Modelling the Effects of Beverage Substitution during Adolescence on Later Obesity Outcomes in Early Adulthood: Results from the Raine Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Anthropometrics
2.3. Dietary and Beverage Intake
2.4. Covariates
2.5. Statistical Methods
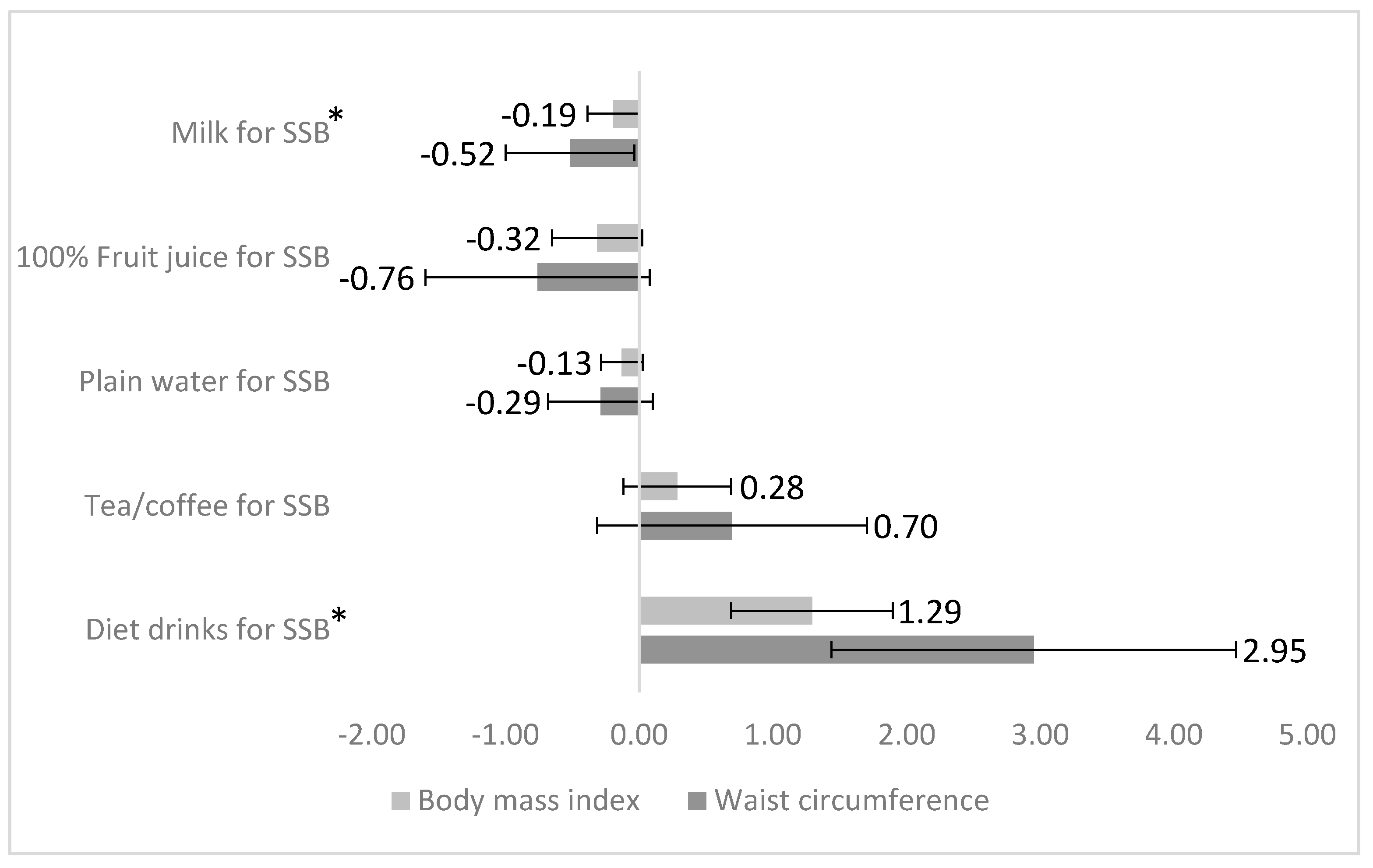
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. (Lond.) 2008, 32, 1431–1437. [Google Scholar] [CrossRef]
- Hill, J.O.; Melanson, E.L. Overview of the determinants of overweight and obesity: Current evidence and research issues. Med. Sci. Sports Exerc. 1999, 31, S515–S521. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Nielsen, S.J. The sweetening of the world’s diet. Obes. Res. 2003, 11, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Massougbodji, J.; Le Bodo, Y.; Fratu, R.; De Wals, P. Reviews examining sugar-sweetened beverages and body weight: Correlates of their quality and conclusions. Am. J. Clin. Nutr. 2014, 99, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organisation: Geneva, The Netherlands, 2003. [Google Scholar]
- World Health Organization. Guideline: Sugar Intake for Adults and Children; World Health Organization: Geneva, The Netherlands, 2015. [Google Scholar]
- Hu, F.B. Resolved: There is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes. Rev. 2013, 14, 606–619. [Google Scholar] [CrossRef]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2013, 346, e7492. [Google Scholar] [CrossRef]
- Bachman, C.M.; Baranowski, T.; Nicklas, T.A. Is there an association between sweetened beverages and adiposity? Nutr. Rev. 2006, 64, 153–174. [Google Scholar] [CrossRef]
- Keller, A.; Bucher Della Torre, S. Sugar-Sweetened Beverages and Obesity among Children and Adolescents: A Review of Systematic Literature Reviews. Childhood obesity (Print) 2015, 11, 338–346. [Google Scholar] [CrossRef]
- Woodward-Lopez, G.; Kao, J.; Ritchie, L. To what extent have sweetened beverages contributed to the obesity epidemic? Public Health Nutr. 2011, 14, 499–509. [Google Scholar] [CrossRef]
- Nissinen, K.; Mikkila, V.; Mannisto, S.; Lahti-Koski, M.; Rasanen, L.; Viikari, J.; Raitakari, O.T. Sweets and sugar-sweetened soft drink intake in childhood in relation to adult BMI and overweight. The Cardiovascular Risk in Young Finns Study. Public Health Nutr. 2009, 12, 2018–2026. [Google Scholar] [CrossRef]
