Ellagic Acid Inhibits Extracellular Acidity-Induced Invasiveness and Expression of COX1, COX2, Snail, Twist 1, and c-myc in Gastric Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Invasion and Migration Assay
2.3. Cytotoxicity Assays
2.4. Western Blot Analysis
2.5. Real-Time Reverse Transcription-Polymerase Chain Reaction
2.6. RNA Interference (RNAi)
2.7. Statistical Analysis
3. Results
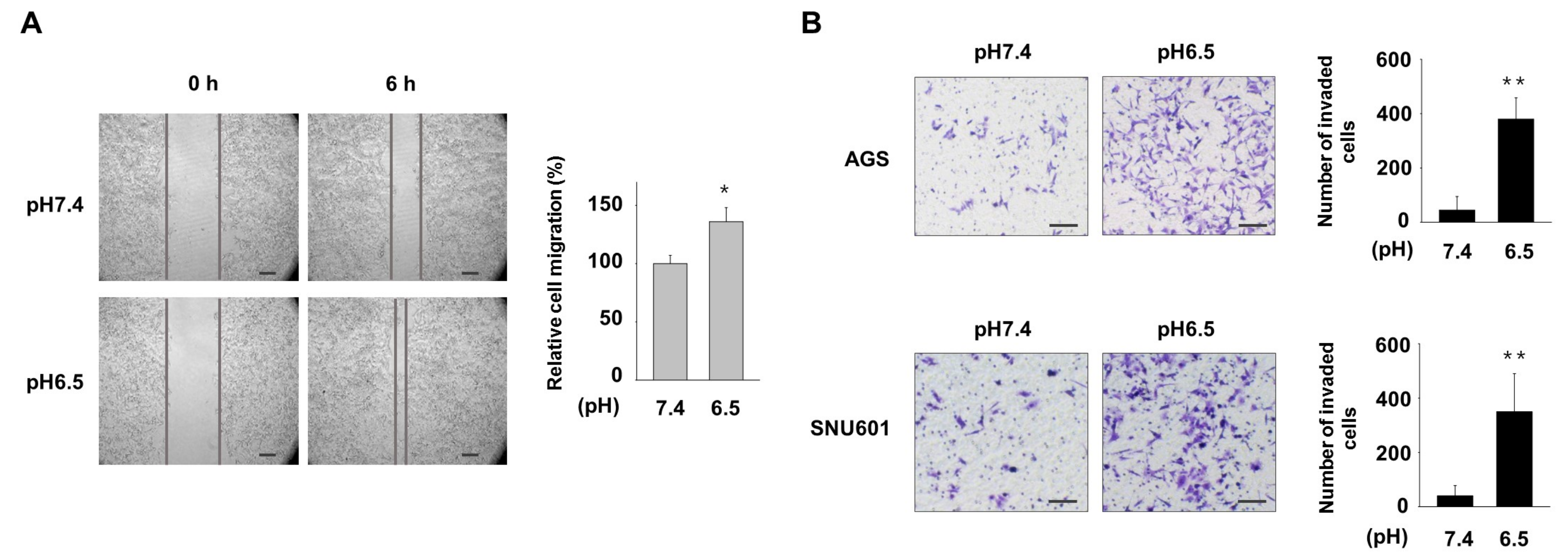
3.1. Acidic Culture Condition Increases Motility and Invasiveness of Gastric Cancer Cells
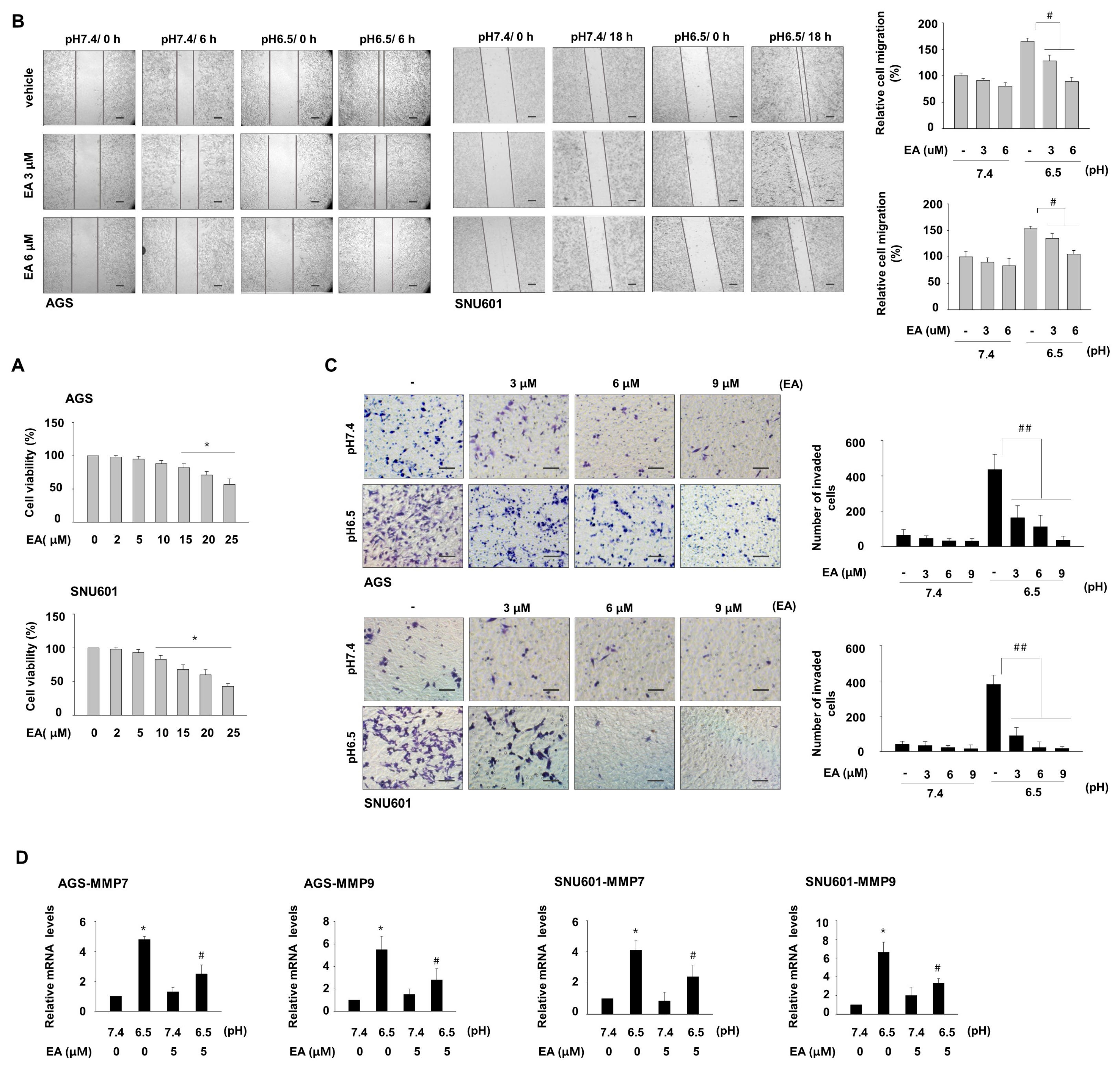
3.2. Ellagic Acid Inhibits Acidity-Mediated Migration and Invasion of Gastric Cancer Cells
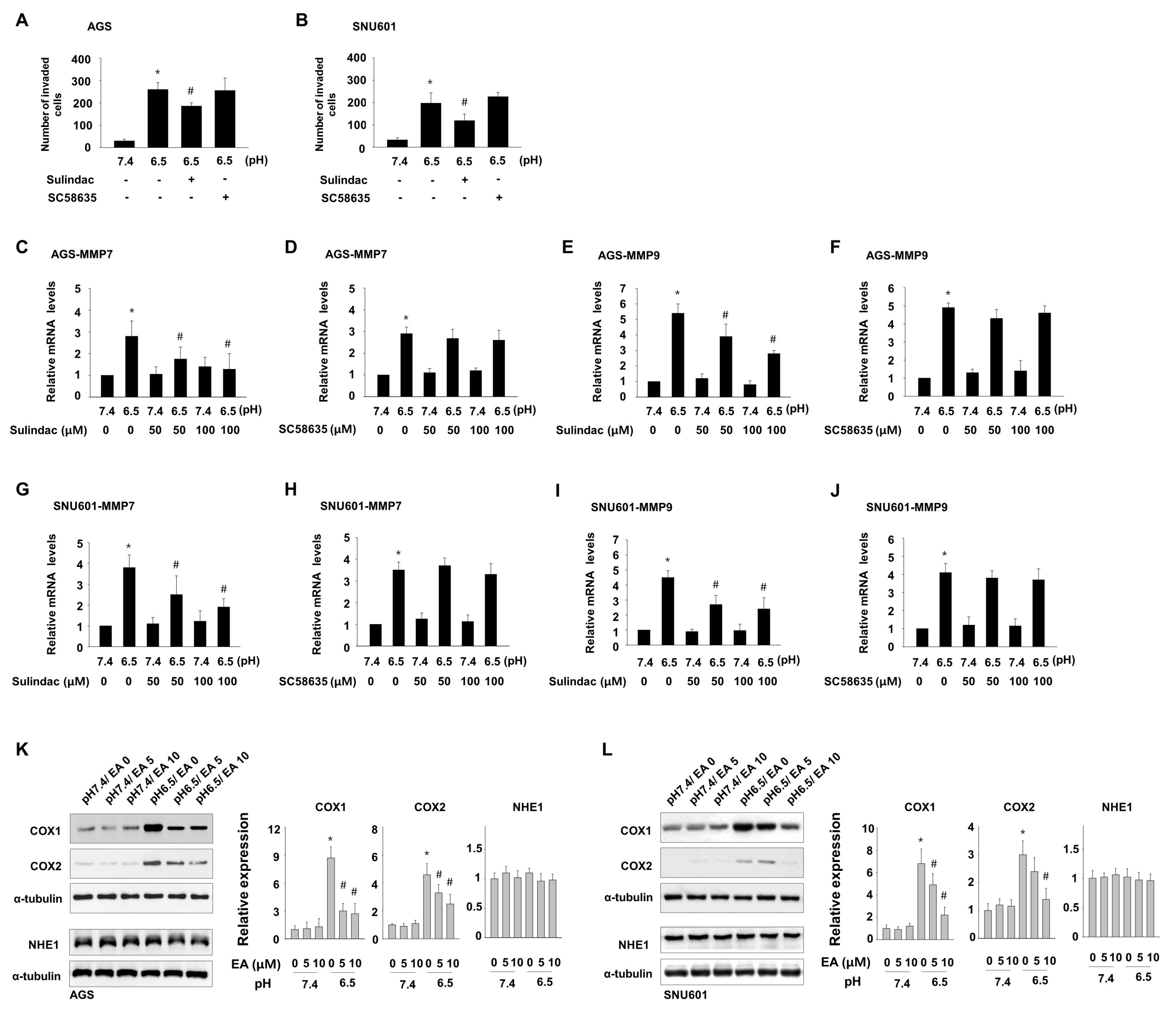
3.3. EA Decreases Induction of COX1 and COX2, Which Are Involved in Acidity-Promoted GC Invasion
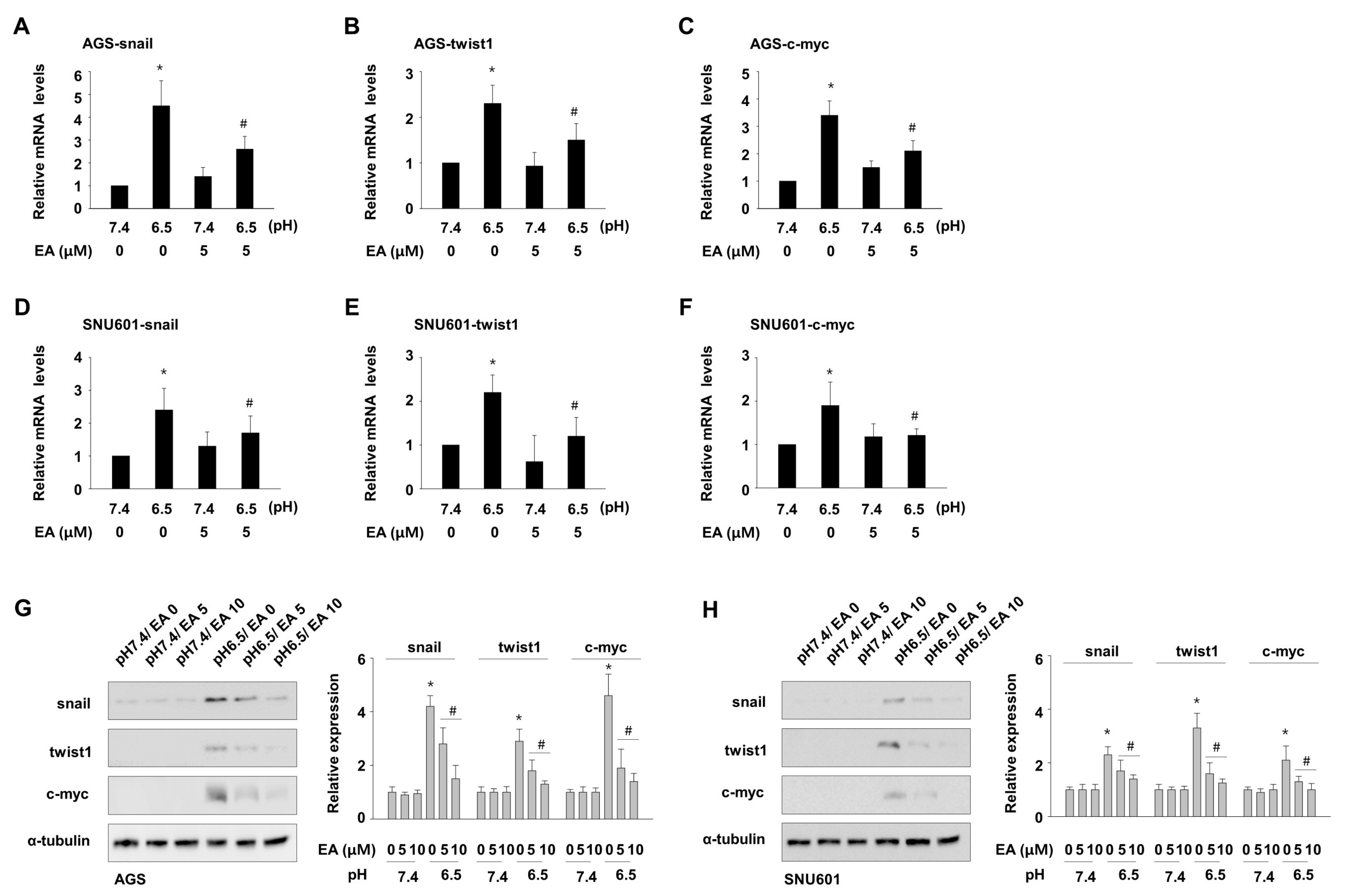
3.4. Ellagic Acid Suppresses Acidity-Mediated Induction of Snail, Twist1, and c-myc
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Justus, C.R.; Dong, L.; Yang, L.V. Acidic tumor microenvironment and ph-sensing g protein-coupled receptors. Front. Physiol. 2013, 4, 354. [Google Scholar] [CrossRef]
- Brisson, L.; Reshkin, S.J.; Gore, J.; Roger, S. Ph regulators in invadosomal functioning: Proton delivery for matrix tasting. Eur. J. Cell Biol. 2012, 91, 847–860. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Thews, O.; Gassner, B.; Kelleher, D.K.; Schwerdt, G.; Gekle, M. Impact of hypoxic and acidic extracellular conditions on cytotoxicity of chemotherapeutic drugs. Adv. Exp. Med. Biol. 2007, 599, 155–161. [Google Scholar]
- Reichert, M.; Steinbach, J.P.; Supra, P.; Weller, M. Modulation of growth and radiochemosensitivity of human malignant glioma cells by acidosis. Cancer 2002, 95, 1113–1119. [Google Scholar] [CrossRef]
