Mechanistic Understanding of Curcumin’s Therapeutic Effects in Lung Cancer
Abstract
1. Introduction
2. Mechanism of Anti-Cancer Effect on Lung Cancer
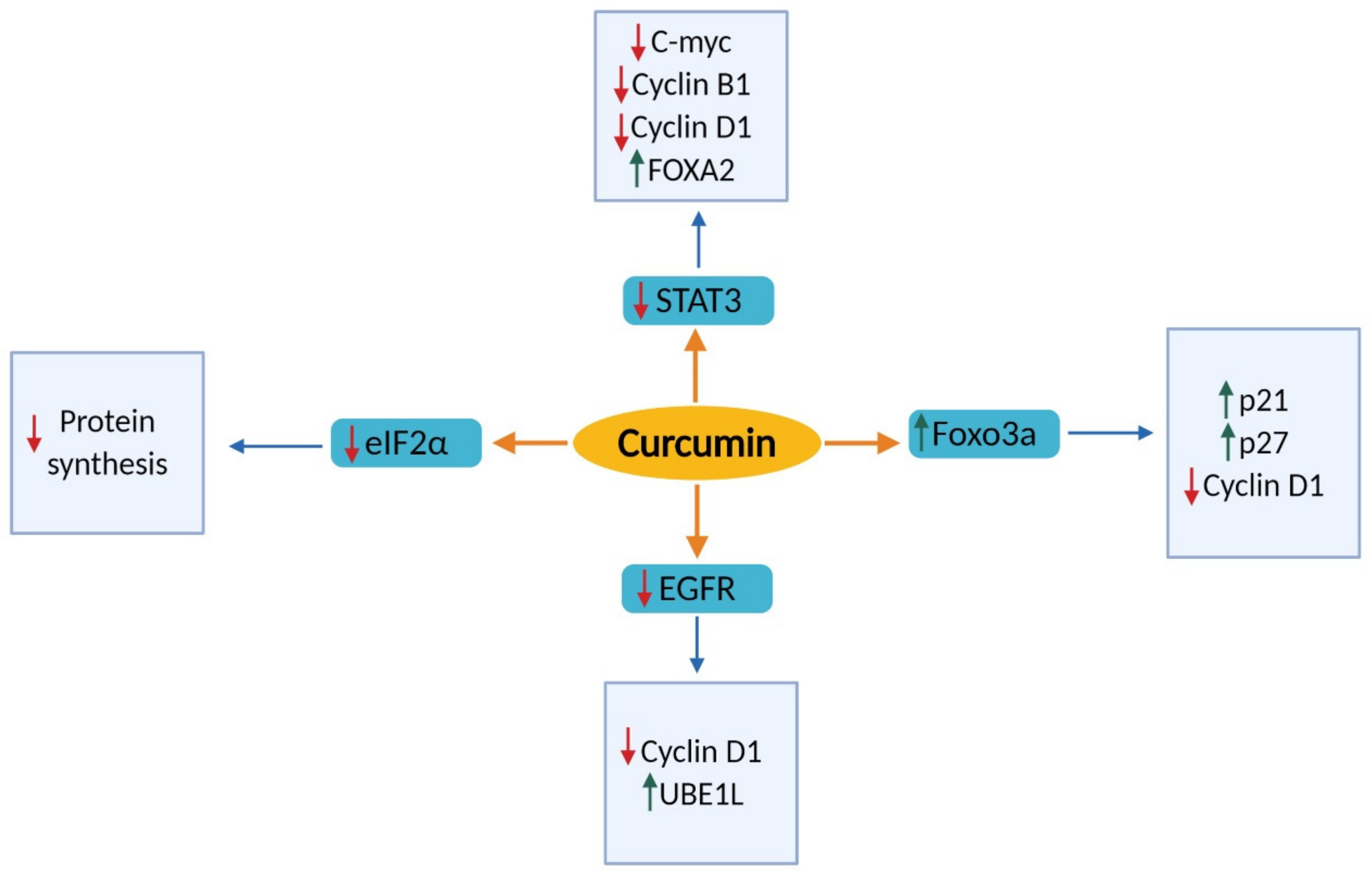
2.1. Effects of Cell Proliferation and Cell Cycle
2.1.1. Signal Transducer and Activator of Transcription 3 (STAT3)
2.1.2. Epidermal Growth Factor Receptor (EGFR)
2.1.3. Forkhead Box O3 (FOXO3a)
2.1.4. Transforming Growth Factor Beta (TGF–β)
2.1.5. Eukaryotic Initiation Factors 2 Alpha (eIF2α)
2.2. Effects on Apoptosis
2.2.1. Cyclooxygenase 2 (COX-2)
2.2.2. B-cell Lymphoma-2 (Bcl-2) Family Member
2.2.3. Phosphatidylinositol-3-kinase-Akt-mTOR (PI3K/Akt/mTOR)
2.2.4. Reactive Oxygen Species (ROS)
2.2.5. Fas–Fas Ligand interactions
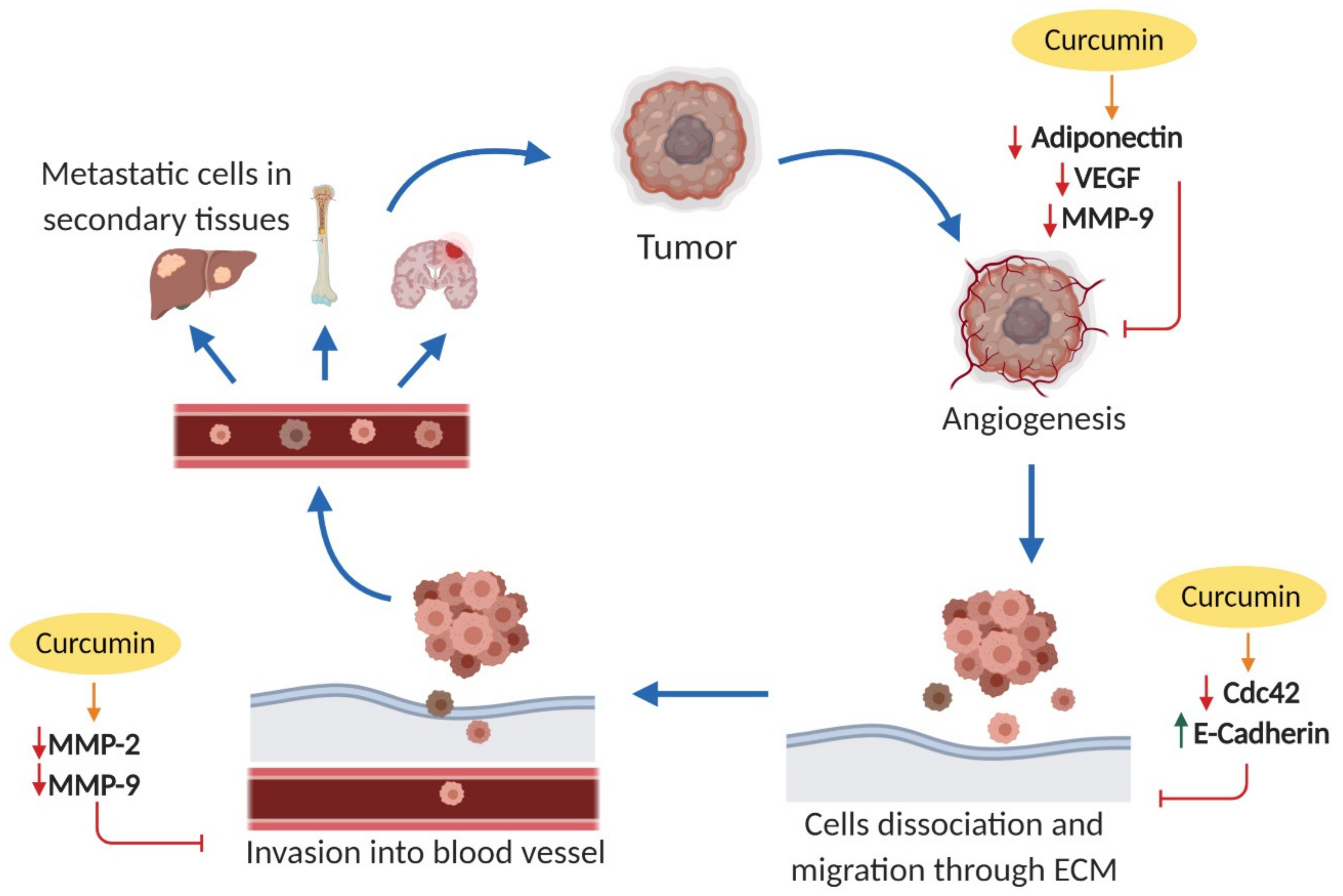
2.3. Effects on Cell Invasion and Metastasis
2.3.1. Cell Division Cycle 42 (Cdc42)
2.3.2. Epithelial Cadherin (E-Cadherin)
2.3.3. Matrix Metalloproteinases (MMPs)
2.3.4. Adiponectin
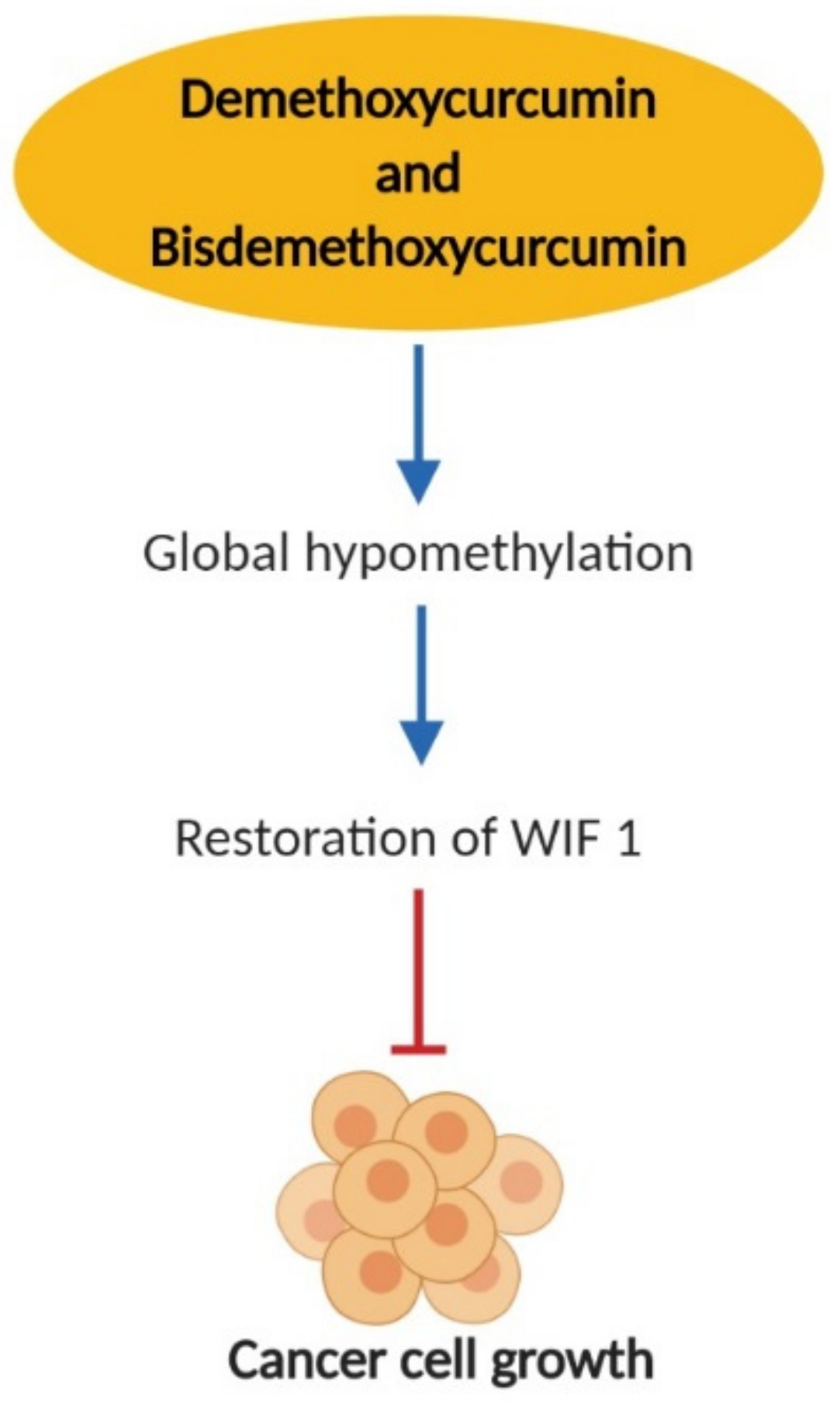
2.4. Effects on Epigenetic Changes
2.5. The Role of MicroRNA (MiRNA)
3. Curcumin Bioavailability Limitation and Strategies to Overcome
4. Curcumin and Its Potential Side Effects
5. Clinical Trials
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA A Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Prev. Biomark. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-B.; Kim, M.-J.; Ham, S.Y.; Park, G.W.; Choi, K.-D.; Jung, S.H.; Yoon, D.-Y. H9 induces apoptosis via the intrinsic pathway in non-small-cell lung cancer A549 cells. J. Microbiol. Biotechnol. 2015, 25, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Kogita, A.; Togashi, Y.; Hayashi, H.; SOGAbE, S.; Terashima, M.; De Velasco, M.A.; Sakai, K.; Fujita, Y.; TOMIdA, S.; Takeyama, Y. Hypoxia induces resistance to ALK inhibitors in the H3122 non-small cell lung cancer cell line with an ALK rearrangement via epithelial-mesenchymal transition. Int. J. Oncol. 2014, 45, 1430–1436. [Google Scholar] [CrossRef]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA A Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015, 33, 1974. [Google Scholar] [CrossRef]
- Arriagada, R.; Dunant, A.; Pignon, J.-P.; Bergman, B.; Chabowski, M.; Grunenwald, D.; Kozlowski, M.; Le Péchoux, C.; Pirker, R.; Pinel, M. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J. Clin. Oncol. 2010, 28, 35–42. [Google Scholar] [CrossRef]
- Kato, H.; Tsuboi, M.; Kato, Y.; Ikeda, N.; Okunaka, T.; Hamada, C. Postoperative adjuvant therapy for completely resected early-stage non-small cell lung cancer. Int. J. Clin. Oncol. 2005, 10, 157–164. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Reck, M.; Heigener, D.F.; Mok, T.; Soria, J.-C.; Rabe, K.F. Management of non-small-cell lung cancer: Recent developments. Lancet 2013, 382, 709–719. [Google Scholar] [CrossRef]
- Imran, M.; Ullah, A.; Saeed, F.; Nadeem, M.; Arshad, M.U.; Suleria, H.A.R. Cucurmin, anticancer, & antitumor perspectives: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1271–1293. [Google Scholar] [PubMed]
- Lestari, M.L.; Indrayanto, G. Curcumin. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 39, pp. 113–204. [Google Scholar]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, M.-C.; Ursoniu, S.; Banach, M. Effect of curcuminoids on oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Funct. Foods 2015, 18, 898–909. [Google Scholar] [CrossRef]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Um, M.Y.; Hwang, K.H.; Choi, W.H.; Ahn, J.; Jung, C.H.; Ha, T.Y. Curcumin attenuates adhesion molecules and matrix metalloproteinase expression in hypercholesterolemic rabbits. Nutr. Res. 2014, 34, 886–893. [Google Scholar] [CrossRef]
- Miriyala, S.; Panchatcharam, M.; Rengarajulu, P. Cardioprotective effects of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: New York, NY, USA, 2007; pp. 359–377. [Google Scholar]
- Vallianou, N.G.; Evangelopoulos, A.; Schizas, N.; Kazazis, C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015, 35, 645–651. [Google Scholar]
- Kumar, G.; Mittal, S.; Sak, K.; Tuli, H.S. Molecular mechanisms underlying chemopreventive potential of curcumin: Current challenges and future perspectives. Life Sci. 2016, 148, 313–328. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Lee, C.-K. What does Stat3 do? J. Clin. Investig. 2002, 109, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wen, Z.; Darnell, J.E. Stat3: A STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 1994, 264, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, S.; Kahl, P.; Buhl, T.M.; Steiner, S.; Wardelmann, E.; Merkelbach-Bruse, S.; Buettner, R.; Heukamp, L.C. Epidermal growth factor receptor mutations in non-small cell lung cancer influence downstream Akt, MAPK and Stat3 signaling. J. Cancer Res. Clin. Oncol. 2009, 135, 723–730. [Google Scholar] [CrossRef]
- Levy, D.E.; Darnell, J., Jr. Signalling: Stats: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798. [Google Scholar] [CrossRef]
- Bromberg, J.F.; Wrzeszczynska, M.H.; Devgan, G.; Zhao, Y.; Pestell, R.G.; Albanese, C.; Darnell, J.E., Jr. Stat3 as an oncogene. Cell 1999, 98, 295–303. [Google Scholar] [CrossRef]
- Zhao, M.; Jiang, B.; Gao, F.-H. Small molecule inhibitors of STAT3 for cancer therapy. Curr. Med. Chem. 2011, 18, 4012–4018. [Google Scholar] [CrossRef]
- Gao, S.P.; Mark, K.G.; Leslie, K.; Pao, W.; Motoi, N.; Gerald, W.L.; Travis, W.D.; Bornmann, W.; Veach, D.; Clarkson, B. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J. Clin. Investig. 2007, 117, 3846–3856. [Google Scholar] [CrossRef]
- Haura, E.B.; Zheng, Z.; Song, L.; Cantor, A.; Bepler, G. Activated epidermal growth factor receptor–Stat-3 signaling promotes tumor survival in vivo in non–small cell lung cancer. Clin. Cancer Res. 2005, 11, 8288–8294. [Google Scholar] [CrossRef]
- Johnson, F.M.; Saigal, B.; Talpaz, M.; Donato, N.J. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non–small cell lung cancer cells. Clin. Cancer Res. 2005, 11, 6924–6932. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Hartmann, T.; Leick, M.; Catusse, J.; Schmitt-Graeff, A.; Burger, M. Alternative implication of CXCR4 in JAK2/STAT3 activation in small cell lung cancer. Br. J. Cancer 2009, 100, 1949. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Sabri, N.; Li, J.; Li, W.X. Role of STAT3 in lung cancer. Jak-Stat 2014, 3, e999503. [Google Scholar] [CrossRef] [PubMed]
- Harada, D.; Takigawa, N.; Kiura, K. The role of STAT3 in non-small cell lung cancer. Cancers 2014, 6, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Gao, F.-H.; Wang, J.-Y.; Liu, F.; Yuan, H.-H.; Zhang, W.-Y.; Jiang, B. JAK2/STAT3 signaling pathway activation mediates tumor angiogenesis by upregulation of VEGF and bFGF in non-small-cell lung cancer. Lung Cancer 2011, 73, 366–374. [Google Scholar] [CrossRef]
- Yang, C.-L.; Liu, Y.-Y.; Ma, Y.-G.; Xue, Y.-X.; Liu, D.-G.; Ren, Y.; Liu, X.-B.; Li, Y.; Li, Z. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PLoS ONE 2012, 7, e37960. [Google Scholar] [CrossRef]