- Striegel-Moore, R.H.; Thompson, D.; Affenito, S.G.; Franko, D.L.; Obarzanek, E.; Barton, B.A.; Schreiber, G.B.; Daniels, S.R.; Schmidt, M.; Crawford, P.B. Correlates of beverage intake in adolescent girls: The National Heart, Lung, and Blood Institute Growth and Health Study. J. Pediatr. 2006, 148, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Rangan, A.; Olsen, N.J.; Bo Andersen, L.; Wedderkopp, N.; Kristensen, P.; Grontved, A.; Ried-Larsen, M.; Lempert, S.M.; Allman-Farinelli, M.; et al. Sugar-sweetened beverages consumption in relation to changes in body fatness over 6 and 12 years among 9-year-old children: the European Youth Heart Study. Eur. J. Clin. Nutr. 2014, 68, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.M.; Cole, T.J. Who changes body mass between adolescence and adulthood? Factors predicting change in BMI between 16 year and 30 years in the 1970 British Birth Cohort. Int. J. Obes. (Lond.) 2006, 30, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Allman-Farinelli, M.; Heitmann, B.L.; Rangan, A. Substitution of sugar-sweetened beverages with other beverage alternatives: a review of long-term health outcomes. J. Acad. Nutr. Diet. 2015, 115, 767–779. [Google Scholar] [CrossRef]
- Langley-Evans, S. Nutrition: A Lifespan Approach; Wiley-Blackwell: Chichester, UK, 2009. [Google Scholar]
- Straker, L.; Mountain, J.; Jacques, A.; White, S.; Smith, A.; Landau, L.; Stanley, F.; Newnham, J.; Pennell, C.; Eastwood, P. Cohort Profile: The Western Australian Pregnancy Cohort (Raine) Study-Generation 2. Int. J. Epidemiol. 2017, 46, 1384–1385j. [Google Scholar] [CrossRef]
- Straker, L.M.; Hall, G.L.; Mountain, J.; Howie, E.K.; White, E.; McArdle, N.; Eastwood, P.R.; Raine Study 22 Year Follow-Up Investigator, G. Rationale, design and methods for the 22 year follow-up of the Western Australian Pregnancy Cohort (Raine) Study. BMC Public Health 2015, 15, 663. [Google Scholar] [CrossRef]
- Newnham, J.P.; Evans, S.F.; Michael, C.A.; Stanley, F.J.; Landau, L.I. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet 1993, 342, 887–891. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatric obesity 2012, 7, 284–294. [Google Scholar] [CrossRef]
- Ambrosini, G.L.; Oddy, W.H.; Robinson, M.; O’Sullivan, T.A.; Hands, B.P.; de Klerk, N.H.; Silburn, S.R.; Zubrick, S.R.; Kendall, G.E.; Stanley, F.J.; et al. Adolescent dietary patterns are associated with lifestyle and family psycho-social factors. Public Health Nutr. 2009, 12, 1807–1815. [Google Scholar] [CrossRef]
- Ambrosini, G.L.; de Klerk, N.H.; O’Sullivan, T.A.; Beilin, L.J.; Oddy, W.H. The reliability of a food frequency questionnaire for use among adolescents. Eur. J. Clin. Nutr. 2009, 63, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.L.; Huang, R.C.; Mori, T.A.; Hands, B.P.; O’Sullivan, T.A.; de Klerk, N.H.; Beilin, L.J.; Oddy, W.H. Dietary patterns and markers for the metabolic syndrome in Australian adolescents. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 274–283. [Google Scholar] [CrossRef]