- Huang, S.; Tang, Y.; Peng, X.; Cai, X.; Wa, Q.; Ren, D.; Li, Q.; Luo, J.; Li, L.; Zou, X.; et al. Acidic extracellular ph promotes prostate cancer bone metastasis by enhancing pc-3 stem cell characteristics, cell invasiveness and vegf-induced vasculogenesis of bm-epcs. Oncol. Rep. 2016, 36, 2025–2032. [Google Scholar] [CrossRef]
- Gilbert, H.T.J.; Hodson, N.; Baird, P.; Richardson, S.M.; Hoyland, J.A. Acidic ph promotes intervertebral disc degeneration: Acid-sensing ion channel -3 as a potential therapeutic target. Sci. Rep. 2016, 6, 37360. [Google Scholar] [CrossRef]
- Chen, B.; Liu, J.; Ho, T.T.; Ding, X.; Mo, Y.Y. Erk-mediated nf-kappab activation through asic1 in response to acidosis. Oncogenesis 2016, 5, e279. [Google Scholar] [CrossRef]
- Riemann, A.; Rauschner, M.; Giesselmann, M.; Reime, S.; Haupt, V.; Thews, O. Extracellular acidosis modulates the expression of epithelial-mesenchymal transition (emt) markers and adhesion of epithelial and tumor cells. Neoplasia 2019, 21, 450–458. [Google Scholar] [CrossRef]
- Peppicelli, S.; Bianchini, F.; Torre, E.; Calorini, L. Contribution of acidic melanoma cells undergoing epithelial-to-mesenchymal transition to aggressiveness of non-acidic melanoma cells. Clin. Exp. Metastasis 2014, 31, 423–433. [Google Scholar] [CrossRef]
- Riemann, A.; Schneider, B.; Gundel, D.; Stock, C.; Thews, O.; Gekle, M. Acidic priming enhances metastatic potential of cancer cells. Pflügers Arch. Eur. J. Physiol. 2014, 466, 2127–2138. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Heber, D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008, 269, 262–268. [Google Scholar] [CrossRef]
- Kresty, L.A.; Morse, M.A.; Morgan, C.; Carlton, P.S.; Lu, J.; Gupta, A.; Blackwood, M.; Stoner, G.D. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001, 61, 6112–6119. [Google Scholar]