- Wu, L.; Guo, L.; Liang, Y.; Liu, X.; Jiang, L.; Wang, L. Curcumin suppresses stem-like traits of lung cancer cells via inhibiting the JAK2/STAT3 signaling pathway. Oncol. Rep. 2015, 34, 3311–3317. [Google Scholar] [CrossRef]
- Alexandrow, M.G.; Song, L.J.; Altiok, S.; Gray, J.; Haura, E.B.; Kumar, N.B. Curcumin: A novel stat 3 pathway inhibitor for chemoprevention of lung cancer. Eur. J. Cancer Prev. 2012, 21, 407. [Google Scholar] [CrossRef]
- Tang, L.; Liu, J.; Zhu, L.; Chen, Q.; Meng, Z.; Sun, L.; Hu, J.; Ni, Z.; Wang, X. Curcumin inhibits growth of human NCI-H292 lung squamous cell carcinoma cells by increasing FOXA2 expression. Front. Pharmacol. 2018, 9, 60. [Google Scholar] [CrossRef]
- Starok, M.; Preira, P.; Vayssade, M.; Haupt, K.; Salomé, L.; Rossi, C. EGFR Inhibition by Curcumin in Cancer Cells: A Dual Mode of Action. Biomacromolecules 2015, 16, 1634–1642. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M. Untangling the ErbB signalling network. Nat. Reviews. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.-X.; Li, Y.; Yin, H.; Zhang, J. Curcumin: Updated molecular mechanisms and intervention targets in human lung cancer. Int. J. Mol. Sci. 2012, 13, 3959–3978. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.; Janne, P.A. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin. Cancer Res. 2008, 14, 2895–2899. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.-M.; Zhao, X.; Christensen, J.; et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007, 316, 1039. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y. The EGF receptor family: Spearheading a merger of signaling and therapeutics. Cytom. Part B Clin. Cytom. 2008, 74B, 388. [Google Scholar]
- Mendelsohn, J.; Baselga, J. The EGF receptor family as targets for cancer therapy. Oncogene 2000, 19, 6550–6565. [Google Scholar] [CrossRef]
- Klinger, B.; Sieber, A.; Fritsche-Guenther, R.; Witzel, F.; Berry, L.; Schumacher, D.; Yan, Y.; Durek, P.; Merchant, M.; Schäfer, R.; et al. Network quantification of EGFR signaling unveils potential for targeted combination therapy. Mol. Syst. Biol. 2013, 9, 673. [Google Scholar] [CrossRef]
- Shafiee, M.; Mohamadzade, E.; ShahidSales, S.; Khakpouri, S.; Maftouh, M.; Alireza Parizadeh, S.; Mahdi Hasanian, S.; Avan, A. Current Status and Perspectives Regarding the Therapeutic Potential of Targeting EGFR Pathway by Curcumin in Lung Cancer. Curr. Pharm. Des. 2017, 23, 2002–2008. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; An, T.; Zhao, J.; Bai, H.; Duan, J.; Wu, M.; Wang, Y.; Wang, J. The Association between EGFR Gene Amplification and the Prognosis in Non-small Cell Lung Cancer: A meta-analysis. Zhongguo Fei Ai Za Zhi 2009, 12, 1247–1254. [Google Scholar]
- Veale, D.; Kerr, N.; Gibson, G.; Kelly, P.; Harris, A.L. The relationship of quantitative epidermal growth-factor receptor expression in nonsmall cell lung-cancer to long-term survival. Br. J. Cancer 1993, 68, 162–165. [Google Scholar] [CrossRef]
- Jiang, A.-P.; Zhou, D.-H.; Meng, X.-L.; Zhang, A.-P.; Zhang, C.; Li, X.-T.; Feng, Q. Down-regulation of epidermal growth factor receptor by curcumin-induced UBE1L in human bronchial epithelial cells. J. Nutr. Biochem. 2014, 25, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, M.; Olmos, Y. The complex biology of FOXO. Curr. Drug Targets 2011, 12, 1322–1350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Graves, D.T. FOXO transcription factors: Their clinical significance and regulation. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Gomes, A.R.; Zhao, F.; Lam, E.W. Role and regulation of the forkhead transcription factors FOXO3a and FOXM1 in carcinogenesis and drug resistance. Chin. J. Cancer 2013, 32, 365. [Google Scholar] [CrossRef]
- Paik, J.-H.; Kollipara, R.; Chu, G.; Ji, H.; Xiao, Y.; Ding, Z.; Miao, L.; Tothova, Z.; Horner, J.W.; Carrasco, D.R. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 2007, 128, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yin, J.; Wang, C.; Gu, Y.; Deng, M.; He, Z. FOXO3a mediates the cytotoxic effects of cisplatin in lung cancer cells. Anti-Cancer Drugs 2014, 25, 898–907. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, B.-H.; Qiu, X.; Wang, H.-S.; Zhang, F.; Fang, R.; Wang, X.-F.; Cai, S.-H.; Du, J.; Bu, X.-Z. T63, a new 4-arylidene curcumin analogue, induces cell cycle arrest and apoptosis through activation of the reactive oxygen species–FOXO3a pathway in lung cancer cells. Free Radic. Biol. Med. 2012, 53, 2204–2217. [Google Scholar] [CrossRef]
- Wrana, J.L. Signaling by the TGFβ superfamily. Cold Spring Harb. Perspect. Biol. 2013, 5, a011197. [Google Scholar] [CrossRef]
- Faler, B.J.; Macsata, R.A.; Plummer, D.; Mishra, L.; Sidawy, A.N. Transforming growth factor-β and wound healing. Perspect. Vasc. Surg. Endovasc. Ther. 2006, 18, 55–62. [Google Scholar] [CrossRef]
- Taylor, M.A.; Lee, Y.-H.; Schiemann, W.P. Role of TGF-β and the tumor microenvironment during mammary tumorigenesis. Gene Expr. J. Liver Res. 2011, 15, 117–132. [Google Scholar] [CrossRef]
- Anumanthan, G.; Halder, S.K.; Osada, H.; Takahashi, T.; Massion, P.; Carbone, D.; Datta, P. Restoration of TGF-β signalling reduces tumorigenicity in human lung cancer cells. Br. J. Cancer 2005, 93, 1157. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Beauchamp, R.D.; Datta, P. Smad7 induces tumorigenicity by blocking TGF-beta-induced growth inhibition and apoptosis. FASEB J. 2004, 18, C109. [Google Scholar]
- Samanta, D.; Gonzalez, A.L.; Nagathihalli, N.; Ye, F.; Carbone, D.P.; Datta, P.K. Smoking attenuates transforming growth factor-β–mediated tumor suppression function through downregulation of Smad3 in lung cancer. Cancer Prev. Res. 2012, 5, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, R.; Li, Y.; Torday, J.S.; Rehan, V.K. Curcumin augments lung maturation, preventing neonatal lung injury by inhibiting TGF-β signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L721–L730. [Google Scholar] [CrossRef] [PubMed]
- Gaedeke, J.; Noble, N.A.; Border, W.A. Curcumin blocks multiple sites of the TGF-β signaling cascade in renal cells. Kidney Int. 2004, 66, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Chen, M.-J.; Yu, Y.-M.; Ko, S.-Y.; Chang, C.-C. Suppression of TGF-β1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: Its potential therapeutic use in the chemoprevention of keloid. Arch. Dermatol. Res. 2010, 302, 717–724. [Google Scholar] [CrossRef]
- Song, K.; Peng, S.; Sun, Z.; Li, H.; Yang, R. Curcumin suppresses TGF-β signaling by inhibition of TGIF degradation in scleroderma fibroblasts. Biochem. Biophys. Res. Commun. 2011, 411, 821–825. [Google Scholar] [CrossRef]
- Datta, R.; Halder, S.K.; Zhang, B. Role of TGF-β signaling in curcumin-mediated inhibition of tumorigenicity of human lung cancer cells. J. Cancer Res. Clin. Oncol. 2013, 139, 563–572. [Google Scholar] [CrossRef]