- Ambrosini, G.L.; Oddy, W.H.; Huang, R.C.; Mori, T.A.; Beilin, L.J.; Jebb, S.A. Prospective associations between sugar-sweetened beverage intakes and cardiometabolic risk factors in adolescents. Am. J. Clin. Nutr. 2013, 98, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: Basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1119–1130. [Google Scholar] [CrossRef]
- Hands, B.P.; Chivers, P.T.; Parker, H.E.; Beilin, L.; Kendall, G.; Larkin, D. The associations between physical activity, screen time and weight from 6 to 14 yrs: the Raine Study. J. Sci. Med. Sport 2011, 14, 397–403. [Google Scholar] [CrossRef]
- Willett, W.C. Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Stookey, J.D.; Constant, F.; Gardner, C.D.; Popkin, B.M. Replacing Sweetened Caloric Beverages with Drinking Water Is Associated with Lower Energy Intake. Obesity 2007, 15, 3013–3022. [Google Scholar] [CrossRef]
- Fiorito, L.; Marini, M.; Francis, L.; Smiciklas-Wright, H.; Birch, L. Beverage intake of girls at age 5 y predicts adiposity and weight status in childhood and adolescence. Am. J. Clin. Nutr. 2009, 90, 935–942. [Google Scholar] [CrossRef]
- Berkey, C.S.; Rockett, H.R.; Field, A.E.; Gillman, M.W.; Colditz, G.A. Sugar-added beverages and adolescent weight change. Obes. Res. 2004, 12, 778–788. [Google Scholar] [CrossRef]
- Warner, M.L.; Harley, K.; Bradman, A.; Vargas, G.; Eskenazi, B. Soda consumption and overweight status of 2-year-old mexican-american children in california. Obesity (Silver Spring) 2006, 14, 1966–1974. [Google Scholar] [CrossRef]
- Zheng, M.; Allman-Farinelli, M.; Heitmann, B.L.; Toelle, B.; Marks, G.; Cowell, C.; Rangan, A. Liquid versus solid energy intake in relation to body composition among Australian children. J. Hum. Nutr. Diet. 2015, 28 (Suppl 2), 70–79. [Google Scholar] [CrossRef]
- Shroff, M.R.; Perng, W.; Baylin, A.; Mora-Plazas, M.; Marin, C.; Villamor, E. Adherence to a snacking dietary pattern and soda intake are related to the development of adiposity: a prospective study in school-age children. Public Health Nutr. 2014, 17, 1507–1513. [Google Scholar] [CrossRef]
- Gopinath, B.; Flood, V.M.; Rochtchina, E.; Baur, L.A.; Louie, J.C.; Smith, W.; Mitchell, P. Carbohydrate nutrition and development of adiposity during adolescence. Obesity (Silver Spring) 2013, 21, 1884–1890. [Google Scholar] [CrossRef]
