Epigenetics: Linking Early Postnatal Nutrition to Obesity Programming?
Abstract
1. Introduction
2. The Early Postnatal Period: A Critical Developmental Epigenetic Window
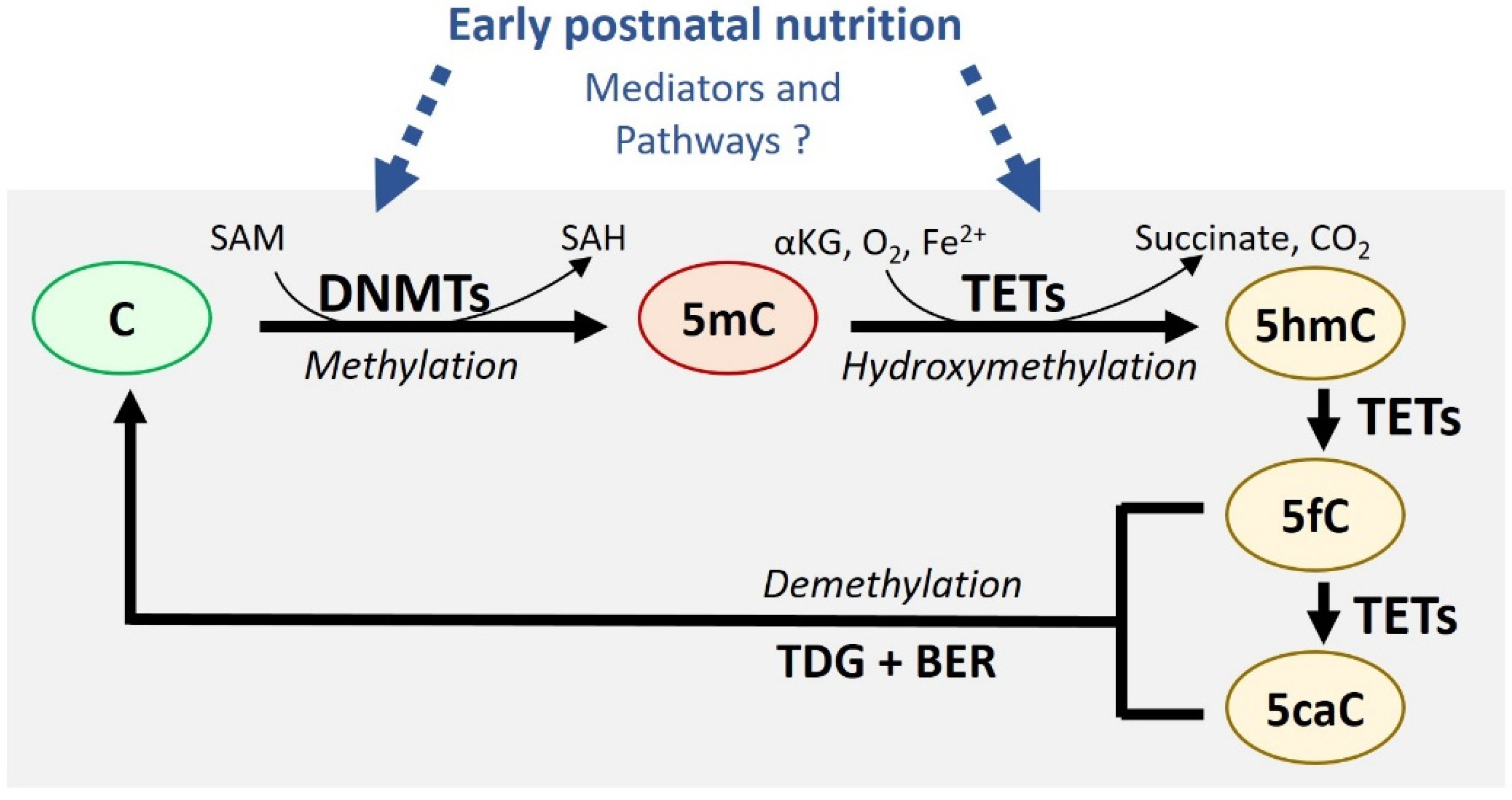
2.1. DNA Methylation and Demethylation
2.2. Postnatal Developmental and Epigenetic Dynamics in Organs of Energy Homeostasis
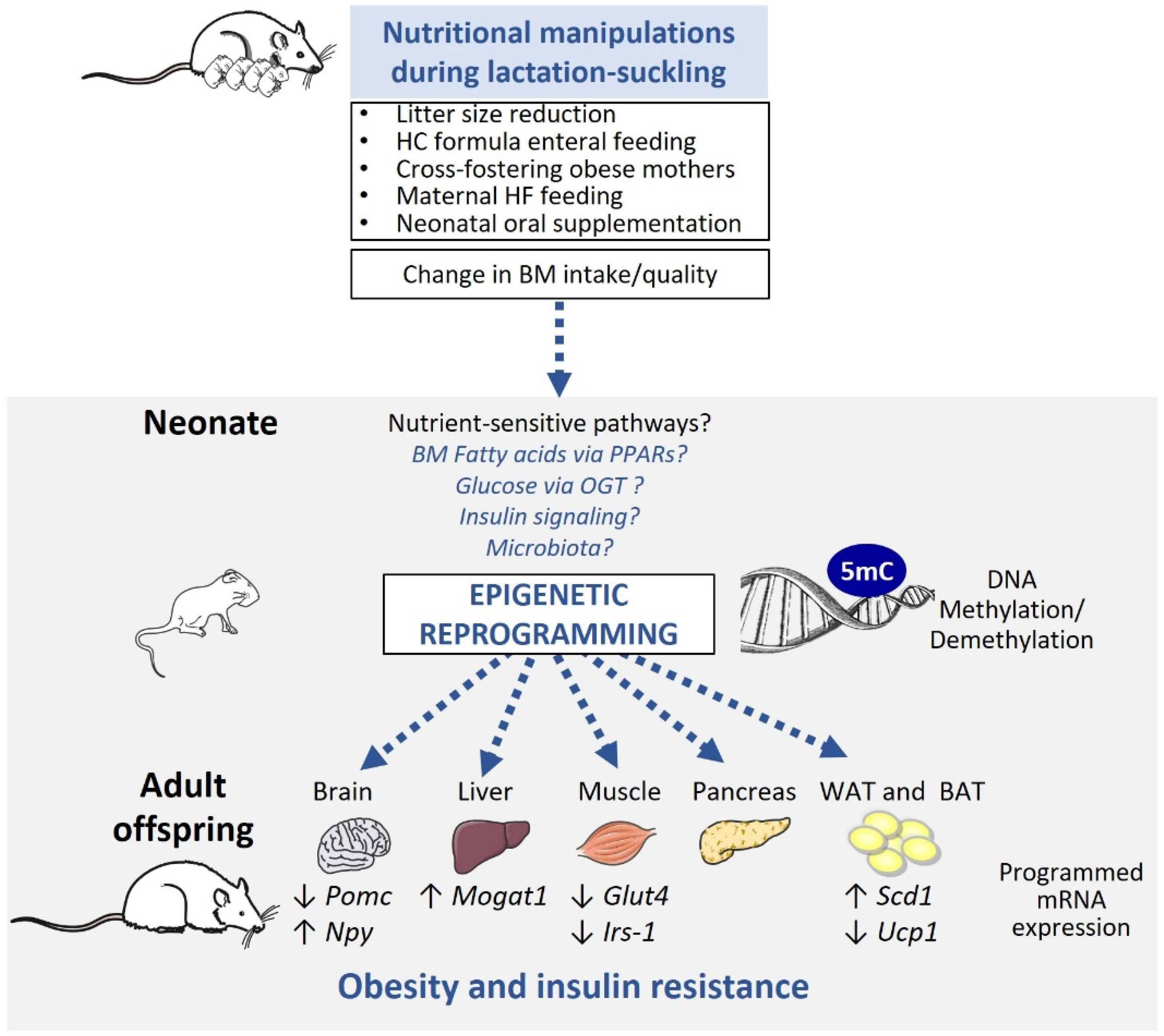
3. Early Postnatal Nutrition Affects Offspring Epigenetic Changes
3.1. Studies in Humans
3.2. Studies in Animal Models
3.2.1. Litter Size Modulation
3.2.2. Artificial Rearing Using Enteral Nutrition
3.2.3. Cross-Fostering
3.2.4. Maternal Nutrition Modification Exclusively during Lactation-Suckling
3.2.5. Neonatal Oral Supplementation
3.3. Limitations and Challenges of Epigenetic Studies in Developmental Programming
3.3.1. Where, When and How to Look for Epigenetic Reprogramming?
3.3.2. Sorting between Correlations and Causality?
4. Candidate Pathways Linking Early Postnatal Nutrition and Epigenetic Programming of Obesity?
4.1. PPARs Nuclear Receptor as Epigenetic Effectors of Breast Milk Fatty Acids?
4.2. Impact of Hormonal and Metabolic Imbalance in Neonates?
4.3. A Role for Gut Microbiota?
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afshin, A.; Reitsma, M.B.; Murray, C.J.L. Health Effects of Overweight and Obesity in 195 Countries. N. Engl. J. Med. 2017, 377, 1496–1497. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Lukaszewski, M.A.; Eberle, D.; Vieau, D.; Breton, C. Nutritional manipulations in the perinatal period program adipose tissue in offspring. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1195–E1207. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.; Levin, B.E.; Ozanne, S.E. Gene-environment interactions controlling energy and glucose homeostasis and the developmental origins of obesity. Physiol. Rev. 2015, 95, 47–82. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Smith, G.D.; Cook, D.G. Effect of infant feeding on the risk of obesity across the life course: A quantitative review of published evidence. Pediatrics 2005, 115, 1367–1377. [Google Scholar] [CrossRef]
- Butte, N.F. Impact of infant feeding practices on childhood obesity. J. Nutr. 2009, 139, 412S–416S. [Google Scholar] [CrossRef]
- Oddy, W.H.; Mori, T.A.; Huang, R.C.; Marsh, J.A.; Pennell, C.E.; Chivers, P.T.; Hands, B.P.; Jacoby, P.; Rzehak, P.; Koletzko, B.V.; et al. Early infant feeding and adiposity risk: From infancy to adulthood. Ann. Nutr. Metab. 2014, 64, 262–270. [Google Scholar] [CrossRef]
- Peneau, S.; Hercberg, S.; Rolland-Cachera, M.F. Breastfeeding, early nutrition, and adult body fat. J. Pediatr. 2014, 164, 1363–1368. [Google Scholar] [CrossRef]
- Horta, B.L.; Loret de Mola, C.; Victora, C.G. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 30–37. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; Franca, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Rudolph, M.C.; Young, B.E.; Lemas, D.J.; Palmer, C.E.; Hernandez, T.L.; Barbour, L.A.; Friedman, J.E.; Krebs, N.F.; MacLean, P.S. Early infant adipose deposition is positively associated with the n-6 to n-3 fatty acid ratio in human milk independent of maternal BMI. Int. J. Obes. 2017, 41, 510–517. [Google Scholar] [CrossRef]
- Young, B.E.; Levek, C.; Reynolds, R.M.; Rudolph, M.C.; MacLean, P.; Hernandez, T.L.; Friedman, J.E.; Krebs, N.F. Bioactive components in human milk are differentially associated with rates of lean and fat mass deposition in infants of mothers with normal vs. elevated BMI. Pediatr. Obes. 2018, 13, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.; Srinivasan, M. Metabolic programming in the immediate postnatal life. Ann. Nutr. Metab. 2011, 58, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Purcell, R.H.; Terrillion, C.E.; Yan, J.; Moran, T.H.; Tamashiro, K.L. Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes 2012, 61, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Jellyman, J.K.; Han, G.; Beall, M.; Lane, R.H.; Ross, M.G. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am. J. Obstet. Gynecol. 2014, 211, 237-e1. [Google Scholar] [CrossRef]
- Vogt, M.C.; Paeger, L.; Hess, S.; Steculorum, S.M.; Awazawa, M.; Hampel, B.; Neupert, S.; Nicholls, H.T.; Mauer, J.; Hausen, A.C.; et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 2014, 156, 495–509. [Google Scholar] [CrossRef]
- Waterland, R.A. Epigenetic mechanisms affecting regulation of energy balance: Many questions, few answers. Annu. Rev. Nutr. 2014, 34, 337–355. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Li, E.; Zhang, Y. DNA methylation in mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a019133. [Google Scholar] [CrossRef]
- Dor, Y.; Cedar, H. Principles of DNA methylation and their implications for biology and medicine. Lancet 2018, 392, 777–786. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef]
- Spruijt, C.G.; Gnerlich, F.; Smits, A.H.; Pfaffeneder, T.; Jansen, P.W.; Bauer, C.; Munzel, M.; Wagner, M.; Muller, M.; Khan, F.; et al. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell 2013, 152, 1146–1159. [Google Scholar] [CrossRef] [PubMed]
