Side Effects Associated with Probiotic Use in Adult Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
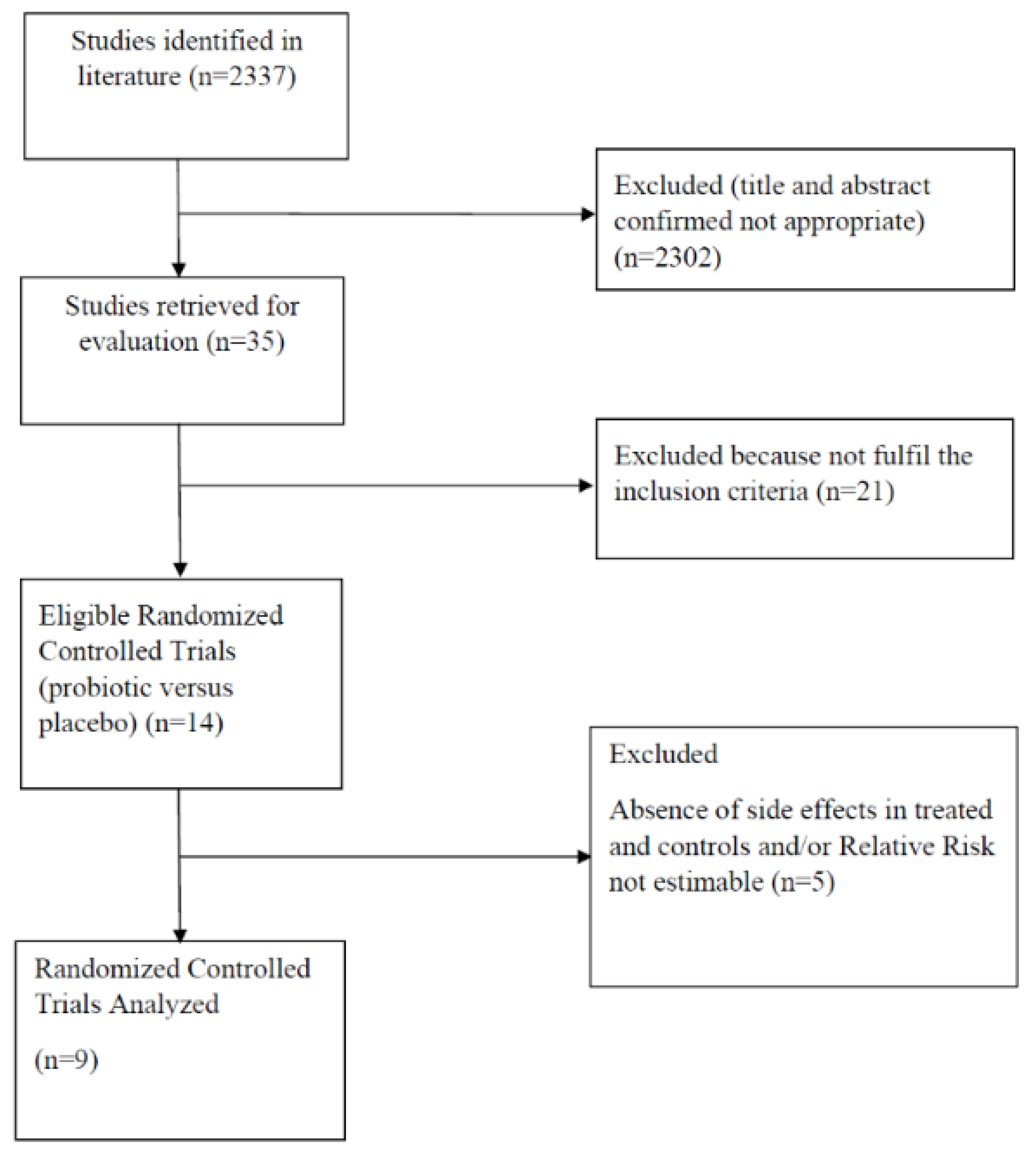
2.1. Search Strategy and Study Selection
2.2. Outcome Assessment
2.3. Data Extraction
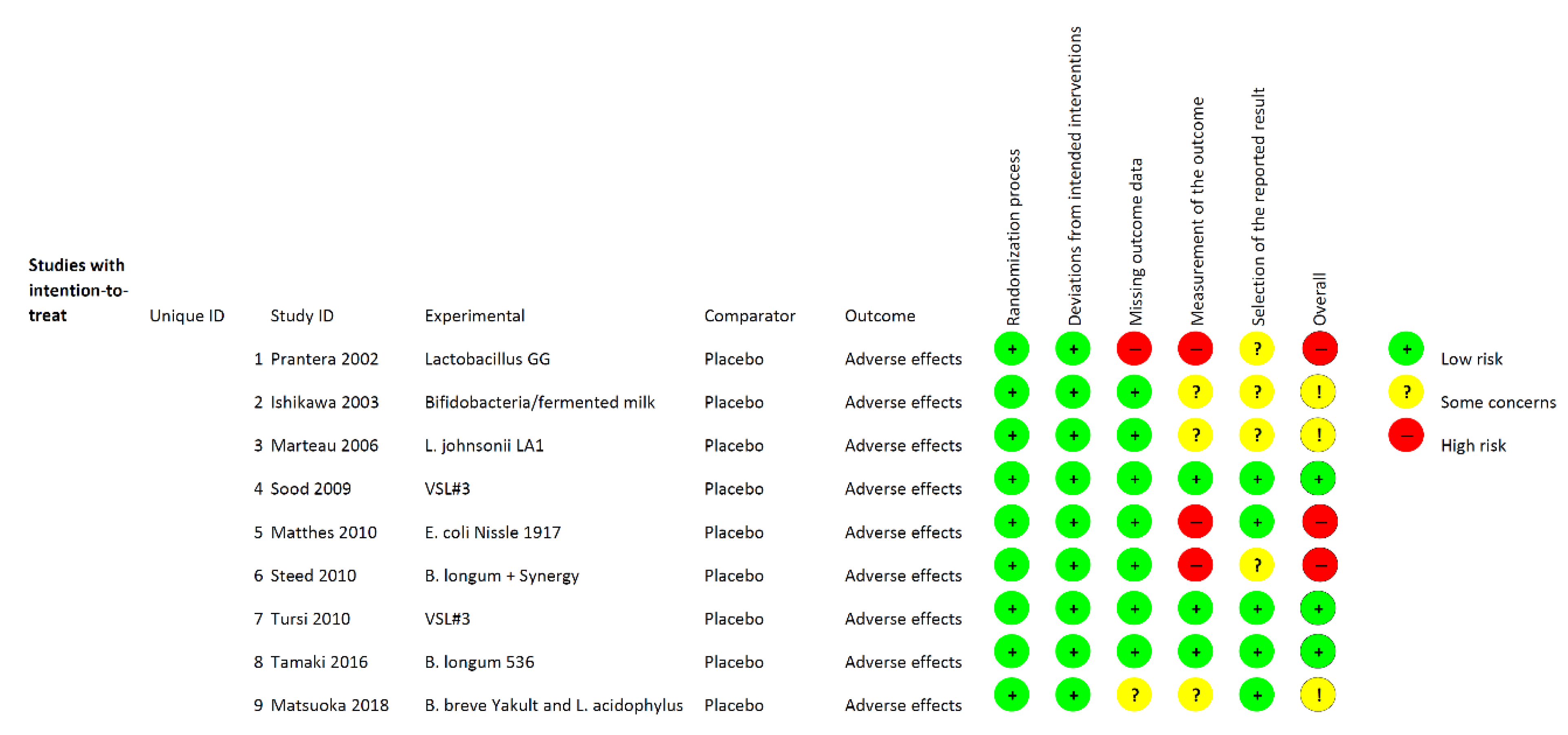
2.4. Data Synthesis and Statistical Analysis
3. Results
3.1. Study Selection and Characteristics
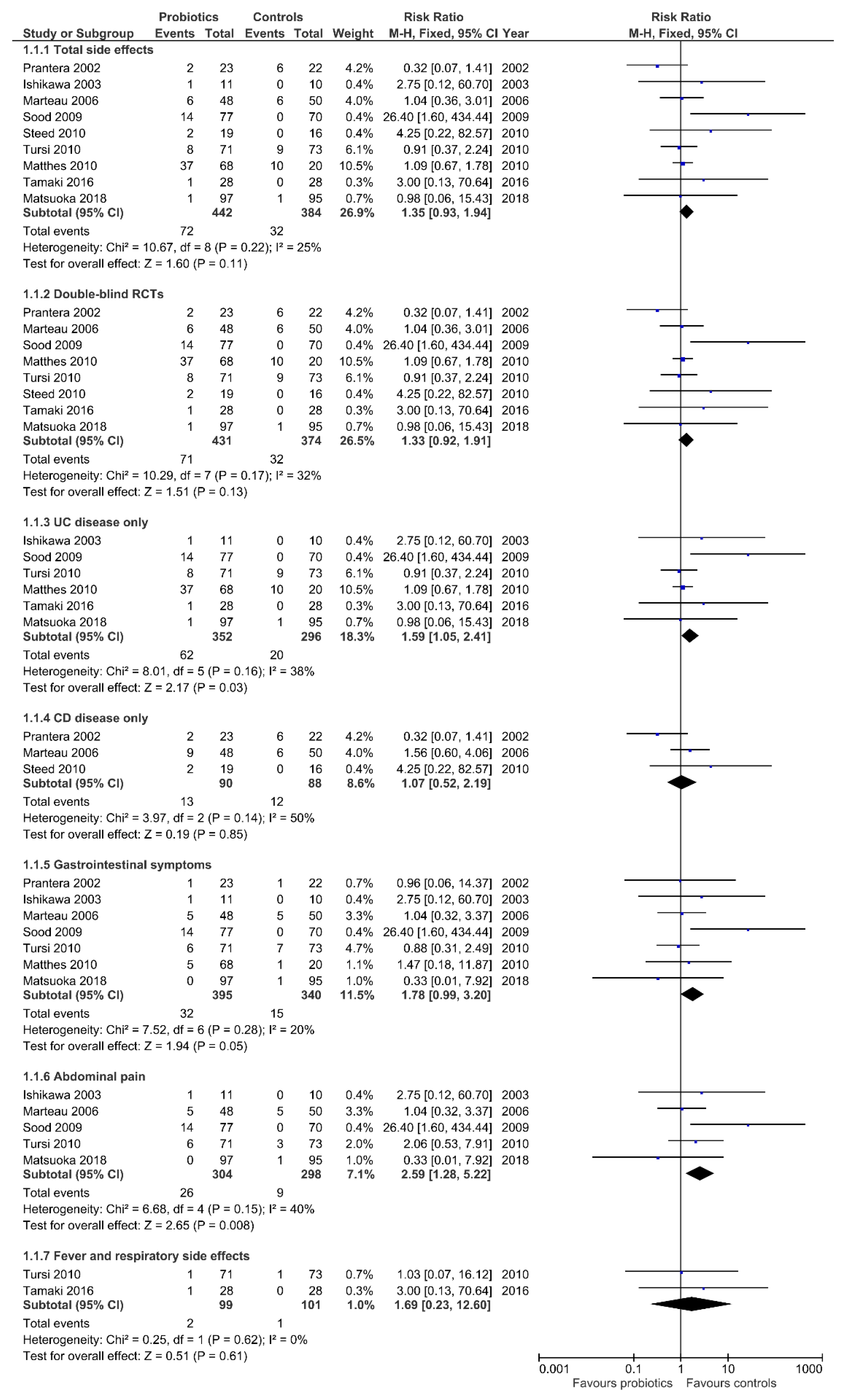
3.2. Total Side Effects
3.3. Side Effects According to IBD Type
3.4. Gastrointestinal Symptoms
3.5. Fever and Respiratory Side Effects
3.6. Excluded Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002, 208, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H. The genetics and immunopathogenesis of inflammatory bowel disease. Nat. Rev. Immunol. 2008, 8, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Stark, P.L.; Lee, A. The microbial ecology of the large bowel of breast‒fed and formula-fed infants during the first year of life. J. Med. Microbiol. 1982, 15, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome, and immune system: Envisioning the future. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.L.; Washington, M.K.; Shi, Y.; Liu, L. Colon-specific delivery of a probiotic‒derived soluble protein ameliorates intestinal inflammation in mice through an EGFR‒dependent mechanism. J. Clin. Investig. 2011, 121, 2242–2253. [Google Scholar] [CrossRef]
- Rembacken, B.J.; Snelling, A.M.; Hawkey, P.M.; Chalmers, D.M.; Axon, A.T. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: A randomised trial. Lancet 1999, 354, 635–639. [Google Scholar] [CrossRef]
- Zocco, M.A.; dal Verme, L.Z.; Cremonini, F.; Piscaglia, A.C.; Nista, E.C.; Candelli, M. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 2006, 23, 1567–1574. [Google Scholar] [CrossRef]
- Miele, E.; Pascarella, F.; Giannetti, E.; Quaglietta, L.; Baldassano, R.N.; Staiano, A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am. J. Gastroenterol. 2009, 104, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Brandimarte, G.; Papa, A.; Giglio, A.; Elisei, W.; Giorgetti, G.M. Treatment of relapsing mild to moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: A double-blind, randomized, placebo-controlled study. Am. J. Gastroenterol. 