- Zheng, Q.; Ye, J.; Cao, J. Translational regulator eIF2α in tumor. Tumor Biol. 2014, 35, 6255–6264. [Google Scholar] [CrossRef]
- Rosenwald, I.B.; Koifman, L.; Savas, L.; Chen, J.-J.; Woda, B.A.; Kadin, M.E. Expression of the translation initiation factors eIF-4E and eIF-2α is frequently increased in neoplastic cells of Hodgkin lymphoma. Hum. Pathol. 2008, 39, 910–916. [Google Scholar] [CrossRef]
- Salehi, Z.; Mashayekhi, F. Expression of the eukaryotic translation initiation factor 4E (eIF4E) and 4E-BP1 in esophageal cancer. Clin. Biochem. 2006, 39, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.V.; Martín, M.E.; Pérez, M.I.; Alonso, F.J.M.; Redondo, C.; Álvarez, M.I.; Salinas, M. Levels, phosphorylation status and cellular localization of translational factor eIF2 in gastrointestinal carcinomas. Histochem. J. 2000, 32, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, I.B.; Wang, S.; Savas, L.; Woda, B.; Pullman, J. Expression of translation initiation factor eIF-2α is increased in benign and malignant melanocytic and colonic epithelial neoplasms. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 98, 1080–1088. [Google Scholar]
- Rosenwald, I.B.; Hutzler, M.J.; Wang, S.; Savas, L.; Fraire, A.E. Expression of eukaryotic translation initiation factors 4E and 2α is increased frequently in bronchioloalveolar but not in squamous cell carcinomas of the lung. Cancer 2001, 92, 2164–2171. [Google Scholar] [CrossRef]
- Chen, L.; Tian, G.; Shao, C.; Cobos, E.; Gao, W. Curcumin modulates eukaryotic initiation factors in human lung adenocarcinoma epithelial cells. Mol. Biol. Rep. 2010, 37, 3105–3110. [Google Scholar] [CrossRef] [PubMed]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Herschman, H.R. Prostaglandin synthase 2. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1996, 1299, 125–140. [Google Scholar] [CrossRef]
- Wolff, H.; Saukkonen, K.; Anttila, S.; Karjalainen, A.; Vainio, H.; Ristimäki, A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998, 58, 4997–5001. [Google Scholar]
- Hosomi, Y.; Yokose, T.; Hirose, Y.; Nakajima, R.; Nagai, K.; Nishiwaki, Y.; Ochiai, A. Increased cyclooxygenase 2 (COX-2) expression occurs frequently in precursor lesions of human adenocarcinoma of the lung. Lung Cancer 2000, 30, 73–81. [Google Scholar] [CrossRef]
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis, N.H.; Naidu, R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef]
- Krysan, K.; Merchant, F.H.; Zhu, L.; Dohadwala, M.; Luo, J.; Lin, Y.; HEUZE-VOURC’H, N.; Põld, M.; Seligson, D.; Chia, D. COX-2-dependent stabilization of survivin in non-small cell lung cancer. FASEB J. 2004, 18, 206–208. [Google Scholar]
- Soslow, R.A.; Dannenberg, A.J.; Rush, D.; Woerner, B.; Khan, K.N.; Masferrer, J.; Koki, A.T. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 2000, 89, 2637–2645. [Google Scholar] [CrossRef]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE 2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [PubMed]
- Sandler, A.B.; Dubinett, S.M. COX-2 inhibition and lung cancer. In Seminars in Oncology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 45–52. [Google Scholar]
- Sobolewski, C.; Cerella, C.; Dicato, M.; Ghibelli, L.; Diederich, M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int. J. Cell Biol. 2010, 2010. [Google Scholar] [CrossRef]
- Masferrer, J.L.; Leahy, K.M.; Koki, A.T.; Zweifel, B.S.; Settle, S.L.; Woerner, B.M.; Edwards, D.A.; Flickinger, A.G.; Moore, R.J.; Seibert, K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000, 60, 1306–1311. [Google Scholar]
- Hida, T.; Yatabe, Y.; Achiwa, H.; Muramatsu, H.; Kozaki, K.-I.; Nakamura, S.; Ogawa, M.; Mitsudomi, T.; Sugiura, T.; Takahashi, T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998, 58, 3761–3764. [Google Scholar]
- Krysan, K.; Reckamp, K.L.; Sharma, S.; Dubinett, S.M. The potential and rationale for COX-2 inhibitors in lung cancer. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2006, 6, 209–220. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Starr, A.; Vexler, A.; Karaush, V.; Loew, V.; Greif, J.; Fenig, E.; Aderka, D.; Ben-Yosef, R. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006, 26, 4423–4430. [Google Scholar]
- Jobin, C.; Bradham, C.A.; Russo, M.P.; Juma, B.; Narula, A.S.; Brenner, D.A.; Sartor, R.B. Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor I-κB kinase activity. J. Immunol. 1999, 163, 3474–3483. [Google Scholar]
- Charalambous, M.; Maihöfner, C.; Bhambra, U.; Lightfoot, T.; Gooderham, N. Upregulation of cyclooxygenase-2 is accompanied by increased expression of nuclear factor-κB and IκB kinase-α in human colorectal cancer epithelial cells. Br. J. Cancer 2003, 88, 1598. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Starr, A.; Katzburg, S.; Berkovich, L.; Rimmon, A.; Ben-Yosef, R.; Vexler, A.; Ron, I.; Earon, G. Curcumin induces apoptosis and inhibits growth of orthotopic human non-small cell lung cancer xenografts. J. Nutr. Biochem. 2014, 25, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.-I.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. The Bcl-2 protein family: Arbiters of cell survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.; Reed, J. Bcl-2 family proteins and cancer. Oncogene 2008, 27, 6398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, S.; Wang, L.; Wang, R.; Chen, S.; Pan, B.; Sun, Y.; Chen, H. Prognostic value of Bcl-2 expression in patients with non-small-cell lung cancer: A meta-analysis and systemic review. Oncotargets Ther. 2015, 8, 3361. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, Y.; Toyoshima, E.; Fujiuchi, S.; Matsui, H.; Hirata, S.; Miyokawa, N.; Kubo, Y.; Kikuchi, K. bcl-2 and p53 protein expression in non-small cell lung cancers: Correlation with survival time. Clin. Cancer Res. 1996, 2, 915–920. [Google Scholar] [PubMed]
- Borner, M.; Brousset, P.; Pfanner-Meyer, B.; Bacchi, M.; Vonlanthen, S.; Hotz, M.; Altermatt, H.; Schlaifer, D.; Reed, J.; Betticher, D. Expression of apoptosis regulatory proteins of the Bcl-2 family and p53 in primary resected non-small-cell lung cancer. Br. J. Cancer 1999, 79, 952. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Luedke, G.H.; Stahel, R.A.; Zangemeister-Wittke, U.; Fabbro, D.; Altmann, K.-H. Induction of apoptosis in small-cell lung cancer cells by an antisense oligodeoxynucleotide targeting the Bcl-2 coding sequence. J. Natl. Cancer Inst. 1997, 89, 1027–1036. [Google Scholar] [CrossRef]
- Kaiser, U.; Schilli, M.; Haag, U.; Neumann, K.; Kreipe, H.; Kogan, E.; Havemann, K. Expression of bcl-2—Protein in small cell lung cancer. Lung Cancer 1996, 15, 31–40. [Google Scholar] [CrossRef]
- Wu, S.-H.; Hang, L.-W.; Yang, J.-S.; Chen, H.-Y.; Lin, H.-Y.; Chiang, J.-H.; Lu, C.-C.; Yang, J.-L.; Lai, T.-Y.; Ko, Y.-C. Curcumin induces apoptosis in human non-small cell lung cancer NCI-H460 cells through ER stress and caspase cascade-and mitochondria-dependent pathways. Anticancer Res. 2010, 30, 2125–2133. [Google Scholar]