- Serandour, A.A.; Avner, S.; Oger, F.; Bizot, M.; Percevault, F.; Lucchetti-Miganeh, C.; Palierne, G.; Gheeraert, C.; Barloy-Hubler, F.; Peron, C.L.; et al. Dynamic hydroxymethylation of deoxyribonucleic acid marks differentiation-associated enhancers. Nucleic Acids Res. 2012, 40, 8255–8265. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Pastor, W.A.; Shen, Y.; Tahiliani, M.; Liu, D.R.; Rao, A. The behaviour of 5-hydroxymethylcytosine in bisulfite sequencing. PLoS ONE 2010, 5, e8888. [Google Scholar] [CrossRef] [PubMed]
- Plongthongkum, N.; Diep, D.H.; Zhang, K. Advances in the profiling of DNA modifications: Cytosine methylation and beyond. Nat. Rev. Genet. 2014, 15, 647–661. [Google Scholar] [CrossRef]
- Zhang, N. Epigenetic modulation of DNA methylation by nutrition and its mechanisms in animals. Anim. Nutr. 2015, 1, 144–151. [Google Scholar] [CrossRef]
- Kadayifci, F.Z.; Zheng, S.; Pan, Y.X. Molecular Mechanisms Underlying the Link between Diet and DNA Methylation. Int. J. Mol. Sci. 2018, 19, 4055. [Google Scholar] [CrossRef]
- Dunsworth, H.M.; Warrener, A.G.; Deacon, T.; Ellison, P.T.; Pontzer, H. Metabolic hypothesis for human altriciality. Proc. Natl. Acad. Sci. USA 2012, 109, 15212–15216. [Google Scholar] [CrossRef]
- Bouret, S.G. Nutritional programming of hypothalamic development: Critical periods and windows of opportunity. Int. J. Obes. Suppl. 2012, 2, S19–S24. [Google Scholar] [CrossRef]
- Li, G.; Kohorst, J.J.; Zhang, W.; Laritsky, E.; Kunde-Ramamoorthy, G.; Baker, M.S.; Fiorotto, M.L.; Waterland, R.A. Early postnatal nutrition determines adult physical activity and energy expenditure in female mice. Diabetes 2013, 62, 2773–2783. [Google Scholar] [CrossRef]
- Gluck, M.E.; Viswanath, P.; Stinson, E.J. Obesity, Appetite, and the Prefrontal Cortex. Curr. Obes. Rep. 2017, 6, 380–388. [Google Scholar] [CrossRef]
- Hill, R.S.; Walsh, C.A. Molecular insights into human brain evolution. Nature 2005, 437, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Bluml, S.; Wisnowski, J.L.; Nelson, M.D., Jr.; Paquette, L.; Gilles, F.H.; Kinney, H.C.; Panigrahy, A. Metabolic maturation of the human brain from birth through adolescence: Insights from in vivo magnetic resonance spectroscopy. Cereb. Cortex 2013, 23, 2944–2955. [Google Scholar] [CrossRef] [PubMed]
- Numata, S.; Ye, T.; Hyde, T.M.; Guitart-Navarro, X.; Tao, R.; Wininger, M.; Colantuoni, C.; Weinberger, D.R.; Kleinman, J.E.; Lipska, B.K. DNA methylation signatures in development and aging of the human prefrontal cortex. Am. J. Hum. Genet. 2012, 90, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Carberry, A.E.; Colditz, P.B.; Lingwood, B.E. Body composition from birth to 4.5 months in infants born to non-obese women. Pediatr. Res. 2010, 68, 84–88. [Google Scholar] [CrossRef]
- Knittle, J.L.; Timmers, K.; Ginsberg-Fellner, F.; Brown, R.E.; Katz, D.P. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J. Clin. Investig. 1979, 63, 239–246. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Naslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Birsoy, K.; Berry, R.; Wang, T.; Ceyhan, O.; Tavazoie, S.; Friedman, J.M.; Rodeheffer, M.S. Analysis of gene networks in white adipose tissue development reveals a role for ETS2 in adipogenesis. Development 2011, 138, 4709–4719. [Google Scholar] [CrossRef]
- Han, J.; Lee, J.E.; Jin, J.; Lim, J.S.; Oh, N.; Kim, K.; Chang, S.I.; Shibuya, M.; Kim, H.; Koh, G.Y. The spatiotemporal development of adipose tissue. Development 2011, 138, 5027–5037. [Google Scholar] [CrossRef]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef]
- Grijalva, J.; Vakili, K. Neonatal liver physiology. In Seminars in Pediatric Surgery; Saunders: Philadelphia, PA, USA, 2013; Volume 22, pp. 185–189. [Google Scholar]
- Reizel, Y.; Spiro, A.; Sabag, O.; Skversky, Y.; Hecht, M.; Keshet, I.; Berman, B.P.; Cedar, H. Gender-specific postnatal demethylation and establishment of epigenetic memory. Genes Dev. 2015, 29, 923–933. [Google Scholar] [CrossRef]
- Cannon, M.V.; Pilarowski, G.; Liu, X.; Serre, D. Extensive Epigenetic Changes Accompany Terminal Differentiation of Mouse Hepatocytes After Birth. G3: Genes Genomes Genet. 2016, 6, 3701–3709. [Google Scholar] [CrossRef] [PubMed]
- Reizel, Y.; Sabag, O.; Skversky, Y.; Spiro, A.; Steinberg, B.; Bernstein, D.; Wang, A.; Kieckhaefer, J.; Li, C.; Pikarsky, E.; et al. Postnatal DNA demethylation and its role in tissue maturation. Nat. Commun. 2018, 9, 2040. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Kellermayer, R.; Rached, M.T.; Tatevian, N.; Gomes, M.V.; Zhang, J.; Zhang, L.; Chakravarty, A.; Zhu, W.; Laritsky, E.; et al. Epigenomic profiling indicates a role for DNA methylation in early postnatal liver development. Hum. Mol. Genet. 2009, 18, 3026–3038. [Google Scholar] [CrossRef] [PubMed]