2010, 105, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Oliva, S.; Di Nardo, G.; Ferrari, F.; Mallardo, S.; Rossi, P.; Patrizi, G. Randomised clinical trial: The effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment. Pharmacol. Ther. 2012, 35, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Derwa, Y.; Gracie, D.J.; Hamlin, P.J.; Ford, A.C. Systematic review with meta‒analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Steed, H.; Macfarlane, G.T.; Blackett, K.L.; Bahrami, B.; Reynolds, N.; Walsh, S.V. Clinical trial: The microbiological and immunological effects of synbiotic consumption—A randomized double-blind placebo controlled study in active Crohn’s disease. Aliment. Pharmacol. Ther. 2010, 32, 872–883. [Google Scholar] [CrossRef]
- Kazemi, A.; Soltani, S.; Ghorabi, S.; Keshtkar, A.; Daneshzad, E.; Nasri, F.; Mazloomi, S.M. Effect of probiotic and synbiotic supplementation on inflammatory markers in health and disease status: A systematic review and meta-analysis of clinical trials. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Dore, M.P.; Rocchi, C.; Longo, N.P.; Scanu, A.M.; Vidili, G.; Padedda, F. Effect of probiotic use on adverse events in adult patients with inflammatory bowel disease: A retrospective cohort study. Probiotics Antimicrob. Proteins 2019. [Google Scholar] [CrossRef]
- Generally Recognized as Safe (GRAS). Available online: http://www.fda.gov/food/IngredientspackagingLabeling/GRAS/ (accessed on 15 September 2019).
- U.S. Food and Drug Administration. Available online: http://www.fda.gov/ohrms/dockets/dockets/95s0316/95s-0316-rpt0282-tab-03-ref-19-joint-faowho-vol219.pdf (accessed on 15 September 2019).
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60, S129–S134. [Google Scholar] [CrossRef]
- Hempel, S.; Newberry, S.; Ruelaz, A.; Wang, Z.; Miles, J.N.; Suttorp, M.J. Safety of probiotics used to reduce risk and prevent or treat disease. Evid. Rep. Technol. Assess. 2011, 200, 1–645. [Google Scholar]
- International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/PROSPERO (accessed on 15 September 2019).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 15 September 2019).
- Bax, L.; Yu, L.M.; Ikeda, N.; Moons, K.G. A systematic comparison of software dedicated to meta-analysis of causal studies. BMC Med. Res. Methodol. 2007, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions: Version 5.1.0. 2011. Available online: http://handbook.cochrane.org/ (accessed on 15 September 2019).