- Nur-E-Kamal, A.; Gross, S.R.; Pan, Z.; Balklava, Z.; Ma, J.; Liu, L.F. Nuclear translocation of cytochrome c during apoptosis. J. Biol. Chem. 2004, 279, 24911–24914. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, S.J. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999, 59 (Suppl. 7), 1693s–1700s. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Geng, J.-X.; Hu, X.-Y. Curcumin inhibits human non-small cell lung cancer A549 cell proliferation through regulation of Bcl-2/Bax and cytochrome C. Asian Pac. J. Cancer Prev. 2013, 14, 4599–4602. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, S.; Patwardhan, R.S.; Pal, D.; Singh, B.; Sharma, D.; Kutala, V.K.; Sandur, S.K. Mitochondrial targeted curcumin exhibits anticancer effects through disruption of mitochondrial redox and modulation of TrxR2 activity. Free Radic. Biol. Med. 2017, 113, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, D.; Ye, B.; Zhong, F.; Chen, G. Curcumin induces the apoptosis of non-small cell lung cancer cells through a calcium signaling pathway. Int. J. Mol. Med. 2015, 35, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.-P.; Aromolaran, A.S.; Bultynck, G.; Zhong, F.; Li, X.; McColl, K.; Matsuyama, S.; Herlitze, S.; Roderick, H.L.; Bootman, M.D. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s inhibition of apoptotic calcium signals. Mol. Cell 2008, 31, 255–265. [Google Scholar] [CrossRef]
- Foyouzi-Youssefi, R.; Arnaudeau, S.; Borner, C.; Kelley, W.L.; Tschopp, J.; Lew, D.P.; Demaurex, N.; Krause, K.-H. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2000, 97, 5723–5728. [Google Scholar] [CrossRef]
- Polivka, J., Jr.; Janku, F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol. Ther. 2014, 142, 164–175. [Google Scholar] [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR signaling in cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef]
- Vara, J.Á.F.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef]
- Fumarola, C.; Bonelli, M.A.; Petronini, P.G.; Alfieri, R.R. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem. Pharmacol. 2014, 90, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Scrima, M.; De Marco, C.; Fabiani, F.; Franco, R.; Pirozzi, G.; Rocco, G.; Ravo, M.; Weisz, A.; Zoppoli, P.; Ceccarelli, M. Signaling networks associated with AKT activation in non-small cell lung cancer (NSCLC): New insights on the role of phosphatydil-inositol-3 kinase. PLoS ONE 2012, 7, e30427. [Google Scholar] [CrossRef] [PubMed]
- Papadimitrakopoulou, V. Development of PI3K/AKT/mTOR pathway inhibitors and their application in personalized therapy for non–small-cell lung cancer. J. Thorac. Oncol. 2012, 7, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Umemura, S.; Mimaki, S.; Makinoshima, H.; Tada, S.; Ishii, G.; Ohmatsu, H.; Niho, S.; Yoh, K.; Matsumoto, S.; Takahashi, A. Therapeutic priority of the PI3K/AKT/mTOR pathway in small cell lung cancers as revealed by a comprehensive genomic analysis. J. Thorac. Oncol. 2014, 9, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gao, S.; Yang, Y.; Zhao, X.; Fan, Y.; Ma, W.; Yang, D.; Yang, A.; Yu, Y. Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol. Rep. 2018, 39, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, J.; Zhang, S.; Zhang, H.; Xu, Z.; Li, X. Curcumin inhibits the development of nonsmall cell lung cancer by inhibiting autophagy and apoptosis. Exp. Ther. Med. 2017, 14, 5075–5080. [Google Scholar]
- Liu, F.; Gao, S.; Yang, Y.; Zhao, X.; Fan, Y.; Ma, W.; Yang, D.; Yang, A.; Yu, Y. Curcumin induced autophagy anticancer effects on human lung adenocarcinoma cell line A549. Oncol. Lett. 2017, 14, 2775–2782. [Google Scholar] [CrossRef]
- Agoulnik, I.U.; Hodgson, M.C.; Bowden, W.A.; Ittmann, M.M. INPP4B: The new kid on the PI3K block. Oncotarget 2011, 2, 321. [Google Scholar] [CrossRef]
- Marsit, C.J.; Zheng, S.; Aldape, K.; Hinds, P.W.; Nelson, H.H.; Wiencke, J.K.; Kelsey, K.T. PTEN expression in non–small-cell lung cancer: Evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Hum. Pathol. 2005, 36, 768–776. [Google Scholar] [CrossRef]
- Soria, J.-C.; Lee, H.-Y.; Lee, J.I.; Wang, L.; Issa, J.-P.; Kemp, B.L.; Liu, D.D.; Kurie, J.M.; Mao, L.; Khuri, F.R. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin. Cancer Res. 2002, 8, 1178–1184. [Google Scholar]
- Forgacs, E.; Biesterveld, E.J.; Sekido, Y.; Fong, K.; Muneer, S.; Wistuba, I.I.; Milchgrub, S.; Brezinschek, R.; Virmani, A.; Gazdar, A.F. Mutation analysis of the PTEN/MMAC1 gene in lung cancer. Oncogene 1998, 17, 1557. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, A.; Tindall, D.J.; Drabkin, H.; Gemmill, R.; Franklin, W.; Yang, P.; Sugio, K.; Smith, D.I.; Liu, W. PTEN/MMAC1 mutations identified in small cell, but not in non-small cell lung cancers. Oncogene 1998, 17, 475. [Google Scholar] [CrossRef]
- Kohno, T.; Takahashi, M.; Manda, R.; Yokota, J. Inactivation of the PTEN/MMAC1/TEP1 gene in human lung cancers. Genes Chromosomes Cancer 1998, 22, 152–156. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, W. MiR-21 suppresses the anticancer activities of curcumin by targeting PTEN gene in human non-small cell lung cancer A549 cells. Clin. Transl. Oncol. 2014, 16, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 50–64. [Google Scholar]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef]
- Yang, C.-L.; Ma, Y.-G.; Xue, Y.-X.; Liu, Y.-Y.; Xie, H.; Qiu, G.-R. Curcumin induces small cell lung cancer NCI-H446 cell apoptosis via the reactive oxygen species-mediated mitochondrial pathway and not the cell death receptor pathway. DNA Cell Biol. 2012, 31, 139–150. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Xu, K.; Lu, G.; Ying, Z.; Wu, L.; Zhan, J.; Fang, R.; Wu, Y.; Zhou, J. Curcumin induces apoptosis in human lung adenocarcinoma A549 cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Oncol. Rep. 2010, 23, 397–403. [Google Scholar] [CrossRef]
- Yao, Q.; Lin, M.; Wang, Y.; Lai, Y.; Hu, J.; Fu, T.; Wang, L.; Lin, S.; Chen, L.; Guo, Y. Curcumin induces the apoptosis of A549 cells via oxidative stress and MAPK signaling pathways. Int. J. Mol. Med. 2015, 36, 1118–1126. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, S.; Ji, Y.; Li, J.; An, P.; Ren, H.; Liang, R.; Yang, J.; Li, Z. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the ERK pathway. PLoS ONE 2013, 8, e72927. [Google Scholar] [CrossRef]
- Rane, M.J.; Song, Y.; Jin, S.; Barati, M.T.; Wu, R.; Kausar, H.; Tan, Y.; Wang, Y.; Zhou, G.; Klein, J.B. Interplay between Akt and p38 MAPK pathways in the regulation of renal tubular cell apoptosis associated with diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2009, 298, F49–F61. [Google Scholar] [CrossRef]