- Ehara, T.; Kamei, Y.; Yuan, X.; Takahashi, M.; Kanai, S.; Tamura, E.; Tsujimoto, K.; Tamiya, T.; Nakagawa, Y.; Shimano, H.; et al. Ligand-activated PPARalpha-dependent DNA demethylation regulates the fatty acid beta-oxidation genes in the postnatal liver. Diabetes 2015, 64, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Tsujimoto, K.; Hashimoto, K.; Kawahori, K.; Hanzawa, N.; Hamaguchi, M.; Seki, T.; Nawa, M.; Ehara, T.; Kitamura, Y.; et al. Epigenetic modulation of Fgf21 in the perinatal mouse liver ameliorates diet-induced obesity in adulthood. Nat. Commun. 2018, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.M.; Kunderfranco, P.; Rubino, M.; Larcher, V.; Carullo, P.; Anselmo, A.; Kurz, K.; Carell, T.; Angius, A.; Latronico, M.V.; et al. DNA hydroxymethylation controls cardiomyocyte gene expression in development and hypertrophy. Nat. Commun. 2016, 7, 12418. [Google Scholar] [CrossRef] [PubMed]
- Szulwach, K.E.; Li, X.; Li, Y.; Song, C.X.; Wu, H.; Dai, Q.; Irier, H.; Upadhyay, A.K.; Gearing, M.; Levey, A.I.; et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat. Neurosci. 2011, 14, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Sheaffer, K.L.; Choi, I.; Won, K.J.; Kaestner, K.H. Epigenetic regulation of intestinal stem cells by Tet1-mediated DNA hydroxymethylation. Genes Dev. 2016, 30, 2433–2442. [Google Scholar] [CrossRef]
- Yu, D.H.; Gadkari, M.; Zhou, Q.; Yu, S.; Gao, N.; Guan, Y.; Schady, D.; Roshan, T.N.; Chen, M.H.; Laritsky, E.; et al. Postnatal epigenetic regulation of intestinal stem cells requires DNA methylation and is guided by the microbiome. Genome Biol. 2015, 16, 211. [Google Scholar] [CrossRef]
- Dhawan, S.; Tschen, S.I.; Zeng, C.; Guo, T.; Hebrok, M.; Matveyenko, A.; Bhushan, A. DNA methylation directs functional maturation of pancreatic beta cells. J. Clin. Investig. 2015, 125, 2851–2860. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Loret de Mola, C.; Davies, N.M.; Victora, C.G.; Relton, C.L. Breastfeeding effects on DNA methylation in the offspring: A systematic literature review. PLoS ONE 2017, 12, e0173070. [Google Scholar] [CrossRef]
- Obermann-Borst, S.A.; Eilers, P.H.; Tobi, E.W.; de Jong, F.H.; Slagboom, P.E.; Heijmans, B.T.; Steegers-Theunissen, R.P. Duration of breastfeeding and gender are associated with methylation of the LEPTIN gene in very young children. Pediatr. Res. 2013, 74, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, W.B.; Bion, V.; Lockett, G.A.; Ziyab, A.H.; Soto-Ramirez, N.; Mukherjee, N.; Kurukulaaratchy, R.J.; Ewart, S.; Zhang, H.; Arshad, S.H.; et al. Duration of breastfeeding is associated with leptin (LEP) DNA methylation profiles and BMI in 10-year-old children. Clin. Epigenet. 2019, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, S.; Symons, L.; Vanautgaerden, E.L.; Ghosh, M.; Duca, R.C.; Bekaert, B.; Freson, K.; Huybrechts, I.; Langie, S.A.S.; Koppen, G.; et al. The Influence of the Duration of Breastfeeding on the Infant’s Metabolic Epigenome. Nutrients 2019, 11, 1408. [Google Scholar] [CrossRef] [PubMed]
- Houde, A.A.; Legare, C.; Biron, S.; Lescelleur, O.; Biertho, L.; Marceau, S.; Tchernof, A.; Vohl, M.C.; Hivert, M.F.; Bouchard, L. Leptin and adiponectin DNA methylation levels in adipose tissues and blood cells are associated with BMI, waist girth and LDL-cholesterol levels in severely obese men and women. BMC Med. Genet. 2015, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, A.; Harder, T.; Brunn, M.; Harder, A.; Roepke, K.; Wittrock-Staar, M.; Ziska, T.; Schellong, K.; Rodekamp, E.; Melchior, K.; et al. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: An epigenetic model of obesity and the metabolic syndrome. J. Physiol. 2009, 587, 4963–4976. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, A.; Roepke, K.; Harder, T.; Brunn, M.; Harder, A.; Wittrock-Staar, M.; Ziska, T.; Schellong, K.; Rodekamp, E.; Melchior, K.; et al. Epigenetic malprogramming of the insulin receptor promoter due to developmental overfeeding. J. Perinat. Med. 2010, 38, 393–400. [Google Scholar] [CrossRef]
- Liu, H.W.; Mahmood, S.; Srinivasan, M.; Smiraglia, D.J.; Patel, M.S. Developmental programming in skeletal muscle in response to overnourishment in the immediate postnatal life in rats. J. Nutr. Biochem. 2013, 24, 1859–1869. [Google Scholar] [CrossRef]
- Ramon-Krauel, M.; Pentinat, T.; Bloks, V.W.; Cebria, J.; Ribo, S.; Perez-Wienese, R.; Vila, M.; Palacios-Marin, I.; Fernandez-Perez, A.; Vallejo, M.; et al. Epigenetic programming at the Mogat1 locus may link neonatal overnutrition with long-term hepatic steatosis and insulin resistance. FASEB J. 2018, 32, 6025–6037. [Google Scholar] [CrossRef]
- Li, G.; Petkova, T.D.; Laritsky, E.; Kessler, N.; Baker, M.S.; Zhu, S.; Waterland, R.A. Early postnatal overnutrition accelerates aging-associated epigenetic drift in pancreatic islets. Environ. Epigenet. 2019, 5, dvz015. [Google Scholar] [CrossRef]