- Kato, K.; Mizuno, S.; Umesaki, Y.; Ishii, Y.; Sugitani, M.; Imaoka, A. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment. Pharmacol. Ther. 2004, 20, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Furrie, E.; Macfarlane, S.; Kennedy, A.; Cummings, J.H.; Walsh, S.V.; O’neil, D.A. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005, 54, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Matsumoto, S.; Ohashi, Y.; Imaoka, A.; Setoyama, H.; Umesaki, Y. Beneficial effects of probiotic Bifidobacterium and galacto-oligosaccharide in patients with ulcerative colitis: A randomized controlled study. Digestion 2011, 84, 128–133. [Google Scholar] [CrossRef]
- Wildt, S.; Nordgaard, I.; Hansen, U.; Brockmann, E.; Rumessen, J.J. A randomized double blind placebo-controlled trial with Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. lactis BB-12 for maintenance of remission in ulcerative colitis. J. Crohns. Colitis. 2001, 5, 115–121. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Yamada, A.; Furukawa, R.; Sono, K.; Osamura, A.; Nakamura, K. Effectiveness of probiotic therapy for the prevention of relapse in patients with inactive ulcerative colitis. World J. Gastroenterol. 2015, 21, 5985–5994. [Google Scholar] [CrossRef]
- Prantera, C.; Scribano, M.L.; Falasco, G.; Andreoli, A.; Luzi, C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: A randomised controlled trial with Lactobacillus GG. Gut 2002, 51, 405–409. [Google Scholar] [CrossRef]
- Ishikawa, H.; Akedo, I.; Umesaki, Y.; Tanaka, R.; Imaoka, A.; Otani, T. Randomized controlled trial of the effect of Bifidobacteria-fermented milk on ulcerative colitis. J. Am. Coll. Nutr. 2003, 22, 56–63. [Google Scholar] [CrossRef]
- Marteau, P.; Lémann, M.; Seksik, P.; Laharie, D.; Colombel, J.F.; Bouhnik, Y. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn’s disease: A randomised, double blind, placebo controlled GETAID trial. Gut 2006, 55, 842–847. [Google Scholar] [CrossRef]
- Sood, A.; Midha, V.; Makharia, G.K.; Ahuja, V.; Singal, D.; Goswami, P. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin. Gastroenterol. Hepatol. 2009, 7, 1202–1209. [Google Scholar] [CrossRef]