- Tarapore, R.S.; Yang, Y.; Katz, J.P. Restoring KLF5 in esophageal squamous cell cancer cells activates the JNK pathway leading to apoptosis and reduced cell survival. Neoplasia (New York NY) 2013, 15, 472. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Kaushik, T.; Yadav, S.K.; Sharma, S.K.; Ranawat, P.; Khanduja, K.L.; Pathak, C.M. Curcumin Sensitizes Lung Adenocarcinoma Cells to Apoptosis Via Intracellular Redox Status Mediated Pathway. Indian. J. Exp. Bio. 2012, 50, 853–861. [Google Scholar]
- Nagata, S.; Golstein, P. The Fas death factor. Science 1995, 267, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Müschen, M.; Warskulat, U.; Beckmann, M. Defining CD95 as a tumor suppressor gene. J. Mol. Med. 2000, 78, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 2008, 19, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Özören, N.; El-Deiry, W.S. Cell surface death receptor signaling in normal and cancer cells. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 135–147. [Google Scholar]
- Lynch, D.H.; Ramsdell, F.; Alderson, M.R. Fas and FasL in the homeostatic regulation of immune responses. Immunol. Today 1995, 16, 569–574. [Google Scholar] [CrossRef]
- Ju, S.-T.; Panka, D.J.; Cui, H.; Ettinger, R.; Maan, E.-K.; Sherr, D.H.; Stanger, B.Z.; Marshak-Rothstein, A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 1995, 373, 444. [Google Scholar] [CrossRef]
- Griffith, T.S.; Brunner, T.; Fletcher, S.M.; Green, D.R.; Ferguson, T.A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 1995, 270, 1189–1192. [Google Scholar] [CrossRef]
- Villa-Morales, M.; Fernandez-Piqueras, J. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 85–101. [Google Scholar] [CrossRef]
- Viard-Leveugle, I.; Veyrenc, S.; French, L.E.; Brambilla, C.; Brambilla, E. Frequent loss of Fas expression and function in human lung tumours with overexpression of FasL in small cell lung carcinoma. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2003, 201, 268–277. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, X.; Sun, T.; Tan, W.; Qu, S.; Xiong, P.; Zhou, Y.; Lin, D. Functional polymorphisms in cell death pathway genes FAS and FASL contribute to risk of lung cancer. J. Med Genet. 2005, 42, 479–484. [Google Scholar] [CrossRef] [PubMed]
- O’connell, J.; O’sullivan, G.C.; Collins, J.K.; Shanahan, F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J. Exp. Med. 1996, 184, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Niehans, G.A.; Brunner, T.; Frizelle, S.P.; Liston, J.C.; Salerno, C.T.; Knapp, D.J.; Green, D.R.; Kratzke, R.A. Human lung carcinomas express Fas ligand. Cancer Res. 1997, 57, 1007–1012. [Google Scholar] [PubMed]
- Bennett, M.W.; O’Connell, J.; O’Sullivan, G.C.; Brady, C.; Roche, D.; Collins, J.K.; Shanahan, F. The Fas counterattack in vivo: Apoptotic depletion of tumor-infiltrating lymphocytes associated with Fas ligand expression by human esophageal carcinoma. J. Immunol. 1998, 160, 5669–5675. [Google Scholar] [PubMed]
- Koyama, S.; Koike, N.; Adachi, S. Fas receptor counterattack against tumor-infiltrating lymphocytes in vivo as a mechanism of immune escape in gastric carcinoma. J. Cancer Res. Clin. Oncol. 2001, 127, 20–26. [Google Scholar] [CrossRef]
- Gupta, G.P.; Massagué, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Sinha, S.; Yang, W. Cellular signaling for activation of Rho GTPase Cdc42. Cell. Signal. 2008, 20, 1927–1934. [Google Scholar] [CrossRef]
- Qadir, M.I.; Parveen, A.; Ali, M. Cdc42: Role in Cancer Management. Chem. Biol. Drug Des. 2015, 86, 432–439. [Google Scholar] [CrossRef]
- Stengel, K.; Zheng, Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell. Signal. 2011, 23, 1415–1423. [Google Scholar] [CrossRef]
- Kodama, A.; Takaishi, K.; Nakano, K.; Nishioka, H.; Takai, Y. Involvement of Cdc42 small G protein in cell-cell adhesion, migration and morphology of MDCK cells. Oncogene 1999, 18, 3996–4006. [Google Scholar] [CrossRef]
- Nakahara, H.; Otani, T.; Sasaki, T.; Miura, Y.; Takai, Y.; Kogo, M. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells 2003, 8, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Arias-Romero, L.E.; Chernoff, J. Targeting Cdc42 in cancer. Expert Opin. Ther. Targets 2013, 17, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Kamai, T.; Yamanishi, T.; Shirataki, H.; Takagi, K.; Asami, H.; Ito, Y.; Yoshida, K.-I. Overexpression of RhoA, Rac1, and Cdc42 GTPases Is Associated with Progression in Testicular Cancer. Clin. Cancer Res. 2004, 10, 4799–4805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Zhang, D.; Wang, E.-H. Overexpression of small GTPases directly correlates with expression of δ-catenin and their coexpression predicts a poor clinical outcome in nonsmall cell lung cancer. Mol. Carcinog. 2013, 52, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Chander, H.; Truesdell, P.; Meens, J.; Craig, A.W.B. Transducer of Cdc42-dependent actin assembly promotes breast cancer invasion and metastasis. Oncogene 2012, 32, 3080. [Google Scholar] [CrossRef] [PubMed]
- Reymond, N.; Im, J.H.; Garg, R.; Vega, F.M.; Borda d’Agua, B.; Riou, P.; Cox, S.; Valderrama, F.; Muschel, R.J.; Ridley, A.J. Cdc42 promotes transendothelial migration of cancer cells through β1 integrin. J. Cell Biol. 2012, 199, 653–668. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Jiao, D.-M.; Yao, Q.-H.; Yan, J.; Song, J.; Chen, F.-Y.; Lu, G.-H.; Zhou, J.-Y. Expression analysis of Cdc42 in lung cancer and modulation of its expression by curcumin in lung cancer cell lines. Int. J. Oncol. 2012, 40, 1561. [Google Scholar] [CrossRef]
- Van Roy, F.; Berx, G. The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 2008, 65, 3756–3788. [Google Scholar] [CrossRef]
- Vergara, D.; Simeone, P.; Latorre, D.; Cascione, F.; Leporatti, S.; Trerotola, M.; Giudetti, A.M.; Capobianco, L.; Lunetti, P.; Rizzello, A.; et al. Proteomics analysis of E-cadherin knockdown in epithelial breast cancer cells. J. Biotechnol. 2015, 202, 3–11. [Google Scholar] [CrossRef]
- Larue, L.; Antos, C.; Butz, S.; Huber, O.; Delmas, V.; Dominis, M.; Kemler, R. A role for cadherins in tissue formation. Development 1996, 122, 3185–3194. [Google Scholar]
- Shiozaki, H.; Oka, H.; Inoue, M.; Tamura, S.; Monden, M. E-cadherin mediated adhesion system in cancer cells. Cancer 1996, 77, 1605–1613. [Google Scholar] [CrossRef]
- Birchmeier, W.; Behrens, J. Cadherin expression in carcinomas: Role in the formation of cell junctions and the prevention of invasiveness. Biochim. Biophys. Acta (BBA) Rev. Cancer 1994, 1198, 11–26. [Google Scholar] [CrossRef]
- Kowalski, P.J.; Rubin, M.A.; Kleer, C.G. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003, 5, R217. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Chu, R.Y.; Hsu, P.-N.; Hsu, P.-I.; Lu, J.-Y.; Lai, K.-H.; Tseng, H.-H.; Chou, N.-H.; Huang, M.-S.; Tseng, C.-J.; et al. Loss of E-cadherin expression correlates with poor differentiation and invasion into adjacent organs in gastric adenocarcinomas. Cancer Lett. 2003, 201, 97–106. [Google Scholar] [CrossRef]
- Takeichi, M. Cadherins in cancer: Implications for invasion and metastasis. Curr. Opin. Cell Biol. 1993, 5, 806–811. [Google Scholar] [CrossRef]