- Mahmood, S.; Smiraglia, D.J.; Srinivasan, M.; Patel, M.S. Epigenetic changes in hypothalamic appetite regulatory genes may underlie the developmental programming for obesity in rat neonates subjected to a high-carbohydrate dietary modification. J. Dev. Orig. Health Dis. 2013, 4, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, N.; Thamotharan, S.; Srinivasan, M.; Mahmood, S.; Patel, M.S.; Devaskar, S.U. Postnatal exposure to a high-carbohydrate diet interferes epigenetically with thyroid hormone receptor induction of the adult male rat skeletal muscle glucose transporter isoform 4 expression. J. Nutr. Biochem. 2014, 25, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yang, Q.; Zhang, L.; Maricelli, J.W.; Rodgers, B.D.; Zhu, M.J.; Du, M. Maternal high-fat diet during lactation impairs thermogenic function of brown adipose tissue in offspring mice. Sci. Rep. 2016, 6, 34345. [Google Scholar] [CrossRef] [PubMed]
- Butruille, L.; Marousez, L.; Pourpe, C.; Oger, F.; Lecoutre, S.; Catheline, D.; Gors, S.; Metges, C.C.; Guinez, C.; Laborie, C.; et al. Maternal high-fat diet during suckling programs visceral adiposity and epigenetic regulation of adipose tissue stearoyl-CoA desaturase-1 in offspring. Int. J. Obes. 2019. [Google Scholar] [CrossRef]
- Palou, M.; Pico, C.; McKay, J.A.; Sanchez, J.; Priego, T.; Mathers, J.C.; Palou, A. Protective effects of leptin during the suckling period against later obesity may be associated with changes in promoter methylation of the hypothalamic pro-opiomelanocortin gene. Br. J. Nutr. 2011, 106, 769–778. [Google Scholar] [CrossRef]
- Pico, C.; Oliver, P.; Sanchez, J.; Miralles, O.; Caimari, A.; Priego, T.; Palou, A. The intake of physiological doses of leptin during lactation in rats prevents obesity in later life. Int. J. Obes. 2007, 31, 1199–1209. [Google Scholar] [CrossRef]
- Arreguin, A.; Ribot, J.; Musinovic, H.; von Lintig, J.; Palou, A.; Bonet, M.L. Dietary vitamin A impacts DNA methylation patterns of adipogenesis-related genes in suckling rats. Arch. Biochem. Biophys. 2018, 650, 75–84. [Google Scholar] [CrossRef]
- Granados, N.; Amengual, J.; Ribot, J.; Musinovic, H.; Ceresi, E.; von Lintig, J.; Palou, A.; Bonet, M.L. Vitamin A supplementation in early life affects later response to an obesogenic diet in rats. Int. J. Obes. 2013, 37, 1169–1176. [Google Scholar] [CrossRef]
- Sefcikova, Z.; Bujnakova, D.; Racek, L.; Kmet, V.; Mozes, S. Developmental changes in gut microbiota and enzyme activity predict obesity risk in rats arising from reduced nests. Physiol. Res. 2011, 60, 337–346. [Google Scholar] [CrossRef]
- Xavier, J.L.P.; Scomparin, D.X.; Pontes, C.C.; Ribeiro, P.R.; Cordeiro, M.M.; Marcondes, J.A.; Mendonca, F.O.; Silva, M.T.D.; Oliveira, F.B.; Franco, G.C.N.; et al. Litter Size Reduction Induces Metabolic and Histological Adjustments in Dams throughout Lactation with Early Effects on Offspring. An. Acad. Bras. Ciências 2019, 91, e20170971. [Google Scholar] [CrossRef]
- Patel, M.S.; Srinivasan, M.; Laychock, S.G. Metabolic programming: Role of nutrition in the immediate postnatal life. J. Inherit. Metab. Dis. 2009, 32, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Glavas, M.M.; Kirigiti, M.A.; Xiao, X.Q.; Enriori, P.J.; Fisher, S.K.; Evans, A.E.; Grayson, B.E.; Cowley, M.A.; Smith, M.S.; Grove, K.L. Early overnutrition results in early-onset arcuate leptin resistance and increased sensitivity to high-fat diet. Endocrinology 2010, 151, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Habbout, A.; Li, N.; Rochette, L.; Vergely, C. Postnatal overfeeding in rodents by litter size reduction induces major short- and long-term pathophysiological consequences. J. Nutr. 2013, 143, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Gali Ramamoorthy, T.; Allen, T.J.; Davies, A.; Harno, E.; Sefton, C.; Murgatroyd, C.; White, A. Maternal overnutrition programs epigenetic changes in the regulatory regions of hypothalamic Pomc in the offspring of rats. Int. J. Obes. 2018, 42, 1431–1444. [Google Scholar] [CrossRef]
- Chen, H.; Simar, D.; Lambert, K.; Mercier, J.; Morris, M.J. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology 2008, 149, 5348–5356. [Google Scholar] [CrossRef]
- Davidowa, H.; Plagemann, A. Insulin resistance of hypothalamic arcuate neurons in neonatally overfed rats. Neuroreport 2007, 18, 521–524. [Google Scholar] [CrossRef]
- Bei, F.; Jia, J.; Jia, Y.Q.; Sun, J.H.; Liang, F.; Yu, Z.Y.; Cai, W. Long-term effect of early postnatal overnutrition on insulin resistance and serum fatty acid profiles in male rats. Lipids Health Dis. 2015, 14, 96. [Google Scholar] [CrossRef]
- Dias, I.; Salviano, I.; Mencalha, A.; de Carvalho, S.N.; Thole, A.A.; Carvalho, L.; Cortez, E.; Stumbo, A.C. Neonatal overfeeding impairs differentiation potential of mice subcutaneous adipose mesenchymal stem cells. Stem Cell Rev. 2018, 14, 535–545. [Google Scholar] [CrossRef]
- Borengasser, S.J.; Zhong, Y.; Kang, P.; Lindsey, F.; Ronis, M.J.; Badger, T.M.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology 2013, 154, 4113–4125. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Wang, B.; Pan, H.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. J. Physiol. 2016, 594, 4453–4466. [Google Scholar] [CrossRef]
