Sophora Tomentosa Extract Prevents MPTP-Induced Parkinsonism in C57BL/6 Mice Via the Inhibition of GSK-3β Phosphorylation and Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Preparation
2.2. Chemicals
2.3. Animals
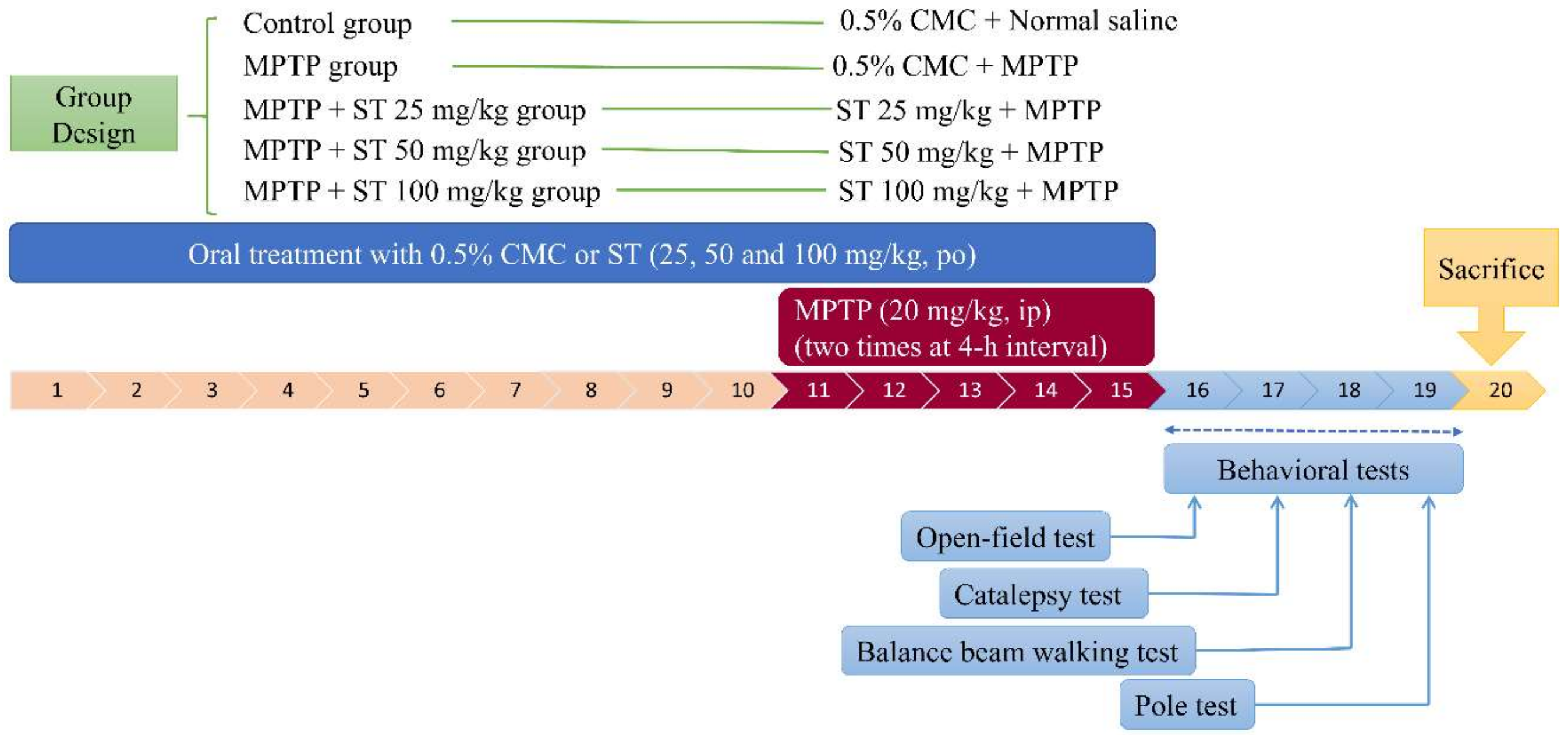
2.4. Drug Treatment and Experimental Design
2.5. Behavioral Tests
2.6. Immunohistochemical Stain of TH-Positive Neurons in the SN
2.7. Measurement of Striatal Dopamine and Its Metabolite Levels
2.8. Biochemical Assays
2.9. Western Blotting
2.10. Determination of Radical Scavenging Activity In Vitro
2.11. Determination of Antioxidant Phytoconstituent Contents by a Spectrophotometric Reader
2.12. Determination of Phytochemical Compounds by HPLC-DAD
2.13. Statistical Analysis
3. Results
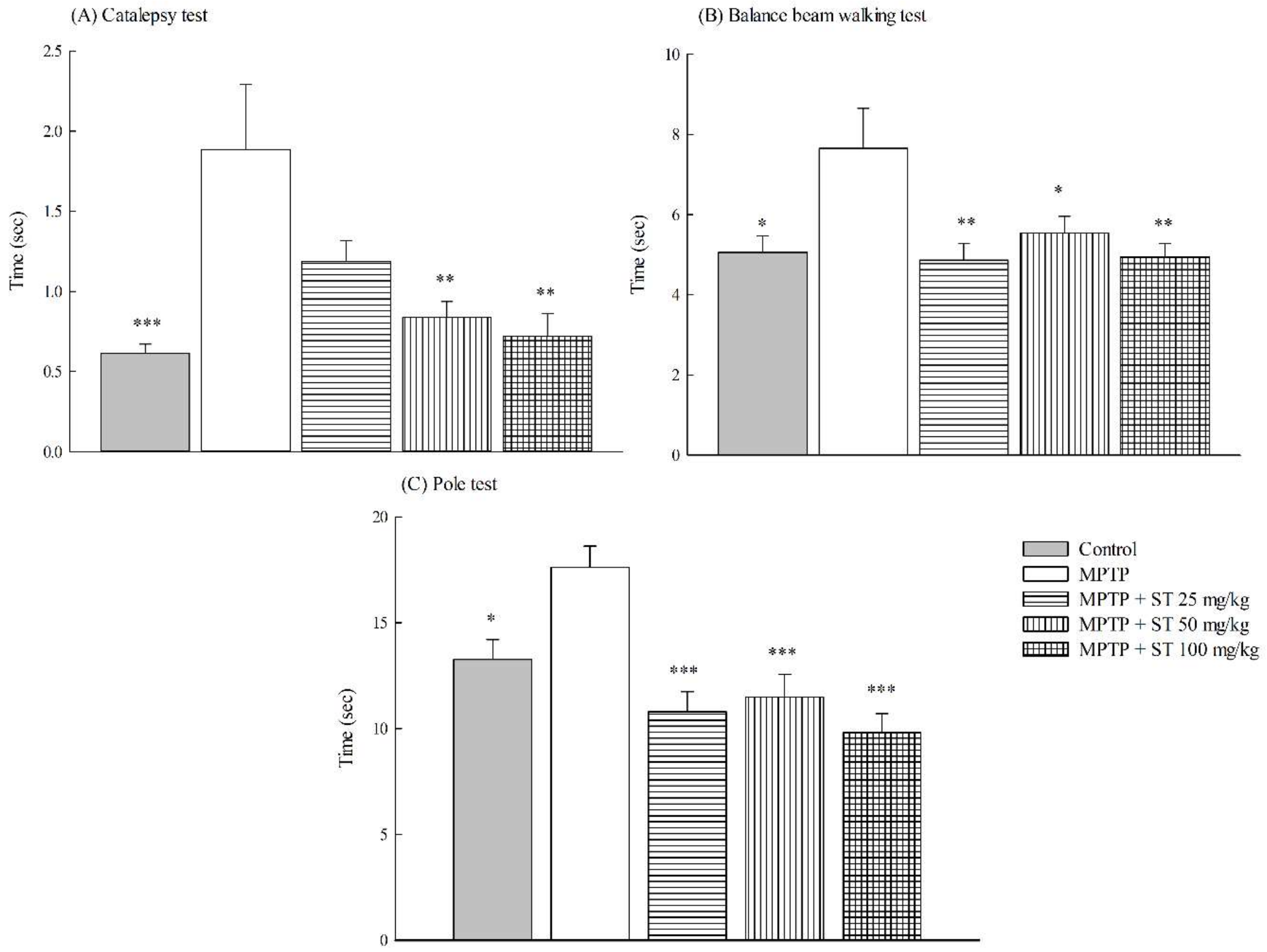
3.1. Effects of ST Extract on the Impairment of Motor Function in an MPTP-Induced PD Mouse Model
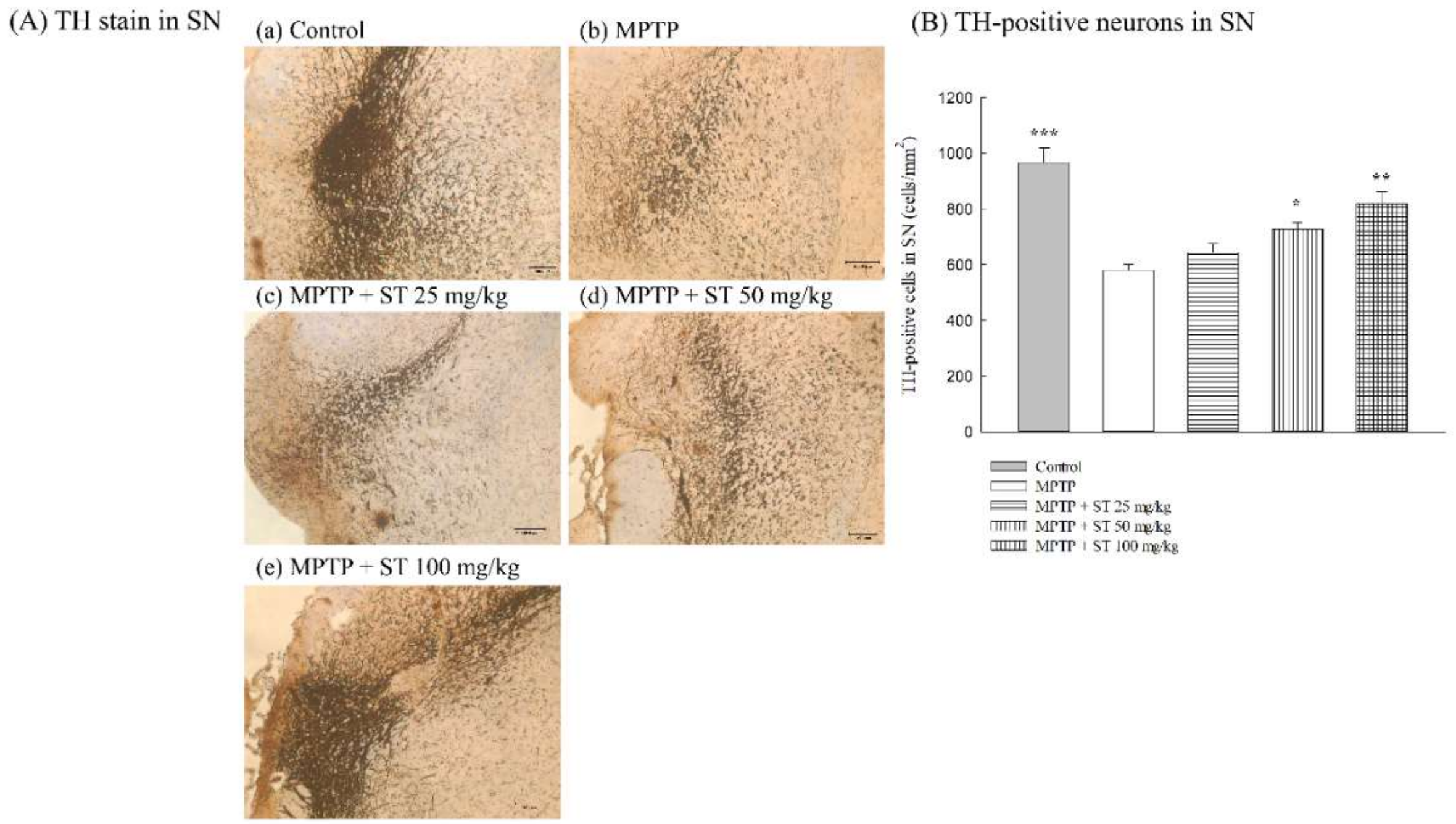
3.2. Effects of ST Extract on the Nigral Dopaminergic Neurons in an MPTP-Induced PD Mouse Model
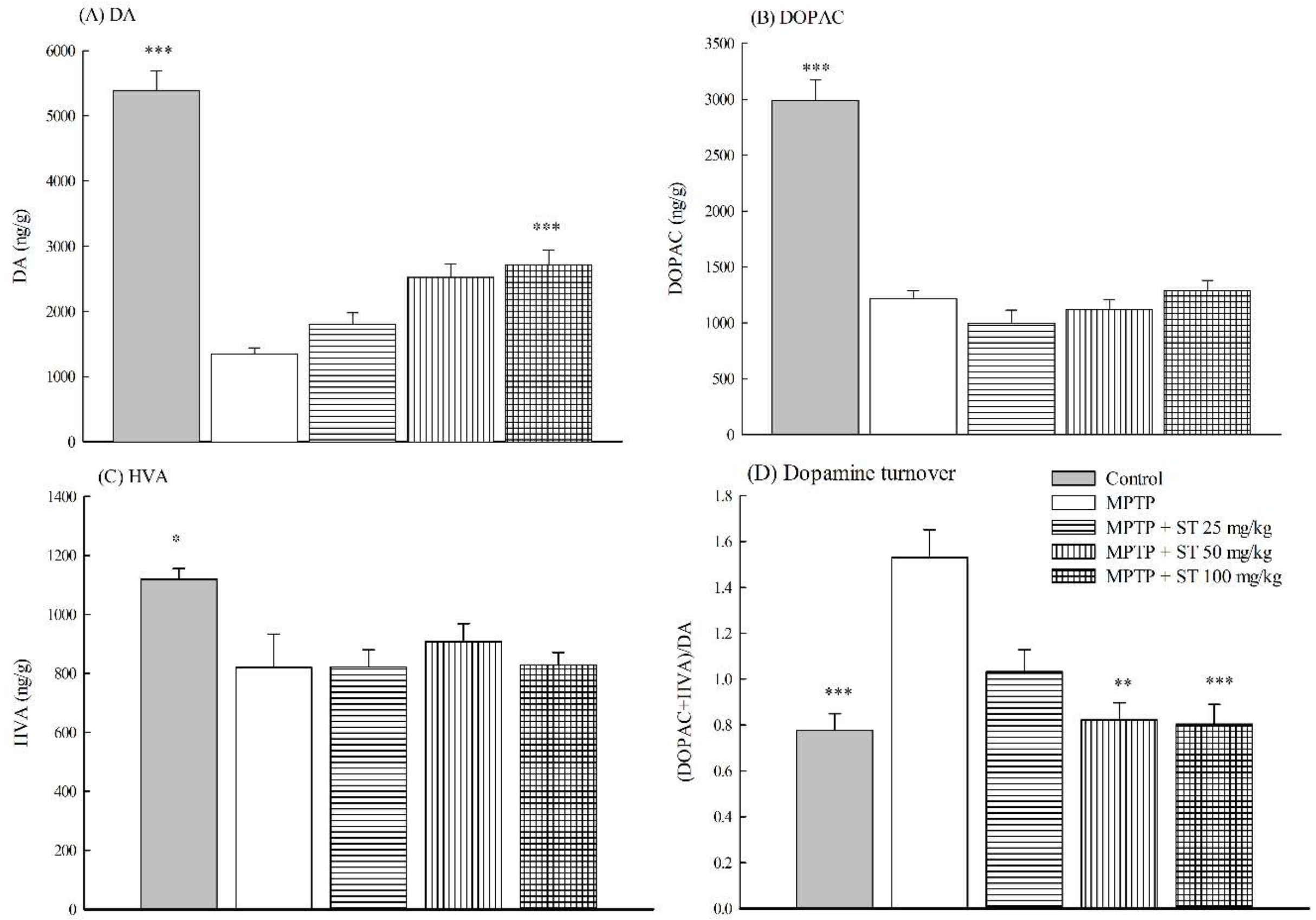
3.3. Effects of ST Extract on the Striatal Neruochemical Alteration in an MPTP-Induced PD Mouse Model
3.4. Effects of ST Extract on the Striatal Oxidative Stress in MPTP-Induced PD Mouse Model
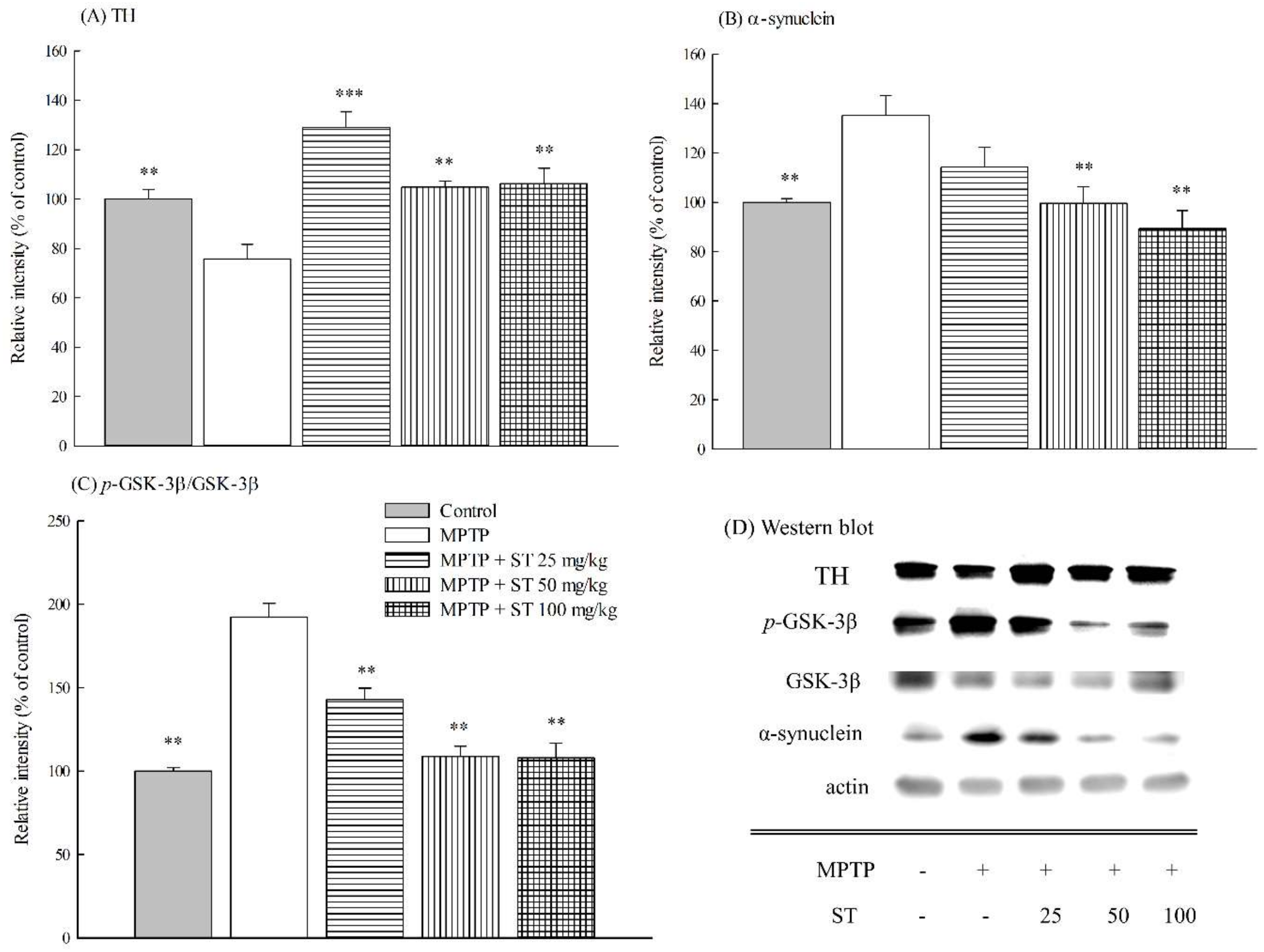
3.5. Effects of ST Extract on the Striatal Protein Expression in MPTP-Induced PD Mouse Model
3.6. Radical Scavenging Capacities
3.7. Antioxidant Phytoconstituents Contents
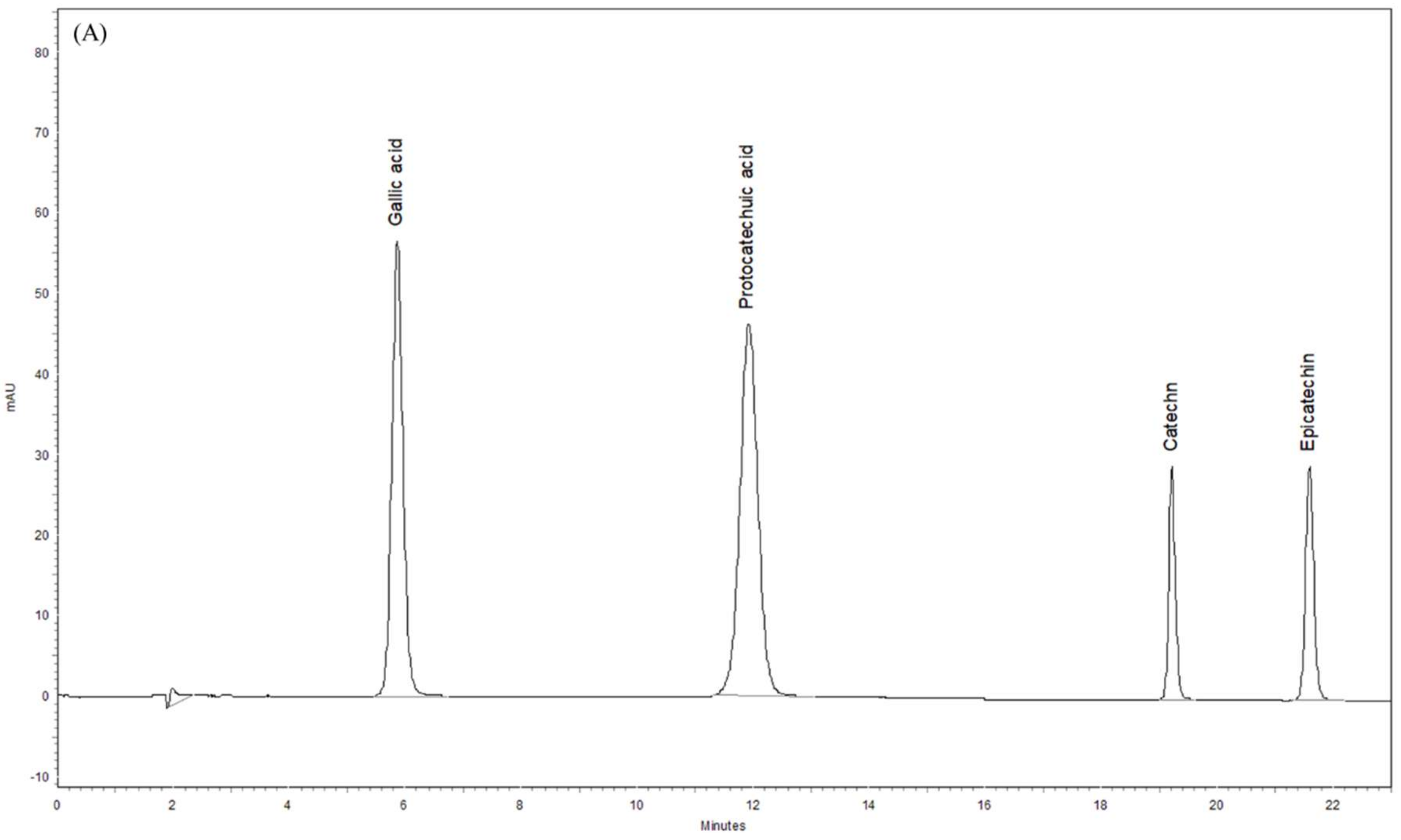
3.8. Phytochemical Profiles of ST Extract by HPLC-DAD
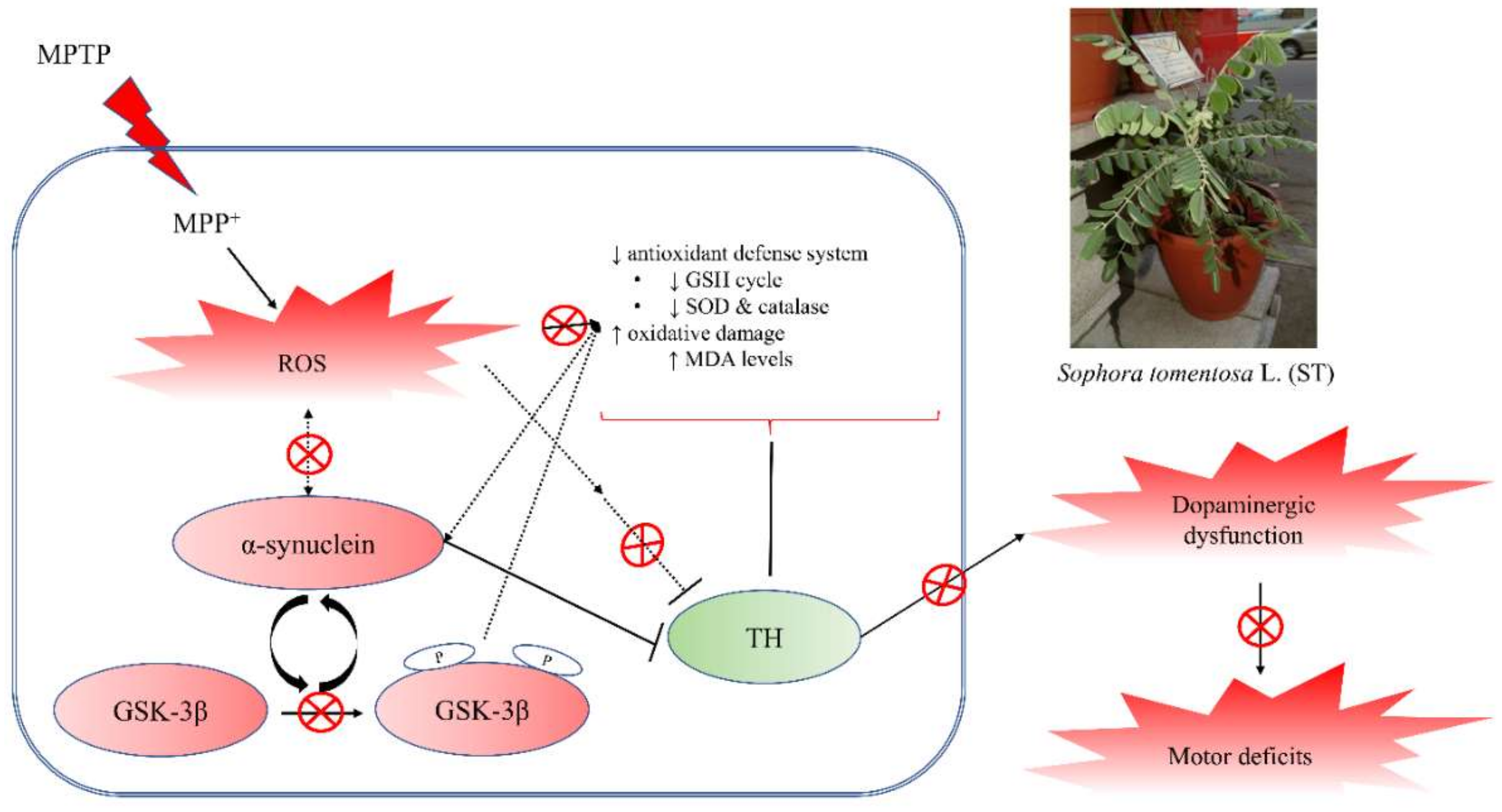
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Dexter, D.T.; Jenner, P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013, 62, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Guo, J.D.; Zhao, X.; Li, Y.; Li, G.R.; Liu, X.L. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease. Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef]