- Onder, T.T.; Gupta, P.B.; Mani, S.A.; Yang, J.; Lander, E.S.; Weinberg, R.A. Loss of E-Cadherin Promotes Metastasis via Multiple Downstream Transcriptional Pathways. Cancer Res. 2008, 68, 3645–3654. [Google Scholar] [CrossRef]
- Canel, M.; Serrels, A.; Frame, M.C.; Brunton, V.G. E-cadherin–integrin crosstalk in cancer invasion and metastasis. J. Cell Sci. 2013, 126, 393–401. [Google Scholar] [CrossRef]
- Böhm, M.; Totzeck, B.; Birchmeier, W.; Wieland, I. Differences of E-cadherin expression levels and patterns in primary and metastatic human lung cancer. Clin. Exp. Metastasis 1994, 12, 55–62. [Google Scholar] [CrossRef]
- Chen, H.-W.; Lee, J.-Y.; Huang, J.-Y.; Wang, C.-C.; Chen, W.-J.; Su, S.-F.; Huang, C.-W.; Ho, C.-C.; Chen, J.J.W.; Tsai, M.-F.; et al. Curcumin Inhibits Lung Cancer Cell Invasion and Metastasis through the Tumor Suppressor HLJ1. Cancer Res. 2008, 68, 7428–7438. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Shay, G.; Lynch, C.C.; Fingleton, B. Moving targets: Emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015, 44–46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Stetler-Stevenson, W.G. The Role of Matrix Metalloproteinases in Tumor Invasion, Metastasis, and Angiogenesis. Surg. Oncol. Clin. 2001, 10, 383–392. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-C.; Tseng, W.-L.; Shiea, J.; Chang, H.-C. Skp2 overexpression increases the expression of MMP-2 and MMP-9 and invasion of lung cancer cells. Cancer Lett. 2010, 288, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Kodate, M.; Kasai, T.; Hashirnoto, H.; Yasumoto, K.; Iwata, Y.; Manabe, H. Expression of matrix metalloproteinase (gelatinase) in T1 adenocarcinoma of the lung. Pathol. Int. 1997, 47, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Swarnakar, S.; Ganguly, K.; Kundu, P.; Banerjee, A.; Maity, P.; Sharma, A.V. Curcumin regulates expression and activity of matrix metalloproteinases 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. J. Biol. Chem. 2005, 280, 9409–9415. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, Q.; Chen, K.; Wang, Y.; Chen, L.; Li, X. Curcumin suppresses migration and invasion of human endometrial carcinoma cells. Oncol. Lett. 2015, 10, 1297–1302. [Google Scholar] [CrossRef]
- Lakka, S.S.; Jasti, S.L.; Gondi, C.; Boyd, D.; Chandrasekar, N.; Dinh, D.H.; Olivero, W.C.; Gujrati, M.; Rao, J.S. Downregulation of MMP-9 in ERK-mutated stable transfectants inhibits glioma invasion in vitro. Oncogene 2002, 21, 5601. [Google Scholar] [CrossRef]
- Mitra, A.; Chakrabarti, J.; Banerji, A.; Chatterjee, A.; Das, B. Curcumin, a potential inhibitor of MMP-2 in human laryngeal squamous carcinoma cells HEp2. J. Environ. Pathol. Toxicol. Oncol. 2006, 25. [Google Scholar] [CrossRef]
- Lin, S.-S.; Lai, K.-C.; Hsu, S.-C.; Yang, J.-S.; Kuo, C.-L.; Lin, J.-P.; Ma, Y.-S.; Wu, C.-C.; Chung, J.-G. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF). Cancer Lett. 2009, 285, 127–133. [Google Scholar] [CrossRef]
- Xiao, L.-J.; Lin, P.; Lin, F.; Liu, X.; Qin, W.; Zou, H.-F.; Guo, L.; Liu, W.; Wang, S.-J.; Yu, X.-G. ADAM17 targets MMP-2 and MMP-9 via EGFR-MEK-ERK pathway activation to promote prostate cancer cell invasion. Int. J. Oncol. 2012, 40, 1714–1724. [Google Scholar] [PubMed]
- Rhee, J.W.; Lee, K.-W.; Sohn, W.-J.; Lee, Y.; Jeon, O.-H.; Kwon, H.-J.; Kim, D.-S. Regulation of matrix metalloproteinase-9 gene expression and cell migration by NF-κB in response to CpG-oligodeoxynucleotides in RAW 264.7 cells. Mol. Immunol. 2007, 44, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000, 2, 737. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-Y.; Zheng, Y.; Jiao, D.-M.; Chen, F.-Y.; Hu, H.-Z.; Wu, Y.-Q.; Song, J.; Yan, J.; Wu, L.-J.; Lv, G.-Y. Curcumin inhibits lung cancer cell migration and invasion through Rac1-dependent signaling pathway. J. Nutr. Biochem. 2014, 25, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Raftopoulou, M.; Hall, A. Cell migration: Rho GTPases lead the way. Dev. Biol. 2004, 265, 23–32. [Google Scholar] [CrossRef]
- Fan, Z.; Duan, X.; Cai, H.; Wang, L.; Li, M.; Qu, J.; Li, W.; Wang, Y.; Wang, J. Curcumin inhibits the invasion of lung cancer cells by modulating the PKCα/Nox-2/ROS/ATF-2/MMP-9 signaling pathway. Oncol. Rep. 2015, 34, 691–698. [Google Scholar] [CrossRef]
- Koerner, A.; Kratzsch, J.; Kiess, W. Adipocytokines: Leptin—The classical, resistin—The controversical, adiponectin—The promising, and more to come. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 525–546. [Google Scholar] [CrossRef]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef]
- Ishikawa, M.; Kitayama, J.; Kazama, S.; Hiramatsu, T.; Hatano, K.; Nagawa, H. Plasma adiponectin and gastric cancer. Clin. Cancer Res. 2005, 11, 466–472. [Google Scholar]
- Goktas, S.; Yilmaz, M.I.; Caglar, K.; Sonmez, A.; Kilic, S.; Bedir, S. Prostate cancer and adiponectin. Urology 2005, 65, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Arisan, E.D.; Arisan, S.; Atis, G.; Palavan-Unsal, N.; Ergenekon, E. Serum adipocytokine levels in prostate cancer patients. Urol. Int. 2009, 82, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Ntikoudi, E.; Kiagia, M.; Boura, P.; Syrigos, K. Hormones of adipose tissue and their biologic role in lung cancer. Cancer Treat. Rev. 2014, 40, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-R.; Liu, P.-L.; Chen, Y.-H.; Chou, S.-H.; Cheng, Y.-J.; Hwang, J.-J.; Chong, I.-W. Curcumin inhibits non-small cell lung cancer cells metastasis through the Adiponectin/NF-κb/MMPs signaling pathway. PLoS ONE 2015, 10, e0144462. [Google Scholar] [CrossRef]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef]
- Jones, P.A. Overview of cancer epigenetics. In Seminars in Hematology; Elsevier: Amsterdam, The Netherlands, 2005; pp. S3–S8. [Google Scholar]
- Sawan, C.; Vaissière, T.; Murr, R.; Herceg, Z. Epigenetic drivers and genetic passengers on the road to cancer. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2008, 642, 1–13. [Google Scholar] [CrossRef]
- Fu, S.; Kurzrock, R. Development of curcumin as an epigenetic agent. Cancer 2010, 116, 4670–4676. [Google Scholar] [CrossRef]
- Teiten, M.H.; Dicato, M.; Diederich, M. Curcumin as a regulator of epigenetic events. Mol. Nutr. Food Res. 2013, 57, 1619–1629. [Google Scholar] [CrossRef]
- Das, P.M.; Singal, R. DNA methylation and cancer. J. Clin. Oncol. 2004, 22, 4632–4642. [Google Scholar] [CrossRef]
- Ehrlich, M. DNA hypomethylation in cancer cells. Epigenomics 2009, 1, 239–259. [Google Scholar] [CrossRef]