- Erion, D.M.; Shulman, G.I. Diacylglycerol-mediated insulin resistance. Nat. Med. 2010, 16, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Pentinat, T.; Ramon-Krauel, M.; Cebria, J.; Diaz, R.; Jimenez-Chillaron, J.C. Transgenerational inheritance of glucose intolerance in a mouse model of neonatal overnutrition. Endocrinology 2010, 151, 5617–5623. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ji, J.; Yu, Y.; Wei, X.; Chai, S.; Liu, D.; Huang, D.; Li, Q.; Dong, Z.; Xiao, X. Neonatal Overfeeding in Female Mice Predisposes the Development of Obesity in their Male Offspring via Altered Central Leptin Signalling. J. Neuroendocrinol. 2015, 27, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Garza, C. Early postnatal nutrition determines adult pancreatic glucose-responsive insulin secretion and islet gene expression in rats. J. Nutr. 2002, 132, 357–364. [Google Scholar] [CrossRef]
- Hall, W.G. Weaning and growth of artificially reared rats. Science 1975, 190, 1313–1315. [Google Scholar] [CrossRef]
- Patel, M.S.; Hiremagalur, B.K. Artificial-rearing technique: Its usefulness in nutrition research. J. Nutr. 1992, 122, 412–419. [Google Scholar] [CrossRef]
- Beierle, E.A.; Chen, M.K.; Hartwich, J.E.; Iyengar, M.; Dai, W.; Li, N.; Demarco, V.; Neu, J. Artificial rearing of mouse pups: Development of a mouse pup in a cup model. Pediatr. Res. 2004, 56, 250–255. [Google Scholar] [CrossRef][Green Version]
- Patel, M.S.; Srinivasan, M. Metabolic programming: Causes and consequences. J. Biol. Chem. 2002, 277, 1629–1632. [Google Scholar] [CrossRef]
- Srinivasan, M.; Laychock, S.G.; Hill, D.J.; Patel, M.S. Neonatal nutrition: Metabolic programming of pancreatic islets and obesity. Exp. Biol. Med. 2003, 228, 15–23. [Google Scholar] [CrossRef]
- Srinivasan, M.; Mitrani, P.; Sadhanandan, G.; Dodds, C.; Shbeir-ElDika, S.; Thamotharan, S.; Ghanim, H.; Dandona, P.; Devaskar, S.U.; Patel, M.S. A high-carbohydrate diet in the immediate postnatal life of rats induces adaptations predisposing to adult-onset obesity. J. Endocrinol. 2008, 197, 565–574. [Google Scholar] [CrossRef]
- Ferre, P.; Decaux, J.F.; Issad, T.; Girard, J. Changes in energy metabolism during the suckling and weaning period in the newborn. Reprod. Nutr. Dev. 1986, 26, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Cremer, J.E.; Teal, H.M. The activity of pyruvate dehydrogenase in rat brain during postnatal development. FEBS Lett. 1974, 39, 17–20. [Google Scholar] [CrossRef]
- Srinivasan, M.; Dodds, C.; Ghanim, H.; Gao, T.; Ross, P.J.; Browne, R.W.; Dandona, P.; Patel, M.S. Maternal obesity and fetal programming: Effects of a high-carbohydrate nutritional modification in the immediate postnatal life of female rats. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E895–E903. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.N.; Dunn-Meynell, A.A.; Hartman, T.G.; Levin, B.E. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R768–R778. [Google Scholar] [CrossRef] [PubMed]
- Oben, J.A.; Mouralidarane, A.; Samuelsson, A.M.; Matthews, P.J.; Morgan, M.L.; McKee, C.; Soeda, J.; Fernandez-Twinn, D.S.; Martin-Gronert, M.S.; Ozanne, S.E.; et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J. Hepatol. 2010, 52, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Wattez, J.S.; Delahaye, F.; Barella, L.F.; Dickes-Coopman, A.; Montel, V.; Breton, C.; Mathias, P.; Foligne, B.; Lesage, J.; Vieau, D. Short- and long-term effects of maternal perinatal undernutrition are lowered by cross-fostering during lactation in the male rat. J. Dev. Orig. Health Dis. 2014, 5, 109–120. [Google Scholar] [CrossRef]
- Matthews, P.A.; Samuelsson, A.M.; Seed, P.; Pombo, J.; Oben, J.A.; Poston, L.; Taylor, P.D. Fostering in mice induces cardiovascular and metabolic dysfunction in adulthood. J. Physiol. 2011, 589, 3969–3981. [Google Scholar] [CrossRef]
- Tsuduki, T.; Kitano, Y.; Honma, T.; Kijima, R.; Ikeda, I. High dietary fat intake during lactation promotes development of diet-induced obesity in male offspring of mice. J. Nutr. Sci. Vitaminol. 2013, 59, 384–392. [Google Scholar] [CrossRef]
- de Los Rios, E.A.; Ruiz-Herrera, X.; Tinoco-Pantoja, V.; Lopez-Barrera, F.; Martinez de la Escalera, G.; Clapp, C.; Macotela, Y. Impaired prolactin actions mediate altered offspring metabolism induced by maternal high-fat feeding during lactation. FASEB J. 2018, 32, 3457–3470. [Google Scholar] [CrossRef]
- Palou, M.; Pico, C.; Palou, A. Leptin as a breast milk component for the prevention of obesity. Nutr. Rev. 2018, 76, 875–892. [Google Scholar] [CrossRef]
- Casabiell, X.; Pineiro, V.; Tome, M.A.; Peino, R.; Dieguez, C.; Casanueva, F.F. Presence of leptin in colostrum and/or breast milk from lactating mothers: A potential role in the regulation of neonatal food intake. J. Clin. Endocrinol. Metab. 1997, 82, 4270–4273. [Google Scholar] [CrossRef] [PubMed]