- Biosa, A.; Arduini, I.; Soriano, M.E.; Giorgio, V.; Bernardi, P.; Bisaglia, M.; Bubacco, L. Dopamine oxidation products as mitochondrial endotoxins, a potential molecular mechanism for preferential neurodegeneration in Parkinson’s disease. ACS Chem. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ghiglieri, V.; Calabrese, V.; Calabresi, P. Alpha-synuclein: From early synaptic dysfunction to neurodegeneration. Front. Neurol. 2018, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Krainc, D. α-synuclein toxicity in neurodegeneration: Mechanism and therapeutic strategies. Nat. Med. 2017, 23, 1–13. [Google Scholar] [CrossRef]
- Perfeito, R.; Ribeiro, M.; Rego, A.C. Alpha-synuclein-induced oxidative stress correlates with altered superoxide dismutase and glutathione synthesis in human neuroblastoma SH-SY5Y cells. Arch. Toxicol. 2017, 91, 1245–1259. [Google Scholar] [CrossRef]
- Credle, J.J.; George, J.L.; Wills, J.; Duka, V.; Shah, K.; Lee, Y.C.; Rodriguez, O.; Simkins, T.; Winter, M.; Moechars, D.; et al. GSK-3β dysregulation contributes to parkinson’s-like pathophysiology with associated region-specific phosphorylation and accumulation of tau and alpha-synuclein. Cell Death Differ. 2015, 22, 838–851. [Google Scholar] [CrossRef]
- Yuan, Y.; Jin, J.; Yang, B.; Zhang, W.; Hu, J.; Zhang, Y.; Chen, N.H. Overexpressed alpha-synuclein regulated the nuclear factor-kappaB signal pathway. Cell. Mol. Neurobiol. 2008, 28, 21–33. [Google Scholar] [CrossRef]