- Wang, N.; Wang, Z.Y.; Mo, S.L.; Loo, T.Y.; Wang, D.M.; Luo, H.B.; Yang, D.P.; Chen, Y.L.; Shen, J.G.; Chen, J.P. Ellagic acid, a phenolic compound, exerts anti-angiogenesis effects via vegfr-2 signaling pathway in breast cancer. Breast Cancer Res. Treat. 2012, 134, 943–955. [Google Scholar] [CrossRef]
- Pitchakarn, P.; Chewonarin, T.; Ogawa, K.; Suzuki, S.; Asamoto, M.; Takahashi, S.; Shirai, T.; Limtrakul, P. Ellagic acid inhibits migration and invasion by prostate cancer cell lines. Asian Pac. J. Cancer Prev. 2013, 14, 2859–2863. [Google Scholar] [CrossRef]
- Ceci, C.; Tentori, L.; Atzori, M.G.; Lacal, P.M.; Bonanno, E.; Scimeca, M.; Cicconi, R.; Mattei, M.; de Martino, M.G.; Vespasiani, G.; et al. Ellagic acid inhibits bladder cancer invasiveness and in vivo tumor growth. Nutrients 2016, 8, 744. [Google Scholar] [CrossRef]
- Abdelazeem, K.N.M.; Singh, Y.; Lang, F.; Salker, M.S. Negative effect of ellagic acid on cytosolic pH regulation and glycolytic flux in human endometrial cancer cells. Cell. Physiol. Biochem. 2017, 41, 2374–2382. [Google Scholar] [CrossRef]
- Huang, H. Matrix metalloproteinase-9 (mmp-9) as a cancer biomarker and mmp-9 biosensors: Recent advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef]
- Szarvas, T.; Becker, M.; vom Dorp, F.; Gethmann, C.; Totsch, M.; Bankfalvi, A.; Schmid, K.W.; Romics, I.; Rubben, H.; Ergun, S. Matrix metalloproteinase-7 as a marker of metastasis and predictor of poor survival in bladder cancer. Cancer Sci. 2010, 101, 1300–1308. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, Z.; Wang, S.; Li, T.; Mastriani, E.; Li, Q.H.; Bao, H.X.; Zhou, Y.J.; Wang, X.; Liu, Y.; et al. Main components of pomegranate, ellagic acid and luteolin, inhibit metastasis of ovarian cancer by down-regulating mmp2 and mmp9. Cancer Biol. Ther. 2017, 18, 990–999. [Google Scholar] [CrossRef]