- Esteller, M. Epigenetics in cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Baylin, S.B. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 2003, 349, 2042–2054. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, Z.; Jones, W.; Pavlovicz, R.E.; Liu, S.; Yu, J.; Li, P.-k.; Lin, J.; Fuchs, J.R.; Marcucci, G. Curcumin is a potent DNA hypomethylation agent. Bioorg. Med. Chem. Lett. 2009, 19, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Parashar, G.; Parashar, N.C.; Capalash, N. Curcumin causes promoter hypomethylation and increased expression of FANCF gene in SiHa cell line. Mol. Cell. Biochem. 2012, 365, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Nikbakht, M.; Parashar, G.; Shrivastava, A.; Capalash, N.; Kaur, J. Reversal of hypermethylation and reactivation of the RARß2 gene by natural compounds in cervical cancer cell lines. Folia Biol. (Praha) 2010, 56, 195–200. [Google Scholar] [PubMed]
- Abusnina, A.; Keravis, T.; Yougbaré, I.; Bronner, C.; Lugnier, C. Anti-proliferative effect of curcumin on melanoma cells is mediated by PDE1A inhibition that regulates the epigenetic integrator UHRF1. Mol. Nutr. Food Res. 2011, 55, 1677–1689. [Google Scholar] [CrossRef]
- Du, L.; Xie, Z.; Wu, L.-C.; Chiu, M.; Lin, J.; Chan, K.K.; Liu, S.; Liu, Z. Reactivation of RASSF1A in breast cancer cells by curcumin. Nutr. Cancer 2012, 64, 1228–1235. [Google Scholar] [CrossRef]
- Shu, L.; Khor, T.O.; Lee, J.-H.; Boyanapalli, S.S.; Huang, Y.; Wu, T.-Y.; Saw, C.L.-L.; Cheung, K.-L.; Kong, A.-N.T. Epigenetic CpG demethylation of the promoter and reactivation of the expression of Neurog1 by curcumin in prostate LNCaP cells. AAPS J. 2011, 13, 606–614. [Google Scholar] [CrossRef]
- Boyanapalli, S.S.; Kong, A.-N.T. “Curcumin, the king of spices”: Epigenetic regulatory mechanisms in the prevention of cancer, neurological, and inflammatory diseases. Curr. Pharmacol. Rep. 2015, 1, 129–139. [Google Scholar] [CrossRef]
- Momparler, R.L.; Bovenzi, V. DNA methylation and cancer. J. Cell. Physiol. 2000, 183, 145–154. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Yang, H.-P.; Gong, L.; Tang, C.-L.; Wang, H.-J. Hypomethylation effects of curcumin, demethoxycurcumin and bisdemethoxycurcumin on WIF-1 promoter in non-small cell lung cancer cell lines. Mol. Med. Rep. 2011, 4, 675–679. [Google Scholar] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Sawan, C.; Herceg, Z. Histone modifications and cancer. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2010; Volume 70, pp. 57–85. [Google Scholar]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381. [Google Scholar] [CrossRef] [PubMed]
- Strahl, B.D.; Allis, C.D. The language of covalent histone modifications. Nature 2000, 403, 41. [Google Scholar] [CrossRef] [PubMed]
- Yasui, W.; Oue, N.; Ono, S.; Mitani, Y.; Ito, R.; Nakayama, H. Histone acetylation and gastrointestinal carcinogenesis. Ann. N. Y. Acad. Sci. 2003, 983, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fu, M.; Mani, S.; Wadler, S.; Senderowicz, A.M.; Pestell, R.G. Histone acetylation and the cell-cycle in cancer. Front Biosci. 2001, 6, 610–629. [Google Scholar] [CrossRef]
- Archer, S.Y.; Hodin, R.A. Histone acetylation and cancer. Curr. Opin. Genet. Dev. 1999, 9, 171–174. [Google Scholar] [CrossRef]
- Kang, S.-K.; Cha, S.-H.; Jeon, H.-G. Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 2006, 15, 165–174. [Google Scholar] [CrossRef]
- Chen, Y.; Shu, W.; Chen, W.; Wu, Q.; Liu, H.; Cui, G. Curcumin, both histone deacetylase and p300/CBP-specific inhibitor, represses the activity of nuclear factor kappa B and Notch 1 in Raji cells. Basic Clin. Pharmacol. Toxicol. 2007, 101, 427–433. [Google Scholar] [CrossRef]
- Marcu, M.G.; Jung, Y.-J.; Lee, S.; Chung, E.-J.; Lee, M.-J.; Trepel, J.; Neckers, L. Curcumin is an inhibitor of p300 histone acetylatransferase. Med. Chem. 2006, 2, 169–174. [Google Scholar]
- Bushati, N.; Cohen, S.M. MicroRNA Functions. Annu. Rev. Cell Dev. Biol. 2007, 23, 175–205. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, O.; Boeri, M.; Verri, C.; Moro, M.; Sozzi, G. Therapeutic use of microRNAs in lung cancer. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Ishikawa, Y. MicroRNA in lung cancer: Novel biomarkers and potential tools for treatment. J. Clin. Med. 2016, 5, 36. [Google Scholar] [CrossRef]
- Johnson, C.D.; Esquela-Kerscher, A.; Stefani, G.; Byrom, M.; Kelnar, K.; Ovcharenko, D.; Wilson, M.; Wang, X.; Shelton, J.; Shingara, J. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007, 67, 7713–7722. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Trang, P.; Wiggins, J.F.; Patrawala, L.; Cheng, A.; Ford, L.; Weidhaas, J.B.; Brown, D.; Bader, A.G.; Slack, F.J. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle 2008, 7, 759–764. [Google Scholar] [CrossRef]
- Garofalo, M.; Jeon, Y.-J.; Nuovo, G.J.; Middleton, J.; Secchiero, P.; Joshi, P.; Alder, H.; Nazaryan, N.; Di Leva, G.; Romano, G. MiR-34a/c-dependent PDGFR-α/β downregulation inhibits tumorigenesis and enhances TRAIL-induced apoptosis in lung cancer. PLoS ONE 2013, 8, e67581. [Google Scholar] [CrossRef]
- Fabbri, M.; Garzon, R.; Cimmino, A.; Liu, Z.; Zanesi, N.; Callegari, E.; Liu, S.; Alder, H.; Costinean, S.; Fernandez-Cymering, C. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA 2007, 104, 15805–15810. [Google Scholar] [CrossRef]
- Bommer, G.T.; Gerin, I.; Feng, Y.; Kaczorowski, A.J.; Kuick, R.; Love, R.E.; Zhai, Y.; Giordano, T.J.; Qin, Z.S.; Moore, B.B. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr. Biol. 2007, 17, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Pedone, C.; Majeed, M.; Sahebkar, A. Curcumin and lung cancer: The role of microRNAs. Curr. Pharm. Des. 2017, 23, 3440–3444. [Google Scholar] [CrossRef] [PubMed]
- Momtazi, A.A.; Shahabipour, F.; Khatibi, S.; Johnston, T.P.; Pirro, M.; Sahebkar, A. Curcumin as a MicroRNA regulator in cancer: A review. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: New York, NY, USA, 2016; Volume 171, pp. 1–38. [Google Scholar]
- Sun, M.; Estrov, Z.; Ji, Y.; Coombes, K.R.; Harris, D.H.; Kurzrock, R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol. Cancer Ther. 2008, 7, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Masoudifar, A.; Sahebkar, A.; Zare, N.; Sadri Nahand, J.; Rashidi, B.; Mehrabian, E.; Mohammadi, M.; Mirzaei, H.R.; Jaafari, M.R. MicroRNA: A novel target of curcumin in cancer therapy. J. Cell. Physiol. 2018, 233, 3004–3015. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Y.; Wu, C.; Ren, X.; Ti, X.; Shi, J.; Zhao, F.; Yin, H. Curcumin promotes apoptosis in human lung adenocarcinoma cells through miR-186* signaling pathway. Oncol. Rep. 2010, 24, 1217–1223. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, J.; Du, Y. Curcumin promoted the apoptosis of cisplain-resistant human lung carcinoma cells A549/DDP through down-regulating miR-186*. Zhongguo Fei Ai Za Zhi= Chin. J. Lung Cancer 2010, 13, 301–306. [Google Scholar]
- Ye, M.; Zhang, J.; Zhang, J.; Miao, Q.; Yao, L.; Zhang, J. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015, 357, 196–205. [Google Scholar] [CrossRef]
- Jin, H.; Qiao, F.; Wang, Y.; Xu, Y.; Shang, Y. Curcumin inhibits cell proliferation and induces apoptosis of human non-small cell lung cancer cells through the upregulation of miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol. Rep. 2015, 34, 2782–2789. [Google Scholar] [CrossRef]