- Zheng, M.; Rangan, A.; Olsen, N.J.; Andersen, L.B.; Wedderkopp, N.; Kristensen, P.; Grøntved, A.; Ried-Larsen, M.; Lempert, S.M.; Allman-Farinelli, M.; et al. Substituting sugar-sweetened beverages with water or milk is inversely associated with body fatness development from childhood to adolescence. Nutrition 2015, 31, 38–44. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D.; Scharf, R.J.; Demmer, R.T. Sugar-sweetened beverages and weight gain in 2- to 5-year-old children. Pediatrics 2013, 132, 413–420. [Google Scholar] [CrossRef]
- Pan, L.; Li, R.; Park, S.; Galuska, D.A.; Sherry, B.; Freedman, D.S. A longitudinal analysis of sugar-sweetened beverage intake in infancy and obesity at 6 years. Pediatrics 2014, 134 (Suppl 1), S29–S35. [Google Scholar] [CrossRef]
- Zheng, M.; Rangan, A.; Allman-Farinelli, M.; Rohde, J.F.; Olsen, N.J.; Heitmann, B.L. Replacing sugary drinks with milk is inversely associated with weight gain among young obesity-predisposed children. Br. J. Nutr. 2015, 114, 1448–1455. [Google Scholar] [CrossRef]
- Soares, M.J.; Chan She Ping-Delfos, W.; Ghanbari, M.H. Calcium and vitamin D for obesity: a review of randomized controlled trials. Eur. J. Clin. Nutr. 2011, 65, 994–1004. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Rumbold, P.L.; Stevenson, E.J. Effect of calcium intake on fat oxidation in adults: a meta-analysis of randomized, controlled trials. Obes. Rev. 2012, 13, 848–857. [Google Scholar] [CrossRef]
- Anderson, G.H.; Luhovyy, B.; Akhavan, T.; Panahi, S. Milk proteins in the regulation of body weight, satiety, food intake and glycemia. Nestle Nutr. Workshop Ser. Pediatr. Program. 2011, 67, 147–159. [Google Scholar] [CrossRef]
- Lowen, T. Sweet debate: do artificial sweeteners contribute to rather than combat obesity? Minn. Med. 2011, 94, 20–23. [Google Scholar]
- Swithers, S.E.; Sample, C.H.; Davidson, T.L. Adverse effects of high-intensity sweeteners on energy intake and weight control in male and obesity-prone female rats. Behav. Neurosci. 2013, 127, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M. Diet beverages and the risk of obesity, diabetes, and cardiovascular disease: a review of the evidence. Nutr. Rev. 2013, 71, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Committee on Nutrition. American Academy of Pediatrics: The use and misuse of fruit juice in pediatrics. Pediatrics 2001, 107, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Eat For Health: Dietary Guidelines; Commonwealth of Australia: Canberra, Australia, 2013.
- US Department of Agriculture; US Department of Health and Human Services. Dietary Guidelines for Americans, 2010; US Government Printing Office: Washington, DC, USA, 2010.