- Resto, M.; O’Connor, D.; Leef, K.; Funanage, V.; Spear, M.; Locke, R. Leptin levels in preterm human breast milk and infant formula. Pediatrics 2001, 108, E15. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Oliver, P.; Miralles, O.; Ceresi, E.; Pico, C.; Palou, A. Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology 2005, 146, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Priego, T.; Palou, M.; Tobaruela, A.; Palou, A.; Pico, C. Oral supplementation with physiological doses of leptin during lactation in rats improves insulin sensitivity and affects food preferences later in life. Endocrinology 2008, 149, 733–740. [Google Scholar] [CrossRef]
- Priego, T.; Sanchez, J.; Palou, A.; Pico, C. Leptin intake during the suckling period improves the metabolic response of adipose tissue to a high-fat diet. Int. J. Obes. 2010, 34, 809–819. [Google Scholar] [CrossRef]
- Palou, A.; Pico, C. Leptin intake during lactation prevents obesity and affects food intake and food preferences in later life. Appetite 2009, 52, 249–252. [Google Scholar] [CrossRef]
- Yura, S.; Itoh, H.; Sagawa, N.; Yamamoto, H.; Masuzaki, H.; Nakao, K.; Kawamura, M.; Mogami, H.; Ogawa, Y.; Fujii, S. Neonatal exposure to leptin augments diet-induced obesity in leptin-deficient Ob/Ob mice. Obesity 2008, 16, 1289–1295. [Google Scholar] [CrossRef]
- Yura, S.; Itoh, H.; Sagawa, N.; Yamamoto, H.; Masuzaki, H.; Nakao, K.; Kawamura, M.; Takemura, M.; Kakui, K.; Ogawa, Y.; et al. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005, 1, 371–378. [Google Scholar] [CrossRef]
- de Oliveira Cravo, C.; Teixeira, C.V.; Passos, M.C.; Dutra, S.C.; de Moura, E.G.; Ramos, C. Leptin treatment during the neonatal period is associated with higher food intake and adult body weight in rats. Horm. Metab. Res. 2002, 34, 400–405. [Google Scholar] [CrossRef]
- Vickers, M.H.; Gluckman, P.D.; Coveny, A.H.; Hofman, P.L.; Cutfield, W.S.; Gertler, A.; Breier, B.H.; Harris, M. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology 2008, 149, 1906–1913. [Google Scholar] [CrossRef]
- Serrano, A.; Asnani-Kishnani, M.; Rodriguez, A.M.; Palou, A.; Ribot, J.; Bonet, M.L. Programming of the Beige Phenotype in White Adipose Tissue of Adult Mice by Mild Resveratrol and Nicotinamide Riboside Supplementations in Early Postnatal Life. Mol. Nutr. Food Res. 2018, 62, e1800463. [Google Scholar] [CrossRef] [PubMed]
- Cazaly, E.; Saad, J.; Wang, W.; Heckman, C.; Ollikainen, M.; Tang, J. Making Sense of the Epigenome Using Data Integration Approaches. Front. Pharmacol. 2019, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Breton, C.V.; Marsit, C.J.; Faustman, E.; Nadeau, K.; Goodrich, J.M.; Dolinoy, D.C.; Herbstman, J.; Holland, N.; LaSalle, J.M.; Schmidt, R.; et al. Small-Magnitude Effect Sizes in Epigenetic End Points are Important in Children’s Environmental Health Studies: The Children’s Environmental Health and Disease Prevention Research Center’s Epigenetics Working Group. Environ. Health Perspect. 2017, 125, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Leenen, F.A.; Muller, C.P.; Turner, J.D. DNA methylation: Conducting the orchestra from exposure to phenotype? Clin. Epigenet. 2016, 8, 92. [Google Scholar] [CrossRef]
- Liu, X.S.; Wu, H.; Ji, X.; Stelzer, Y.; Wu, X.; Czauderna, S.; Shu, J.; Dadon, D.; Young, R.A.; Jaenisch, R. Editing DNA Methylation in the Mammalian Genome. Cell 2016, 167, 233–247. [Google Scholar] [CrossRef]
- Innis, S.M. Metabolic programming of long-term outcomes due to fatty acid nutrition in early life. Matern. Child Nutr. 2011, 7, 112–123. [Google Scholar] [CrossRef]
- Muhlhausler, B.S.; Ailhaud, G.P. Omega-6 polyunsaturated fatty acids and the early origins of obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 56–61. [Google Scholar] [CrossRef]
- Ailhaud, G. Omega-6 fatty acids and excessive adipose tissue development. World Rev. Nutr. Diet. 2008, 98, 51–61. [Google Scholar] [CrossRef]
- Jump, D.B. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr. Opin. Lipidol. 2008, 19, 242–247. [Google Scholar] [CrossRef]
- Silva-Martinez, G.A.; Rodriguez-Rios, D.; Alvarado-Caudillo, Y.; Vaquero, A.; Esteller, M.; Carmona, F.J.; Moran, S.; Nielsen, F.C.; Wickstrom-Lindholm, M.; Wrobel, K.; et al. Arachidonic and oleic acid exert distinct effects on the DNA methylome. Epigenetics 2016, 11, 321–334. [Google Scholar] [CrossRef]
- Perfilyev, A.; Dahlman, I.; Gillberg, L.; Rosqvist, F.; Iggman, D.; Volkov, P.; Nilsson, E.; Riserus, U.; Ling, C. Impact of polyunsaturated and saturated fat overfeeding on the DNA-methylation pattern in human adipose tissue: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, K.; Shinoda, A.; Kano, F.; Sato, R.; Shirahige, K.; Murata, M. PPARgamma-induced PARylation promotes local DNA demethylation by production of 5-hydroxymethylcytosine. Nat. Commun. 2013, 4, 2262. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Hasegawa, H.; Matsumoto, T.; Yasukawa, M. Peroxisome proliferator-activated receptor alpha and gamma agonists together with TGF-beta convert human CD4+CD25- T cells into functional Foxp3+ regulatory T cells. J. Immunol. 2010, 185, 7186–7198. [Google Scholar] [CrossRef] [PubMed]
- Pazienza, V.; Tavano, F.; Benegiamo, G.; Vinciguerra, M.; Burbaci, F.P.; Copetti, M.; di Mola, F.F.; Andriulli, A.; di Sebastiano, P. Correlations among PPARgamma, DNMT1, and DNMT3B Expression Levels and Pancreatic Cancer. PPAR Res. 2012, 2012, 461784. [Google Scholar] [CrossRef]