- Ahmad, A.; Sayed, A.; Ginnebaugh, K.R.; Sharma, V.; Suri, A.; Saraph, A.; Padhye, S.; Sarkar, F.H. Molecular docking and inhibition of matrix metalloproteinase-2 by novel difluorinatedbenzylidene curcumin analog. Am. J. Transl. Res. 2015, 7, 298. [Google Scholar]
- Wu, G.-Q.; Chai, K.-Q.; Zhu, X.-M.; Jiang, H.; Wang, X.; Xue, Q.; Zheng, A.-H.; Zhou, H.-Y.; Chen, Y.; Chen, X.-C. Anti-cancer effects of curcumin on lung cancer through the inhibition of EZH2 and NOTCH1. Oncotarget 2016, 7, 26535. [Google Scholar] [CrossRef]
- Liu, W.-L.; Chang, J.-M.; Chong, I.-W.; Hung, Y.-L.; Chen, Y.-H.; Huang, W.-T.; Kuo, H.-F.; Hsieh, C.-C.; Liu, P.-L. Curcumin Inhibits LIN-28A through the Activation of miRNA-98 in the Lung Cancer Cell Line A549. Molecules 2017, 22, 929. [Google Scholar] [CrossRef] [PubMed]
- Devassy, J.G.; Nwachukwu, I.D.; Jones, P.J. Curcumin and cancer: Barriers to obtaining a health claim. Nutr. Rev. 2015, 73, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Ireson, C.R.; Jones, D.J.L.; Orr, S.; Coughtrie, M.W.H.; Boocock, D.J.; Williams, M.L.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Metabolism of the Cancer Chemopreventive Agent Curcumin in Human and Rat Intestine. Cancer Epidemiol. Biomark. AMP Prev. 2002, 11, 105–111. [Google Scholar]
- Pan, M.-H.; Huang, T.-M.; Lin, J.-K. Biotransformation of Curcumin through Reduction and Glucuronidation in Mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar]
- Somparn, P.; Phisalaphong, C.; Nakornchai, S.; Unchern, S.; Morales, N.P. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol. Pharm. Bull. 2007, 30, 74–78. [Google Scholar] [CrossRef]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The Molecular Basis for the Pharmacokinetics and Pharmacodynamics of Curcumin and Its Metabolites in Relation to Cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef]
- Pal, A.; Sung, B.; Prasad, B.A.B.; Schuber, P.T., Jr.; Prasad, S.; Aggarwal, B.B.; Bornmann, W.G. Curcumin glucuronides: Assessing the proliferative activity against human cell lines. Bioorg. Med. Chem. 2014, 22, 435–439. [Google Scholar] [CrossRef]
- Ireson, C.; Orr, S.; Jones, D.J.; Verschoyle, R.; Lim, C.-K.; Luo, J.-L.; Howells, L.; Plummer, S.; Jukes, R.; Williams, M. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1058–1064. [Google Scholar]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S.S.R. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Mohan Yallapu, M.; Ray Dobberpuhl, M.; Michele Maher, D.; Jaggi, M.; Chand Chauhan, S. Design of Curcumin loaded Cellulose Nanoparticles for Prostate Cancer. Curr. Drug Metab. 2012, 13, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Bisht, S.; Mizuma, M.; Feldmann, G.; Ottenhof, N.A.; Hong, S.-M.; Pramanik, D.; Chenna, V.; Karikari, C.; Sharma, R.; Goggins, M.G.; et al. Systemic Administration of Polymeric Nanoparticle-Encapsulated Curcumin (NanoCurc) Blocks Tumor Growth and Metastases in Preclinical Models of Pancreatic Cancer. Mol. Cancer Ther. 2010, 9, 2255–2264. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Liu, Y.-K.; Tsai, N.-M.; Hsieh, J.-H.; Chen, C.-H.; Lin, C.-M.; Liao, K.-W. A Lipo-PEG-PEI complex for encapsulating curcumin that enhances its antitumor effects on curcumin-sensitive and curcumin-resistance cells. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 318–327. [Google Scholar] [CrossRef]
- Ma, Z.; Shayeganpour, A.; Brocks, D.R.; Lavasanifar, A.; Samuel, J. High-performance liquid chromatography analysis of curcumin in rat plasma: Application to pharmacokinetics of polymeric micellar formulation of curcumin. Biomed. Chromatogr. 2007, 21, 546–552. [Google Scholar] [CrossRef]
- Liu, A.; Lou, H.; Zhao, L.; Fan, P. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J. Pharm. Biomed. Anal. 2006, 40, 720–727. [Google Scholar] [CrossRef]
- Mosley, C.A.; Liotta, D.C.; Snyder, J.P. Highly active anticancer curcumin analogues. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Aggarwal, B.B., Surh, Y.-J., Shishodia, S., Eds.; Springer: Boston, MA, USA, 2007; pp. 77–103. [Google Scholar]
- Mehta, H.J.; Patel, V.; Sadikot, R.T. Curcumin and lung cancer—A review. Target. Oncol. 2014, 9, 295–310. [Google Scholar] [CrossRef]
- López-Lázaro, M. Anticancer and carcinogenic properties of curcumin: Considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008, 52, S103–S127. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Turmeric Oleoresin (CAS No. 8024-37-1) (Major Component 79%-85% Curcumin, CAS No. 458-37-7) in F344/N Rats and B6C3F1 Mice (Feed Studies). Natl. Toxicol. Program Tech. Rep. Ser. 1993, 427, 1–275. [Google Scholar]
- Somasundaram, S.; Edmund, N.A.; Moore, D.T.; Small, G.W.; Shi, Y.Y.; Orlowski, R.Z. Dietary Curcumin Inhibits Chemotherapy-induced Apoptosis in Models of Human Breast Cancer. Cancer Res. 2002, 62, 3868–3875. [Google Scholar] [PubMed]
- López-Lázaro, M. Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Lu, J.; Holmgren, A. Thioredoxin reductase is irreversibly modified by curcumin: A novel molecular mechanism for its anticancer activity. J. Biol. Chem. 2005, 280, 25284–25290. [Google Scholar] [CrossRef] [PubMed]
- Syng-ai, C.; Kumari, A.L.; Khar, A. Effect of curcumin on normal and tumor cells: Role of glutathione and bcl-2. Mol. Cancer Ther. 2004, 3, 1101–1108. [Google Scholar]
- Cao, J.; Jia, L.; Zhou, H.-M.; Liu, Y.; Zhong, L.-F. Mitochondrial and Nuclear DNA Damage Induced by Curcumin in Human Hepatoma G2 Cells. Toxicol. Sci. 2006, 91, 476–483. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I Clinical Trial of Oral Curcumin: Biomarkers of Systemic Activity and Compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef]
- Pavan, A.R.; Silva, G.D.; Jornada, D.H.; Chiba, D.E.; Fernandes, G.F.; Man Chin, C.; Dos Santos, J.L. Unraveling the Anticancer Effect of Curcumin and Resveratrol. Nutrients 2016, 8, 628. [Google Scholar] [CrossRef]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch. Der Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan Mohd Tajuddin, W.N.B.; Lajis, N.H.; Abas, F.; Othman, I.; Naidu, R. Mechanistic Understanding of Curcumin’s Therapeutic Effects in Lung Cancer. Nutrients 2019, 11, 2989. https://doi.org/10.3390/nu11122989
Wan Mohd Tajuddin WNB, Lajis NH, Abas F, Othman I, Naidu R. Mechanistic Understanding of Curcumin’s Therapeutic Effects in Lung Cancer. Nutrients. 2019; 11(12):2989. https://doi.org/10.3390/nu11122989
Chicago/Turabian StyleWan Mohd Tajuddin, Wan Nur Baitty, Nordin H. Lajis, Faridah Abas, Iekhsan Othman, and Rakesh Naidu. 2019. "Mechanistic Understanding of Curcumin’s Therapeutic Effects in Lung Cancer" Nutrients 11, no. 12: 2989. https://doi.org/10.3390/nu11122989
APA StyleWan Mohd Tajuddin, W. N. B., Lajis, N. H., Abas, F., Othman, I., & Naidu, R. (2019). Mechanistic Understanding of Curcumin’s Therapeutic Effects in Lung Cancer. Nutrients, 11(12), 2989. https://doi.org/10.3390/nu11122989