- Wills, J.; Jones, J.; Haggerty, T.; Duka, V.; Joyce, J.N.; Sidhu, A. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson’s disease brains with and without dementia. Exp. Neurol. 2010, 225, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, A.P.; Gaisina, I.N.; Petukhov, P.A.; Sridhar, J.; King, L.T.; Blond, S.Y.; Duka, T.; Rusnak, M.; Sidhu, A. Highly potent and specific GSK-3beta inhibitors that block tau phosphorylation and decrease alpha-synuclein protein expression in a cellular model of Parkinson’s disease. ChemMedChem 2006, 1, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Pain, S.; Gochard, A.; Bodard, S.; Gulhan, Z.; Prunier-Aesch, C.; Chalon, S. Toxicity of MPTP on neurotransmission in three mouse models of Parkinson’s disease. Exp. Toxicol. Pathol. 2013, 65, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Visanji, N.P.; Brotchie, J.M. MPTP-induced models of Parkinson’s disease in mice and non-human primates. Curr. Protoc. Pharmacol. 2005, 29, 5–42. [Google Scholar]
- He, X.; Bai, Y.; Zhao, Z.; Wang, X.; Fang, J.; Huang, L.; Zeng, M.; Zhang, Q.; Zhang, Y.; Zheng, X. Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L.: A review. J. Ethnopharmacol. 2016, 187, 160–182. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Huang, L.; Wang, J.; Huang, X. Sophora flavescens Ait.: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2015, 172, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Jeon, H.; Kim, H.; Koo, S.; Kim, S. Sophora flavescens Aiton decreases MPP(+)-induced mitochondrial dysfunction in SH-SY5Y cells. Front. Aging Neurosci. 2018, 10, 119. [Google Scholar] [CrossRef]
- Jeong, G.S.; Li, B.; Lee, D.S.; Byun, E.; An, R.B.; Pae, H.O.; Chung, H.T.; Youn, K.H.; Kim, Y.C. Lavandulyl flavanones from Sophora flavescens protect mouse hippocampal cells against glutamate-induced neurotoxicity via the induction of heme oxygenase-1. Biol. Pharm. Bull. 2008, 31, 1964–1967. [Google Scholar] [CrossRef]