- Eskra, J.N.; Dodge, A.; Schlicht, M.J.; Bosland, M.C. Effects of black raspberries and their constituents on rat prostate carcinogenesis and human prostate cancer cell growth in vitro. Nutr. Cancer 2019, 1–14. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Q.; Tang, H.L.; Zhang, F.X.; Zheng, Y.F.; Wang, S.Q.; Zhang, J.; Wang, Z.Y.; Xie, X.M. Direct inhibition of actn4 by ellagic acid limits breast cancer metastasis via regulation of beta-catenin stabilization in cancer stem cells. J. Exp. Clin. Cancer Res. 2017, 36, 172. [Google Scholar] [CrossRef]
- Chung, Y.C.; Lu, L.C.; Tsai, M.H.; Chen, Y.J.; Chen, Y.Y.; Yao, S.P.; Hsu, C.P. The inhibitory effect of ellagic acid on cell growth of ovarian carcinoma cells. Evid. Based Complement. Altern. Med. 2013, 2013, 306705. [Google Scholar] [CrossRef]
- Naiki-Ito, A.; Chewonarin, T.; Tang, M.; Pitchakarn, P.; Kuno, T.; Ogawa, K.; Asamoto, M.; Shirai, T.; Takahashi, S. Ellagic acid, a component of pomegranate fruit juice, suppresses androgen-dependent prostate carcinogenesis via induction of apoptosis. Prostate 2015, 75, 151–160. [Google Scholar] [CrossRef]
- Yuan, J.; Kramer, A.; Matthess, Y.; Yan, R.; Spankuch, B.; Gatje, R.; Knecht, R.; Kaufmann, M.; Strebhardt, K. Stable gene silencing of cyclin b1 in tumor cells increases susceptibility to taxol and leads to growth arrest in vivo. Oncogene 2006, 25, 1753–1762. [Google Scholar] [CrossRef]
- Malik, A.; Mukhtar, H. Prostate cancer prevention through pomegranate fruit. Cell Cycle 2006, 5, 371–373. [Google Scholar]
- Huang, S.T.; Wang, C.Y.; Yang, R.C.; Chu, C.J.; Wu, H.T.; Pang, J.H. Phyllanthus urinaria increases apoptosis and reduces telomerase activity in human nasopharyngeal carcinoma cells. Complement. Med. Res. 2009, 16, 34–40. [Google Scholar] [CrossRef]
