.6 | 87 | 16.4 ± 3.1 | 89 | 16.7 ± 3.1 | 0.02 |
| Task self-efficacy (range 3–30) | |||||||||
| Healthy eating | 88 | 19.9 ± 4.9 | 83 | 22.1 ± 5.5 | 88 | 21.5 ± 4.4 | 89 | 20.8 ± 5.8 | 0.03 |
| Physical activity | 88 | 17.4 ± 5.7 | 84 | 19.4 ± 5.7 | 87 | 19.4 ± 5.7 | 87 | 19.7 ± 6.0 | 0.03 |
| Satisfaction with social support (range 1–10) | |||||||||
| Healthy eating | 89 | 7.4 ± 2.1 | 86 | 7.7 ± 2.1 | 88 | 8.0 ± 1.7 | 89 | 7.7 ± 2.1 | 0.26 |
| Physical activity | 89 | 7.4 ± 2.2 | 86 | 7.7 ± 2.2 | 86 | 7.9 ± 1.9 | 89 | 8.2 ± 1.9 | 0.10 |
| Lifestyle behaviours 24–28 weeks | |||||||||
| MVPA, MET-h/week * | 101 | 29.7 (15.3; 70.3) | 96 | 43.1 (21.0; 77.8) | 104 | 35.7 (17.1; 83.5) | 101 | 51.2 (25.3; 83.5) | 0,03 |
| Sedentary behaviour, MET-h/week | 101 | 13.4 ± 9.7 | 96 | 10.8 ± 8.9 | 104 | 11.8 ± 7.8 | 101 | 12.3 ± 7.3 | 0.19 |
| Sugary drinks, portions/week | 96 | 4.8 ± 5.0 | 90 | 5.3 ± 7.6 | 99 | 3.2 ± 4.2 | 96 | 7.4 ± 9.6 | <0.001 |
| Vegetables, portions/week | 97 | 11.6 ± 9.2 | 91 | 13.8 ± 8.5 | 98 | 15.2 ± 9.7 | 95 | 12.7 ± 9.2 | 0.04 |
| Carbohydrates, portions /week | 92 | 35.8 ± 19.0 | 87 | 37.0 ± 17.5 | 98 | 32.6 ± 15.6 | 90 | 35.4 ± 19.6 | 0.40 |
| Portion size, portions/week | 97 | 18.1 ± 13.1 | 91 | 19.9 ± 17.7 | 97 | 14.1 ±10.1 | 94 | 20.1 ± 14.4 | 0.01 |
| 35–37 weeks | |||||||||
| MVPA, MET-h/week * | 89 | 21.6 (11.6; 42.0) | 86 | 27.2 (7.8; 54.2) | 88 | 21.1 (9.9; 59.6) | 88 | 35.4 (15.8; 60.4) | 0.18 |
| Sedentary behaviour, MET-h/week | 89 | 13.8 ± 9.9 | 86 | 10.7 ± 7.7 | 88 | 13.2 ± 9.6 | 88 | 13.9 ± 8.2 | 0.06 |
| Sugary drinks, portions/week | 77 | 5.4 ± 5.9 | 79 | 3.6 ± 4.7 | 82 | 3.3 ± 5.1 | 76 | 6.2 ± 6.7 | 0.002 |
| Vegetables, portions/week | 86 | 12.9 ± 9.5 | 81 | 12.6 ± 9.2 | 82 | 14.2 ± 10.1 | 82 | 12.6 ± 11.0 | 0.69 |
| Carbohydrates, portions/week | 77 | 37.3 ± 19.7 | 79 | 32.9 ± 18.4 | 80 | 29.6 ± 13.9 | 77 | 36.5 ± 20.4 | 0.03 |
| Portion size, portions /week | 81 | 17.9 ± 13.8 | 80 | 16.7 ± 14.1 | 81 | 14.8 ± 11.3 | 80 | 21.3 ± 17.4 | 0.03 |
| 24–28 Weeks | HE&PA vs. UC Estimate (95% CI) | p | HE vs. UC Estimate (95% CI) | p | PA vs. UC Estimate (95% CI) | p |
| Perceived risk for GDM (range 1–5) | 0.04 (−0.19; 0.27) | 0.74 | 0.002 (−0.22; 0.23) | 0.99 | −0.01 (−0.24; 0.22) | 0.92 |
| Outcome expectancy GDM risk reduction with physical activity (range 2–20) | 1.31 (0.47; 2.14) | 0.002 | 1.24 (0.43; 2.05) | 0.003 | 1.38 (0.56; 2.21) | 0.001 |
| Outcome expectancy GDM risk reduction with healthy eating (range 2–20) | 1.25 (0.43; 2.07) | 0.003 | 1.24 (0.43; 2.03) | 0.003 | 1.12 (0.30; 1.93) | 0.007 |
| Outcome expectancy risk reduction baby with physical activity (range 2–20) | 0.83 (0.03; 1.63) | 0.04 | 0.92 (0.14; 1.70) | 0.02 | 0.96 (0.17; 1.75) | 0.02 |
| Outcome expectancy risk reduction baby with healthy eating (range 2–20) | 0.89 (0.13; 1.66) | 0.02 | 0.91 (0.16; 1.66) | 0.02 | 0.95 (0.19; 1.72) | 0.01 |
| Task self-efficacy for physical activity (range 3–30) | 2.11 (0.81; 3.42) | 0.002 | 1.09 (−0.18; 2.37) | 0.09 | 1.89 (0.59; 3.18) | 0.004 |
| Task self-efficacy for healthy eating (range 3–30) | 2.19 (0.94; 3.43) | 0.001 | 2.32 (1.10; 3.54) | 0.0002 | 0.49 (−0.75; 1.73) | 0.44 |
| Satisfaction with social support for physical activity (range 1–10) | 0.57 (0.06; 1.08) | 0.03 | 0.41 (−0.09; 0.90) | 0.11 | 0.49 (−0.01; 1.00) | 0.054 |