- Chen, H.N.; Hsieh, C.L. Effects of Sophora japonica flowers (Huaihua) on cerebral infarction. Chin. Med. 2010, 5, 34. [Google Scholar] [CrossRef]
- Park, S.J.; Nam, K.W.; Lee, H.J.; Cho, E.Y.; Koo, U.; Mar, W. Neuroprotective effects of an alkaloid-free ethyl acetate extract from the root of Sophora flavescens Ait. against focal cerebral ischemia in rats. Phytomedicine 2009, 16, 1042–1051. [Google Scholar] [CrossRef]
- Komatsu, M.; Yokoe, I.; Shirataki, Y. Studies on the constituents of sophora species. XIII. Constituents of the aerial parts of Sophora tomentosa L. Chem. Pharm. Bull. 1978, 26, 3863–3870. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, H.; Yokoyama, H.; Kimoto, H.; Kato, H.; Araki, T. Biochemical alterations of the striatum in an MPTP-treated mouse model of Parkinson’s disease. Metab. Brain Dis. 2010, 25, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Shiao, Y.J.; Su, M.H.; Lin, H.C.; Wu, C.R. Echinacoside ameliorates the memory impairment and cholinergic deficit induced by amyloid beta peptides via the inhibition of amyloid deposition and toxicology. Food Funct. 2017, 8, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, A.; Essa, M.M.; Manivasagam, T. Therapeutic attenuation of neuroinflammation and apoptosis by black tea theaflavin in chronic MPTP/probenecid model of Parkinson’s disease. Neurotox. Res. 2013, 23, 166–173. [Google Scholar] [CrossRef]
- Hou, X.; Yuan, Y.; Sheng, Y.; Yuan, B.; Wang, Y.; Zheng, J.; Liu, C.F.; Zhang, X.; Hu, L.F. GYY4137, an H2S slow-releasing donor, prevents nitrative stress and alpha-synuclein nitration in an MPTP mouse model of Parkinson’s disease. Front. Pharmacol. 2017, 8, 741. [Google Scholar] [CrossRef]
- Matsuura, K.; Kabuto, H.; Makino, H.; Ogawa, N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J. Neurosci. Methods 1997, 73, 45–48. [Google Scholar] [CrossRef]