- Muckelbauer, R.; Sarganas, G.; Gruneis, A.; Muller-Nordhorn, J. Association between water consumption and body weight outcomes: a systematic review. Am. J. Clin. Nutr. 2013, 98, 282–299. [Google Scholar] [CrossRef]
- Muckelbauer, R.; Barbosa, C.L.; Mittag, T.; Burkhardt, K.; Mikelaishvili, N.; Muller-Nordhorn, J. Association between water consumption and body weight outcomes in children and adolescents: a systematic review. Obesity (Silver Spring) 2014, 22, 2462–2475. [Google Scholar] [CrossRef]
- Bhatti, S.K.; O’Keefe, J.H.; Lavie, C.J. Coffee and tea: perks for health and longevity? Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 688–697. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S. Green tea catechins, caffeine and body-weight regulation. Physiol. Behav. 2010, 100, 42–46. [Google Scholar] [CrossRef]
- Subar, A.F.; Freedman, L.S.; Tooze, J.A.; Kirkpatrick, S.I.; Boushey, C.; Neuhouser, M.L.; Thompson, F.E.; Potischman, N.; Guenther, P.M.; Tarasuk, V.; et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. J. Nutr. 2015, 145, 2639–2645. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Grant, S.W.; Takkenberg, J.J.M.; Mokhles, M.M. Statistical primer: how to deal with missing data in scientific research? Interact. Cardiovasc. Thorac. Surg. 2018, 27, 153–158. [Google Scholar] [CrossRef]
| Sugar-Sweetened Beverage Intake | ||||||
|---|---|---|---|---|---|---|
| Non-Consumer (n = 40) | ≤1 Serving (n = 363) | >1 Serving (n = 264) | ||||
| Continuous variables | Mean | SD | Mean | SD | Mean | SD |
| Age (years) | 14.0 | 0.2 | 14.0 | 0.2 | 14.0 | 0.2 |
| † Body mass index (kg/m2) | 21.1 | 3.9 | 20.6 | 3.4 | 21.7 | 4.4 |
| † Waist circumference (cm) | 74.1 | 10.0 | 73.6 | 9.2 | 76.7 | 11.5 |
| † Plain water (g) | 785.2 | 608.4 | 809.8 | 521.9 | 649.2 | 545.2 |
| Tea/coffee (g) | 38.1 | 84.7 | 46.7 | 109.1 | 41.0 | 78.3 |
| † Diet drinks (g) | 50.1 | 122.0 | 15.0 | 48.6 | 17.8 | 63.3 |
| 100% fruit juice (g) | 89.5 | 118.1 | 92.1 | 117.6 | 89.4 | 132.5 |
| Milk (g) | 398.9 | 332.1 | 398.2 | 320.5 | 387.3 | 311.1 |
| † Total energy intake (MJ) | 8.4 | 2.2 | 8.9 | 2.7 | 10.3 | 3.1 |
| † Healthy dietary pattern z-score | 0.3 | 0.8 | 0.1 | 0.8 | −0.1 | 0.8 |
| † Western dietary pattern z-score | −0.7 | 0.5 | −0.3 | 0.7 | 0.3 | 0.9 |
| Categorical variables | n (%) | n (%) | n (%) | |||
| † Overweight or obese | 5 (12.5) | 34 (9.4) | 48 (18.2) | |||
| Sex | ||||||
| Males | 20 (50.0) | 175 (48.2) | 123 (46.6) | |||
| Females | 20 (50.0) | 188 (51.8) | 141 (53.4) | |||
| † Dietary misreporting | ||||||
| Under-reporters | 14 (35.0) | 107 (29.5) | 59 (22.3) | |||
| Plausible reporters | 25 (62.5) | 229 (63.1) | 162 (61.4) | |||
| Over-reporters | 1 (2.5) | 27 (7.4) | 43 (16.3) | |||
| Physical activity | ||||||
| Low active | 10 (25.0) | 158 (43.5) | 110 (41.8) | |||
| Active | 30 (75.0) | 205 (56.5) | 154 (58.2) | |||
| † Maternal education | ||||||