- Hervouet, E.; Vallette, F.M.; Cartron, P.F. Dnmt3/transcription factor interactions as crucial players in targeted DNA methylation. Epigenetics 2009, 4, 487–499. [Google Scholar] [CrossRef]
- Hervouet, E.; Vallette, F.M.; Cartron, P.F. Dnmt1/Transcription factor interactions: An alternative mechanism of DNA methylation inheritance. Genes Cancer 2010, 1, 434–443. [Google Scholar] [CrossRef]
- Whitmore, T.J.; Trengove, N.J.; Graham, D.F.; Hartmann, P.E. Analysis of insulin in human breast milk in mothers with type 1 and type 2 diabetes mellitus. Int. J. Endocrinol. 2012, 2012, 296368. [Google Scholar] [CrossRef]
- Dearden, L.; Bouret, S.G.; Ozanne, S.E. Sex and gender differences in developmental programming of metabolism. Mol. Metab. 2018, 15, 8–19. [Google Scholar] [CrossRef]
- Dehennaut, V.; Leprince, D.; Lefebvre, T. O-GlcNAcylation, an Epigenetic Mark. Focus on the Histone Code, TET Family Proteins, and Polycomb Group Proteins. Front. Endocrinol. 2014, 5, 155. [Google Scholar] [CrossRef]
- Berthier, A.; Vinod, M.; Porez, G.; Steenackers, A.; Alexandre, J.; Yamakawa, N.; Gheeraert, C.; Ploton, M.; Marechal, X.; Dubois-Chevalier, J.; et al. Combinatorial regulation of hepatic cytoplasmic signaling and nuclear transcriptional events by the OGT/REV-ERBalpha complex. Proc. Natl. Acad. Sci. USA 2018, 115, E11033–E11042. [Google Scholar] [CrossRef]
- Alderete, T.L.; Autran, C.; Brekke, B.E.; Knight, R.; Bode, L.; Goran, M.I.; Fields, D.A. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 2015, 102, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Rea, A.; Tie, W.J.; Lai, C.T.; Kugananthan, S.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Carbohydrates in Human Milk and Body Composition of Term Infants during the First 12 Months of Lactation. Nutrients 2019, 11, 1472. [Google Scholar] [CrossRef] [PubMed]
- Koleva, P.T.; Bridgman, S.L.; Kozyrskyj, A.L. The infant gut microbiome: Evidence for obesity risk and dietary intervention. Nutrients 2015, 7, 2237–2260. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Prentice, P.M.; Schoemaker, M.H.; Vervoort, J.; Hettinga, K.; Lambers, T.T.; van Tol, E.A.F.; Acerini, C.L.; Olga, L.; Petry, C.J.; Hughes, I.A.; et al. Human Milk Short-Chain Fatty Acid Composition is Associated with Adiposity Outcomes in Infants. J. Nutr. 2019, 149, 716–722. [Google Scholar] [CrossRef]
- Miro-Blanch, J.; Yanes, O. Epigenetic Regulation at the Interplay Between Gut Microbiota and Host Metabolism. Front. Genet. 2019, 10, 638. [Google Scholar] [CrossRef]
- Pan, W.H.; Sommer, F.; Falk-Paulsen, M.; Ulas, T.; Best, P.; Fazio, A.; Kachroo, P.; Luzius, A.; Jentzsch, M.; Rehman, A.; et al. Exposure to the gut microbiota drives distinct methylome and transcriptome changes in intestinal epithelial cells during postnatal development. Genome Med. 2018, 10, 27. [Google Scholar] [CrossRef]
| Study | Cohort | Sample Type and Analysis | Findings |
|---|---|---|---|
| Obermann-Borst et al., 2013 [54] | 98 infants at 1.4 years-old; BF duration groups (number/group): No BF (24), <1 month (14), >1–3 months (21), >3–6 months (21), >6 months (18) | Whole blood 10 CpGs of LEP promoter; Mass spectrometry-based method with bisulfite DNA conversion |
|
| Pauwels et al., 2019 [56] | 101 infants at 1 year-old (42.5% girls); BF duration groups (number/group): No BF (5), 1–3 months (31), 4–6 months (29), 7–9 months (19), 10–12 months (17) | Buccal epithelial cells; 1 CpG LEP promoter 5 CpG RXRA promoter; Pyrosequencing |
|
| Sherwood et al., 2019 [55] | 259 infants at 10 years-old; 257 infants at 18 years-old Groups (number/group not available): exclusive BF vs. mixed feeding (BF despite formula feeding or solid food introduction) | Whole blood 16 to 23 CpGs of LEP; Infinium methylation EPIC BeadChips or Infinium Human Methylation450 |
|
| Model | Study Details | Metabolic Outcomes | Epigenetic Modifications Gene Expression |
|---|---|---|---|
| Litter Size Adjustment | |||
| Plagemann et al., 2009 [58]; Plagemann et al., 2010 [59] | Rat 12 vs. 3 pups per litter | At 21 days old: ↑ BW, adiposity ↑ Plasma glucose, insulin, leptinInsulin resistance | Hypothalamus Pomc: ↑methylation at CpGs 12–13 (Sp1 and NF-Kb binding sites), but no change in mRNA Npy: ↔ methylation at CpGs 1–17 InsR: ↑ mean CpGs methylation in the −322bp upstream CGI of InsR promoter, but no change in mRNA |
| Liu et al., 2013 [60] | Rat (F) 12 vs. 3 pups per litter | At 21 days old: ↑ BW ↑ Plasma insulin | Muscle Irs1: ↔ methylation at CpGs 4–21 ↓ mRNA Glut4: ↓ methylation at CpG 5 ↑ mRNA |
| At 4.5 months old: ↑ BW, food intake ↑ Plasma glucose, insulin, leptin | Muscle Irs1: ↑ methylation at CpGs 8, 9–12, 15–17 ↓ mRNA Glut4: ↑ methylation at CpGs 13–14 ↓ mRNA | ||
| Ramon-Krauel et al., 2018 [61] | Mouse (M) 8 vs. 4 pups per litter | At 14 days old: ↑ BW, food intake ↑ eWAT ↔ Plasma glucose, insulin, TGs; ↑ Plasma NEFA | Liver Mogat1: ↔ methylation at CpGs 1–21 ↓ mRNA |