- Wu, C.R.; Chang, H.C.; Cheng, Y.D.; Lan, W.C.; Yang, S.E.; Ching, H. Aqueous extract of Davallia mariesii attenuates 6-hydroxydopamine-induced oxidative damage and apoptosis in B35 cells through inhibition of caspase cascade and activation of PI3K/AKT/GSK-3beta pathway. Nutrients 2018, 10, 1449. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Chang, J.C.; Lin, L.W.; Tsai, F.H.; Chang, H.C.; Wu, C.R. Comparison of the hepatoprotective effects of four endemic Cirsium species extracts from Taiwan on CCl(4)-induced acute liver damage in C57BL/6 mice. Int. J. Mol. Sci. 2018, 19, 1329. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; Batista, R.S.d.A.; Medeiros, F.D.d.; Santos, F.S.; Medeiros, A.C.D. Development of a rapid and simple HPLC-UV method for determination of gallic acid in Schinopsis brasiliensis. Rev. Bras. Farmacogn. 2015, 25, 208–211. [Google Scholar] [CrossRef]
- Mingazov, E.R.; Khakimova, G.R.; Kozina, E.A.; Medvedev, A.E.; Buneeva, O.A.; Bazyan, A.S.; Ugrumov, M.V. MPTP mouse model of preclinical and clinical Parkinson’s disease as an instrument for translational medicine. Mol. Neurobiol. 2018, 55, 2991–3006. [Google Scholar] [CrossRef]
- Li, X.H.; Dai, C.F.; Chen, L.; Zhou, W.T.; Han, H.L.; Dong, Z.F. 7,8-dihydroxyflavone ameliorates motor deficits Via suppressing alpha-synuclein expression and oxidative stress in the MPTP-induced mouse model of Parkinson’s disease. CNS Neurosci. Ther. 2016, 22, 617–624. [Google Scholar] [CrossRef]
- Macedo, D.; Tavares, L.; McDougall, G.J.; Vicente Miranda, H.; Stewart, D.; Ferreira, R.B.; Tenreiro, S.; Outeiro, T.F.; Santos, C.N. (Poly)phenols protect from alpha-synuclein toxicity by reducing oxidative stress and promoting autophagy. Hum. Mol. Genet. 2015, 24, 1717–1732. [Google Scholar] [CrossRef]