- Matthes, H.; Krummenerl, T.; Giensch, M.; Wolff, C.; Schulze, J. Clinical trial: Probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN). BMC Complement. Altern. Med. 2010, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, H.; Nakase, H.; Inoue, S.; Kawanami, C.; Itani, T.; Ohana, M. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Dig. Endosc. 2016, 28, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Uemura, Y.; Kanai, T.; Kunisaki, R.; Suzuki, Y.; Yokoyama, K. Efficacy of Bifidobacterium breve fermented milk in maintaining remission of ulcerative colitis. Dig. Dis. Sci. 2018, 63, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001, 291, 881–884. [Google Scholar] [CrossRef]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef]
- Satta, R.; Pes, G.M.; Rocchi, C.; Pes, M.C.; Dore, M.P. Is probiotic use beneficial for skin lesions in patients with inflammatory bowel disease? J. Dermatolog. Treat. 2019, 30, 612–616. [Google Scholar] [CrossRef]
- Patton, T.J.; Guandalini, S. Are probiotic effects dose‒related. In World Review of Nutrition and Dietetics, Probiotic Bacteria and Their Effect on Human Health and Wellbeing; Guarino, A., Quigley, E.M.M., Walker, W.A., Eds.; Karger: Basel, Switzerland, 2013; Volume 107, pp. 151–160. [Google Scholar]
- Van Niel, C.W.; Feudtner, C.; Garrison, M.M.; Christakis, D.A. Lactobacillus therapy for acute infectious diarrhea in children: A meta-analysis. Pediatrics 2002, 109, 678–684. [Google Scholar] [CrossRef]
- Mangalat, N.; Liu, Y.; Fatheree, N.Y.; Ferris, M.J.; Van Arsdall, M.R.; Chen, Z. Safety and tolerability of Lactobacillus reuteri DSM 17938 and effects on biomarkers in healthy adults: Results from a randomized masked trial. PLoS ONE 2012, 7, e43910. [Google Scholar] [CrossRef]
- Williams, N.T. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef]
- Karpa, K.D. Probiotics for Clostridium difficile diarrhea: Putting it into perspective. Ann. Pharmacother. 2007, 41, 1284–1287. [Google Scholar] [CrossRef]
- Pessione, E.; Cirrincione, S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef] [PubMed]
- Squires, R.F. Multiple forms of monoamine oxidase in intact mitochondria as characterized by selective inhibitors and thermal stability: A comparison of eight mammalian species. Adv. Biochem. Psychopharmacol. 1972, 5, 355–370. [Google Scholar] [PubMed]