- Aiyer, H.S.; Warri, A.M.; Woode, D.R.; Hilakivi-Clarke, L.; Clarke, R. Influence of berry polyphenols on receptor signaling and cell-death pathways: Implications for breast cancer prevention. J. Agric. Food Chem. 2012, 60, 5693–5708. [Google Scholar] [CrossRef]
- Edderkaoui, M.; Lugea, A.; Hui, H.; Eibl, G.; Lu, Q.Y.; Moro, A.; Lu, X.; Li, G.; Go, V.L.; Pandol, S.J. Ellagic acid and embelin affect key cellular components of pancreatic adenocarcinoma, cancer, and stellate cells. Nutr. Cancer 2013, 65, 1232–1244. [Google Scholar] [CrossRef]
- Umesalma, S.; Nagendraprabhu, P.; Sudhandiran, G. Ellagic acid inhibits proliferation and induced apoptosis via the akt signaling pathway in hct-15 colon adenocarcinoma cells. Mol. Cell. Biochem. 2015, 399, 303–313. [Google Scholar] [CrossRef]
- Larrosa, M.; Tomas-Barberan, F.A.; Espin, J.C. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma caco-2 cells by using the mitochondrial pathway. J. Nutr. Biochem. 2006, 17, 611–625. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, S.N.; Marsh, J.L.; Shankar, S.; Srivastava, R.K. Ellagic acid inhibits human pancreatic cancer growth in balb c nude mice. Cancer Lett. 2013, 337, 210–217. [Google Scholar] [CrossRef]
- Losso, J.N.; Bansode, R.R.; Trappey, A., II; Bawadi, H.A.; Truax, R. In vitro anti-proliferative activities of ellagic acid. J. Nutr. Biochem. 2004, 15, 672–678. [Google Scholar] [CrossRef]
- Okajima, F. Regulation of inflammation by extracellular acidification and proton-sensing gpcrs. Cell. Signal. 2013, 25, 2263–2271. [Google Scholar] [CrossRef]
- Riemann, A.; Reime, S.; Thews, O. Tumor acidosis and hypoxia differently modulate the inflammatory program: Measurements in vitro and in vivo. Neoplasia 2017, 19, 1033–1042. [Google Scholar] [CrossRef]