- Fox, S.H.; Brotchie, J.M. The MPTP-lesioned non-human primate models of Parkinson’s disease. Past, present, and future. Prog. Brain Res. 2010, 184, 133–157. [Google Scholar] [PubMed]
- Mani, S.; Sekar, S.; Barathidasan, R.; Manivasagam, T.; Thenmozhi, A.J.; Sevanan, M.; Chidambaram, S.B.; Essa, M.M.; Guillemin, G.J.; Sakharkar, M.K. Naringenin decreases alpha-synuclein expression and neuroinflammation in MPTP-induced Parkinson’s disease model in mice. Neurotox. Res. 2018, 33, 656–670. [Google Scholar] [CrossRef]
- Jo, M.G.; Ikram, M.; Jo, M.H.; Yoo, L.; Chung, K.C.; Nah, S.Y.; Hwang, H.; Rhim, H.; Kim, M.O. Gintonin mitigates MPTP-induced loss of nigrostriatal dopaminergic neurons and accumulation of alpha-synuclein via the Nrf2/HO-1 pathway. Mol. Neurobiol. 2018. [Google Scholar] [CrossRef]
- Golpich, M.; Amini, E.; Hemmati, F.; Ibrahim, N.M.; Rahmani, B.; Mohamed, Z.; Raymond, A.A.; Dargahi, L.; Ghasemi, R.; Ahmadiani, A. Glycogen synthase kinase-3 β (GSK-3β) signaling: Implications for Parkinson’s disease. Pharmacol. Res. 2015, 97, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Huttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Szeto, S.S.W.; Chong, C.M.; Quan, Q.; Huang, C.; Cui, W.; Guo, B.; Wang, Y.; Han, Y.; et al. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic. Biol. Med. 2015, 84, 331–343. [Google Scholar] [CrossRef]
- Zhang, H.N.; An, C.N.; Xu, M.; Guo, D.A.; Li, M.; Pu, X.P. Protocatechuic acid inhibits rat pheochromocytoma cell damage induced by a dopaminergic neurotoxin. Biol. Pharm. Bull. 2009, 32, 1866–1869. [Google Scholar] [CrossRef]
- Liu, Y.M.; Jiang, B.; Bao, Y.M.; An, L.J. Protocatechuic acid inhibits apoptosis by mitochondrial dysfunction in rotenone-induced PC12 cells. Toxicol. In Vitro 2008, 22, 430–437. [Google Scholar] [CrossRef]