- Minderhoud, I.M.; Oldenburg, B.; Schipper, M.E.; ter Linde, J.J.; Samsom, M. Serotonin synthesis and uptake in symptomatic patients with Crohn’s disease in remission. Clin. Gastroenterol. Hepatol. 2007, 5, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Pugin, B.; Barcik, W.; Westermann, P.; Heider, A.; Wawrzyniak, M.; Hellings, P. A wide diversity of bacteria from the human gut produces and degrades biogenic amines. Microb. Ecol. Health. Dis. 2017, 28, 1353881. [Google Scholar] [CrossRef]
- Bennett, W.E., Jr. Quantitative risk-benefit analysis of probiotic use for irritable bowel syndrome and inflammatory bowel disease. Drug Saf. 2016, 39, 295–305. [Google Scholar] [CrossRef]
| Author, Year and Country | IBD Type | Intervention | No. of Patients | Dose and Duration | Side Effects | Study Design |
|---|---|---|---|---|---|---|
| Prantera et al., 2002 Italy [32] | CD | Lactobacillus GG | 45 | 12 billion CFU/day for 52 weeks | Not described | DB, RCT |
| Ishikawa et al., 2003 Japan [33] | UC | Bifidobacteria-fermented milk (B. breve, and B. bifidum, and L. acidophilus YIT 0168 Yakult) | 21 | at least 10 billion per 100 mL bottle for 1 year | Coryza, Abdominal pain | RCT |
| Kato et al., 2004 Japan [27] | UC | B. bifidum + B. breve Yakult + L. acidophillus | 20 | 10 billion per 100 mL/day for 12 weeks | No side effects in both arms | RCT |
| Furrie et al., 2005 UK [28] | UC | B. longum + Synergy | 18 | 2 × 1011 CFU twice daily + 6 g fructo-oligosaccharide/inulin for 4 weeks. | No side effects in both arms | DB, RCT |
| Marteau et al., 2006 France [34] | CD | L. johnsonii LA1 | 98 | 2 × 109 CFU 2packets/day for 6 months | Digestive disorders | DB, RCT |
| Sood et al., 2009 North India [35] | UC | VSL#3 | 147 | 3600 billion CFU/day for 12 weeks | Abdominal pain, Unpleasant taste | MC, DB, RCT |
| Matthes et al., 2010 Germany [36] | UC | E. coli Nissle 1917 | 90 | 108 viable EcN/mL in 40, or 20 or 10 mL enemas/day for at least 2 weeks | Gastrointestinal disorders, Flatulence | Explorative, MC, DB, RCT |
| Steed et al., 2010 UK [15] | CD | B. longum + Synergy | 35 | 2 × 1011 CFU twice daily + 6 g fructo-oligosaccharide/inulin for 6 months | Not better specified (2 patients unable to tolerate synbiotic) | DB, RCT |