| High | 33 (82.5) | 267 (74.1) | 164 (62.1) | |||
| Low | 7 (17.5) | 94 (25.9) | 100 (37.9) | |||
| † Family income (rank) | ||||||
| 0 | 9 (22.5) | 77 (21.2) | 78 (29.5) | |||
| 1 | 13 (32.5) | 95 (26.2) | 89 (33.7) | |||
| 2 | 5 (12.5) | 98 (27.0) | 56 (21.2) | |||
| 3 | 13 (32.5) | 93 (25.6) | 41 (15.5) | |||
| BMI | WC | Overweight or Obesity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | OR | 95% CI | P | |
| Continuous variable (100 g/day) | |||||||||
| Crude model | 0.21 | (0.08, 0.33) | 0.001 | 0.41 | (0.09, 0.73) | 0.01 | 1.08 | (1.02, 1.14) | 0.01 |
| Model 1 | 0.19 | (0.04, 0.33) | 0.01 | 0.41 | (0.04, 0.78) | 0.03 | 1.10 | (1.02, 1.18) | 0.01 |
| Model 2 | 0.16 | (0.01, 0.30) | 0.04 | 0.34 | (−0.04, 0.71) | 0.08 | 1.10 | (1.02, 1.18) | 0.01 |
| Categorical variable | |||||||||
| Crude | |||||||||
| Non-consumer | ref | ||||||||
| ≤1 serve | −0.18 | (−1.72, 1.37) | 0.82 | −1.34 | (−5.27, 2.60) | 0.51 | 0.92 | (0.43, 1.98) | 0.83 |
| >1 serve | 1.69 | (1.21, 3.25) | 0.04 | 2.68 | (−1.32, 6.67) | 0.19 | 1.77 | (0.81, 3.84) | 0.15 |
| Model 1 | |||||||||
| Non-consumer | ref | ||||||||
| ≤1 serve | 0.32 | (−1.27, 1.90) | 0.70 | 0.39 | (−3.49, 4.27) | 0.84 | 0.96 | (0.41, 2.25) | 0.92 |
| >1 serve | 2.00 | (0.32, 3.67) | 0.02 | 4.47 | (0.35, 8.58) | 0.03 | 1.87 | (0.76, 4.62) | 0.18 |
| Model 2 | |||||||||
| Non-consumer | ref | ||||||||
| ≤1 serve | 0.41 | (−1.17, 2.00) | 0.61 | 0.35 | (−3.56, 4.26) | 0.86 | 0.93 | (0.41, 2.25) | 0.93 |
| >1 serve | 1.98 | (0.31, 3.64) | 0.02 | 4.09 | (−0.04, 8.22) | 0.05 | 1.84 | (0.75, 4.75) | 0.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Rangan, A.; Huang, R.-C.; Beilin, L.J.; Mori, T.A.; Oddy, W.H.; Ambrosini, G.L. Modelling the Effects of Beverage Substitution during Adolescence on Later Obesity Outcomes in Early Adulthood: Results from the Raine Study. Nutrients 2019, 11, 2928. https://doi.org/10.3390/nu11122928
Zheng M, Rangan A, Huang R-C, Beilin LJ, Mori TA, Oddy WH, Ambrosini GL. Modelling the Effects of Beverage Substitution during Adolescence on Later Obesity Outcomes in Early Adulthood: Results from the Raine Study. Nutrients. 2019; 11(12):2928. https://doi.org/10.3390/nu11122928
Chicago/Turabian StyleZheng, Miaobing, Anna Rangan, Rae-Chi Huang, Lawrence Joseph Beilin, Trevor Anthony Mori, Wendy Hazel Oddy, and Gina Leslie Ambrosini. 2019. "Modelling the Effects of Beverage Substitution during Adolescence on Later Obesity Outcomes in Early Adulthood: Results from the Raine Study" Nutrients 11, no. 12: 2928. https://doi.org/10.3390/nu11122928
APA StyleZheng, M., Rangan, A., Huang, R.-C., Beilin, L. J., Mori, T. A., Oddy, W. H., & Ambrosini, G. L. (2019). Modelling the Effects of Beverage Substitution during Adolescence on Later Obesity Outcomes in Early Adulthood: Results from the Raine Study. Nutrients, 11(12), 2928. https://doi.org/10.3390/nu11122928