| Satisfaction with social support for healthy eating (range 1–10) | 0.10 (−0.38; 0.59) | 0.67 | 0.38 (−0.09; 0.84) ** | 0.12 | 0.07 (−0.41; 0.54) | 0.78 |
| 35–37 weeks | HE&PA vs. UC Estimate (95% CI) | p | HE vs. UC Estimate (95% CI) | p | PA vs. UC Estimate (95% CI) | p |
| Perceived risk for GDM (range 1–5) | −0.02 (−0.35; 0.31) | 0.74 | −0.001 (−0.33; 0.32) | 0.99 | −0.15 (−0.47; 0.18) | 0.37 |
| Outcome expectancy GDM risk reduction with physical activity (range 2–20) | 1.99 (0.62; 3.36) | 0.002 | 0.87 (−0.49; 2.22) | 0.21 | 1.74 (0.39; 3.10) | 0.01 |
| Outcome expectancy GDM risk reduction with healthy eating (range 2–20) | 2.09 (0.69; 3.49) | 0.004 | 0.97 (−0.41; 2.35) | 0.17 | 1.32 (−0.05; 2.69) | 0.06 |
| Outcome expectancy risk reduction baby with physical activity (range 2–20) | 1.29 (0.39; 2.18) | 0.04 | 0.85 (−0.03; 1.74) | 0.06 | 1.25 (0.36; 2.13) | 0.01 |
| Outcome expectancy risk reduction baby with healthy eating (range 2–20) | 1.46 (0.56; 2.36) | 0.002 | 0.82 (−0.07; 1.71) | 0.07 | 0.82 (−0.06; 1.71) | 0.07 |
| Task self-efficacy for physical activity (range 3–30) | 2.02 (0.38; 3.67) | 0.002 | 1.98 (0.35; 3.60) | 0.02 | 1.90 (0.27; 3.53) | 0.02 |
| Task self-efficacy for healthy eating (range 3–30) | 2.01 (0.49; 3.53) | 0.01 | 1.43 (−0.07; 2.93) | 0.06 | 0.37 (−1.12; 1.86) | 0.63 |
| Satisfaction with social support for physical activity (range 1–10) | 0.63 (0.07; 1.19) | 0.03 | 0.63 (0.08; 1.18) | 0.03 | 0.78 (0.23; 1.33) | 0.01 |
| Satisfaction with social support for healthy eating (range 1–10) | 0.54 (−0.02; 1.10) | 0.06 | 0.65 (0.10; 1.21) | 0.02 | 0.36 (−0.19; 0.91) | 0.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Poppel, M.N.M.; Jelsma, J.G.M.; Simmons, D.; Devlieger, R.; Jans, G.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Harreiter, J.; et al. Mediators of Lifestyle Behaviour Changes in Obese Pregnant Women. Secondary Analyses from the DALI Lifestyle Randomised Controlled Trial. Nutrients 2019, 11, 311. https://doi.org/10.3390/nu11020311
van Poppel MNM, Jelsma JGM, Simmons D, Devlieger R, Jans G, Galjaard S, Corcoy R, Adelantado JM, Dunne F, Harreiter J, et al. Mediators of Lifestyle Behaviour Changes in Obese Pregnant Women. Secondary Analyses from the DALI Lifestyle Randomised Controlled Trial. Nutrients. 2019; 11(2):311. https://doi.org/10.3390/nu11020311
Chicago/Turabian Stylevan Poppel, Mireille N. M., Judith G. M. Jelsma, David Simmons, Roland Devlieger, Goele Jans, Sander Galjaard, Rosa Corcoy, Juan M. Adelantado, Fidelma Dunne, Jürgen Harreiter, and et al. 2019. "Mediators of Lifestyle Behaviour Changes in Obese Pregnant Women. Secondary Analyses from the DALI Lifestyle Randomised Controlled Trial" Nutrients 11, no. 2: 311. https://doi.org/10.3390/nu11020311
APA Stylevan Poppel, M. N. M., Jelsma, J. G. M., Simmons, D., Devlieger, R., Jans, G., Galjaard, S., Corcoy, R., Adelantado, J. M., Dunne, F., Harreiter, J., Kautzky-Willer, A., Damm, P., Mathiesen, E. R., Jensen, D. M., Andersen, L.-L., Tanvig, M., Lapolla, A., Dalfra, M.-G., Bertolotto, A., ... Snoek, F. J. (2019). Mediators of Lifestyle Behaviour Changes in Obese Pregnant Women. Secondary Analyses from the DALI Lifestyle Randomised Controlled Trial. Nutrients, 11(2), 311. https://doi.org/10.3390/nu11020311