- Shui, G.; Bao, Y.M.; Bo, J.; An, L.J. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur. J. Pharmacol. 2006, 538, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Alvarez-Fernandez, M.A.; Cerezo, A.B.; Richard, T.; Troncoso, A.M.; Garcia-Parrilla, M.A. Protocatechuic Acid: Inhibition of Fibril Formation, Destabilization of Preformed Fibrils of Amyloid-beta and alpha-Synuclein, and Neuroprotection. J. Agric. Food Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.N.; An, C.N.; Zhang, H.N.; Pu, X.P. Protocatechuic acid inhibits neurotoxicity induced by MPTP in vivo. Neurosci. Lett. 2010, 474, 99–103. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, J.H.; Seo, Y.H.; Jang, J.H.; Jeong, C.H.; Lee, S.; Jeong, G.S.; Park, B. Epicatechin Prevents Methamphetamine-Induced Neuronal Cell Death via Inhibition of ER Stress. Biomol. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Osornio, M.; Gorostieta-Salas, E.; Montes, S.; Perez-Severiano, F.; Rubio, C.; Gomez, C.; Rios, C.; Guevara, J. Epicatechin Reduces Striatal MPP(+)-Induced Damage in Rats through Slight Increases in SOD-Cu,Zn Activity. Oxid. Med. Cell. Longev. 2015, 2015, 276039. [Google Scholar] [CrossRef]
- Bitu Pinto, N.; da Silva Alexandre, B.; Neves, K.R.; Silva, A.H.; Leal, L.K.; Viana, G.S. Neuroprotective Properties of the Standardized Extract from Camellia sinensis (Green Tea) and Its Main Bioactive Components, Epicatechin and Epigallocatechin Gallate, in the 6-OHDA Model of Parkinson’s Disease. Evid. Based Complement. Alternat. Med. 2015, 2015, 161092. [Google Scholar] [CrossRef] [PubMed]


| Samples | GSH (nmoL/mg of protein) | GR (mU/mg of protein) | GPx (mU/mg of protein) | SOD (U/mg of protein) | Catalase (U/mg of protein) | MDA (nmoL/mg of protein) |
|---|---|---|---|---|---|---|