- Riemann, A.; Ihling, A.; Thomas, J.; Schneider, B.; Thews, O.; Gekle, M. Acidic environment activates inflammatory programs in fibroblasts via a camp-mapk pathway. Biochim. Biophys. Acta 2015, 1853, 299–307. [Google Scholar] [CrossRef]
- Ma, C.; Zheng, C.; Bai, E.; Yang, K. Mir-101 inhibits glioma cell invasion via the downregulation of cox-2. Oncol. Lett. 2016, 12, 2538–2544. [Google Scholar] [CrossRef][Green Version]
- Singh, B.; Berry, J.A.; Shoher, A.; Ramakrishnan, V.; Lucci, A. Cox-2 overexpression increases motility and invasion of breast cancer cells. Int. J. Oncol. 2005, 26, 1393–1399. [Google Scholar] [CrossRef]
- Iwata, C.; Kano, M.R.; Komuro, A.; Oka, M.; Kiyono, K.; Johansson, E.; Morishita, Y.; Yashiro, M.; Hirakawa, K.; Kaminishi, M.; et al. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res. 2007, 67, 10181–10189. [Google Scholar] [CrossRef]
- Karlsson, S.; Nanberg, E.; Fjaeraa, C.; Wijkander, J. Ellagic acid inhibits lipopolysaccharide-induced expression of enzymes involved in the synthesis of prostaglandin e2 in human monocytes. Br. J. Nutr. 2010, 103, 1102–1109. [Google Scholar] [CrossRef]
- Cornelio Favarin, D.; Martins Teixeira, M.; Lemos de Andrade, E.; de Freitas Alves, C.; Lazo Chica, J.E.; Arterio Sorgi, C.; Faccioli, L.H.; Paula Rogerio, A. Anti-inflammatory effects of ellagic acid on acute lung injury induced by acid in mice. Mediat. Inflamm. 2013, 2013, 164202. [Google Scholar] [CrossRef]
- Pannunzio, A.; Coluccia, M. Cyclooxygenase-1 (cox-1) and cox-1 inhibitors in cancer: A review of oncology and medicinal chemistry literature. Pharmaceuticals 2018, 11, 101. [Google Scholar] [CrossRef]
- Zidar, N.; Odar, K.; Glavac, D.; Jerse, M.; Zupanc, T.; Stajer, D. Cyclooxygenase in normal human tissues—Is cox-1 really a constitutive isoform, and cox-2 an inducible isoform? J. Cell. Mol. Med. 2009, 13, 3753–3763. [Google Scholar] [CrossRef]