| At 6 months old: (At 4 months-old): ↑ Plasma insulin, TGs, liver TGs content; ↔ Plasma glucose, NEFA | Liver Mogat1: ↔ methylation at CpGs 1–21 ↑ enrichment H3K4me3, H3K9ac ↑ mRNA | ||
| Li et al., 2013 [30] Li et al., 2019 [62] | Mouse (F & M) 9 vs. 4 pups per litter | At 21–25 days old: ↑ BW, adiposity (F & M) | Hypothalamus Aqp14: ↑ methylation (p = 0.06, F only), but no change in mRNA Nolz1: ↑ methylation (p = 0.07, F only), but no change in mRNA Gadd45b: ↑ methylation (M only) ↓ mRNA Pancreas (only M studied): ↑ global genomic methylation Akt1: ↑ methylation Cacna1i: ↑ methylation Scn10a: ↑ methylation |
| At 6 months old: ↑ BW, adiposity (F & M) ↓ Energy expenditure (F only) | Hypothalamus Aqp14: ↑ methylation (p = 0.06, F only), but no change in mRNA Nolz1: ↑ methylation (p = 0.07, F only), but no change in mRNA Gadd45b: ↑ methylation (M only) ↓ mRNA Pancreas (only males studied) ↑ global genomic methylation Akt1: ↔ methylation Cacna1i: ↑ methylation Scn10a: ↑ methylation | ||
| Artificial Rearing | |||
| Mahmood et al., 2013 [63] | Rat (F) HC artificial formula (56% carbohydrate, 20% fat, 24% protein in kcal) vs. maternal rearing (maternal milk: 8% carbohydrate, 68% fat, 24% protein in kcal) | At 16 days old: ↔ BW ↑ Plasma insulin ↓ Plasma leptin | Hypothalamus Pomc: ↔ methylation at CpGs 2–23 ↓ H3K9ac enrichment ↔ H3K9me2 enrichment ↓ mRNA Npy: ↑ methylation at CpG21 ↓ mean methylation at CpGs 1–24 (p = 0.06) ↑ H3K9ac enrichment ↔ H3K9me2 enrichment ↑ mRNA |
| At 3 months old: ↑ BW ↑ Plasma insulin, leptin | Hypothalamus Pomc: ↔ methylation CpGs 2–23 ↔ mRNA Npy: ↑ methylation CpG 21 ↓ methylation CpGs 1–2, 16–17, 20, 24 ↑ mRNA | ||
| Raychaudhuri et al., 2014 [64] | Rat (M) HC artificial formula (56% carbohydrate, 20% fat, 24% protein in kcal) vs. maternal rearing (maternal milk: 8% carbohydrate, 68% fat, 24% protein in kcal) | At 12 days old: ↓ Plasma TSH, T4 | Muscle Glut4: ↔ mRNA |
| At 3 months old: ↓ Plasma TSH | Muscle Glut4: ↑ mean CpG methylation ↑ DNMT3b (CpG1-2), DNMT3a (CpG3) binding ↓ TR, SRC-1 and CBP binding ↑ MeCP2, HDAC4 binding ↓ H4K16ac enrichment ↓ mRNA | ||
| Maternal Nutrition | |||
| Liang et al., 2016 [65] | Mouse (M) Maternal HF (60% kcal fat) vs. C (10% kcal fat) diet6 pups per litter | At 21 days old: ↑ BW ↑ eWAT, iWAT, BAT ↑ Plasma glucose, TGs | BAT Ucp1: ↑ PPARa binding (ChIP) ↑ mRNA |
| At 4 months old: ↑ BW ↑ eWAT, iWAT, BAT Insulin resistance, glucose intolerance | BAT Ucp1: ↓ mRNA | ||
| Butruille et al., 2019 [66] | Rat (M) Maternal HF (60% kcal fat) vs. C (10% kcal fat) diet 8 pups per litter | At 12 days-old: ↑ BW, eWAT, iWAT (hypertrophy) ↔ Plasma glucose, TGs ↑ Plasma leptin, insulin (p = 0.08) | eWAT Scd1: ↓ methylation CpG33 (p = 0.08) ↓ mRNA |
| At 6 month-old: ↑ BW, eWAT (hyperplasia) ↔ iWAT ↔ Plasma glucose, insulin, leptin ↑ Plasma adiponectin (p = 0.08) | eWAT Scd1: ↓ methylation CpG33 ↑ PPARg binding (ChIP) ↑ mRNA | ||
| Neonatal Supplementation | |||
| Palou et al., 2011 [67] Picó et al., 2007 [68] | Rat (M) Daily leptin gavage (5 fold BM physiological dose) vs. C gavage (water) from postnatal day 1 to 20 | At 6 months old (adult C or HF diet): ↓ BW, food intake (postnatal leptin protective effect against age-induced and HF-induced obesity) ↔ Plasma glucose, insulin, leptin, ghrelin | Hypothalamus Pomc: ↓ methylation CpG6 ↓ mRNA But for offspring exposed to HF during adulthood: ↑ methylation CpG6 ↑ mRNA LepR: ↔ methylation CpGs 1–19 ↔ mRNA Socs3: ↔ methylation CpGs 1–13 ↔ mRNA |
| Arreguín et al., 2018 [69] Granados et al., 2012 [70] | Rat (M) Daily retinyl ester gavage (3–5 fold BM physiological dose) vs. C gavage (olive oil) from postnatal day 1 to 20 | At 21 days old: ↔ BW, fat mass ↑ iWAT proportions of small adipocytes (At 4.5 months old: ↑ iWAT, eWAT mass following HF diet during adulthood) | iWAT Pparg2: ↑ methylation CpGs 1–4 ↓ mRNA Rbp4: ↔ methylation CpGs 1–9/13–37 ↓ mRNA Zfp423: ↓ methylation CpGs 1–3 ↑ mRNA Pcna: ↓ methylation CpGs 1–17 ↔ mRNA |
| Rat (M) Daily β-carotene gavage (3–5 fold BM physiological dose) vs. C gavage (olive oil) from postnatal day 1 to 20 | At 21 days old: (NA) | iWAT Pparg2: ↔ methylation CpGs 1–4 ↔ mRNA Rbp4: ↓ methylation CpGs 1–9/13–37 ↔ mRNA Zfp423: ↓ methylation CpGs 1–3 ↑ mRNA Pcna: ↑ methylation CpGs 1–17 ↔ mRNA | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marousez, L.; Lesage, J.; Eberlé, D. Epigenetics: Linking Early Postnatal Nutrition to Obesity Programming? Nutrients 2019, 11, 2966. https://doi.org/10.3390/nu11122966
Marousez L, Lesage J, Eberlé D. Epigenetics: Linking Early Postnatal Nutrition to Obesity Programming? Nutrients. 2019; 11(12):2966. https://doi.org/10.3390/nu11122966
Chicago/Turabian StyleMarousez, Lucie, Jean Lesage, and Delphine Eberlé. 2019. "Epigenetics: Linking Early Postnatal Nutrition to Obesity Programming?" Nutrients 11, no. 12: 2966. https://doi.org/10.3390/nu11122966
APA StyleMarousez, L., Lesage, J., & Eberlé, D. (2019). Epigenetics: Linking Early Postnatal Nutrition to Obesity Programming? Nutrients, 11(12), 2966. https://doi.org/10.3390/nu11122966