| Control | 2.54 ± 0.35 * | 210.08 ± 12.44 * | 4.86 ± 0.43 * | 21.90 ± 2.71 * | 11.11 ± 1.47 * | 4.28 ± 0.23 ** |
| MPTP | 1.55 ± 0.25 | 144.02 ± 9.05 | 3.30 ± 0.23 | 12.16 ± 1.35 | 5.79 ± 0.82 | 8.11 ± 0.76 |
| MPTP + ST 25 mg/kg | 1.82 ± 0.22 | 145.75 ± 14.98 | 3.89 ± 0.27 | 14.48 ± 2.00 | 5.52 ± 1.02 | 8.25 ± 0.65 |
| MPTP + ST 50 mg/kg | 2.06 ± 0.22 | 180.04 ± 18.24 | 4.47 ± 0.54 | 18.10 ± 2.93 | 8.69 ± 1.93 | 6.43 ± 1.12 |
| MPTP + ST 100 mg/kg | 2.65 ± 0.14 * | 249.23 ± 19.10 *** | 5.08 ± 0.47 * | 25.69 ± 2.12 *** | 11.79 ± 1.17 * | 4.52 ± 0.47 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-C.; Liu, K.-F.; Teng, C.-J.; Lai, S.-C.; Yang, S.-E.; Ching, H.; Wu, C.-R. Sophora Tomentosa Extract Prevents MPTP-Induced Parkinsonism in C57BL/6 Mice Via the Inhibition of GSK-3β Phosphorylation and Oxidative Stress. Nutrients 2019, 11, 252. https://doi.org/10.3390/nu11020252
Chang H-C, Liu K-F, Teng C-J, Lai S-C, Yang S-E, Ching H, Wu C-R. Sophora Tomentosa Extract Prevents MPTP-Induced Parkinsonism in C57BL/6 Mice Via the Inhibition of GSK-3β Phosphorylation and Oxidative Stress. Nutrients. 2019; 11(2):252. https://doi.org/10.3390/nu11020252
Chicago/Turabian StyleChang, Hung-Chi, Keng-Fan Liu, Chia-Jen Teng, Shu-Chen Lai, Shu-Er Yang, Hui Ching, and Chi-Rei Wu. 2019. "Sophora Tomentosa Extract Prevents MPTP-Induced Parkinsonism in C57BL/6 Mice Via the Inhibition of GSK-3β Phosphorylation and Oxidative Stress" Nutrients 11, no. 2: 252. https://doi.org/10.3390/nu11020252
APA StyleChang, H.-C., Liu, K.-F., Teng, C.-J., Lai, S.-C., Yang, S.-E., Ching, H., & Wu, C.-R. (2019). Sophora Tomentosa Extract Prevents MPTP-Induced Parkinsonism in C57BL/6 Mice Via the Inhibition of GSK-3β Phosphorylation and Oxidative Stress. Nutrients, 11(2), 252. https://doi.org/10.3390/nu11020252