- Li, Z.; Dong, L.; Dean, E.; Yang, L.V. Acidosis decreases c-myc oncogene expression in human lymphoma cells: A role for the proton-sensing g protein-coupled receptor tdag8. Int. J. Mol. Sci. 2013, 14, 20236–20255. [Google Scholar] [CrossRef]
- Wolfer, A.; Wittner, B.S.; Irimia, D.; Flavin, R.J.; Lupien, M.; Gunawardane, R.N.; Meyer, C.A.; Lightcap, E.S.; Tamayo, P.; Mesirov, J.P.; et al. Myc regulation of a “poor-prognosis” metastatic cancer cell state. Proc. Natl. Acad. Sci. USA 2010, 107, 3698–3703. [Google Scholar] [CrossRef]
- Wong, D.J.; Liu, H.; Ridky, T.W.; Cassarino, D.; Segal, E.; Chang, H.Y. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell 2008, 2, 333–344. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.-C.; Hwang, H.; Han, S.I. Ellagic Acid Inhibits Extracellular Acidity-Induced Invasiveness and Expression of COX1, COX2, Snail, Twist 1, and c-myc in Gastric Carcinoma Cells. Nutrients 2019, 11, 3023. https://doi.org/10.3390/nu11123023
Lim S-C, Hwang H, Han SI. Ellagic Acid Inhibits Extracellular Acidity-Induced Invasiveness and Expression of COX1, COX2, Snail, Twist 1, and c-myc in Gastric Carcinoma Cells. Nutrients. 2019; 11(12):3023. https://doi.org/10.3390/nu11123023
Chicago/Turabian StyleLim, Sung-Chul, Hyoin Hwang, and Song Iy Han. 2019. "Ellagic Acid Inhibits Extracellular Acidity-Induced Invasiveness and Expression of COX1, COX2, Snail, Twist 1, and c-myc in Gastric Carcinoma Cells" Nutrients 11, no. 12: 3023. https://doi.org/10.3390/nu11123023
APA StyleLim, S.-C., Hwang, H., & Han, S. I. (2019). Ellagic Acid Inhibits Extracellular Acidity-Induced Invasiveness and Expression of COX1, COX2, Snail, Twist 1, and c-myc in Gastric Carcinoma Cells. Nutrients, 11(12), 3023. https://doi.org/10.3390/nu11123023