| Tursi et al., 2010 Italy [12] | UC | VSL#3(L. paracasei, L. plantarum, L. acidophilus, and L. delbrueckii subsp bulgaricus B. longum, B. breve, and B. infantis, Streptococcus thermophilus) | 144 | 3600 billion CFU/day for 8 weeks | Abdominal bloating and discomfort, Unpleasant taste, Dizziness, Flu-like symptoms | MC *, DB #, RCT † |
| Wildt et al., 2011 Denmark [30] | UC | L. acidophilus LA-5 + B. animalis subsp. lactis BB-12 | 32 | 1.5 × 1011 CFU/day for 52 weeks | Gastrointestinal symptoms equally reported in both arms | DB, RCT |
| Ishikawa et al., 2011 Japan [29] | UC | B. breve Yakult + GOS | 41 | 109 CFU/g three times daily + 5.5 g of galacto-oligosaccharide once a day for 1 year | diarrhea or abdominal pain. (without given number) | RCT |
| Yoshimatsu et al., 2015 Japan [31] | UC | 1 tablet = Streptococcus faecalis T-110 (2 mg) + Clostridium butyricum TO-A (10 mg) + Bacillus mesentericus TO-A (10 mg) | 60 | 9 tablets daily for 1 year | No side effects in both arms | DB, RCT |
| Tamaki et al., 2016 Japan [37] | UC | B. longum 536 | 56 | 2.3 × 1011 three times daily for 8 weeks | Dry cough | MC, DB, RCT |
| Matsuoka et al., 2018 Japan [38] | UC | B. breve Yakult (10 × 109) + L. acidophilus (109) | 195 | 11 billion/day for 48 weeks | Body odor, Abdominal bloating, Stress | MC, DB, RCT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dore, M.P.; Bibbò, S.; Fresi, G.; Bassotti, G.; Pes, G.M. Side Effects Associated with Probiotic Use in Adult Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 2913. https://doi.org/10.3390/nu11122913
Dore MP, Bibbò S, Fresi G, Bassotti G, Pes GM. Side Effects Associated with Probiotic Use in Adult Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2019; 11(12):2913. https://doi.org/10.3390/nu11122913
Chicago/Turabian StyleDore, Maria Pina, Stefano Bibbò, Gianni Fresi, Gabrio Bassotti, and Giovanni Mario Pes. 2019. "Side Effects Associated with Probiotic Use in Adult Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 11, no. 12: 2913. https://doi.org/10.3390/nu11122913
APA StyleDore, M. P., Bibbò, S., Fresi, G., Bassotti, G., & Pes, G. M. (2019). Side Effects Associated with Probiotic Use in Adult Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 11(12), 2913. https://doi.org/10.3390/nu11122913








