High Hydration Factor in Older Hispanic-American Adults: Possible Implications for Accurate Body Composition Estimates
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Design
2.3. Anthropometry
2.4. Body Composition and HF Measurements
2.5. Hydration Factor
2.6. Obesity Classification
2.7. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- He, W.; Goodkind, D.; Kowal, P.R. An Aging World: 2015; U.S. Census Bureau: Washington, DC, USA, 2016.
- Pencharz, P.B.; Azcue, M. Use of bioelectrical impedance analysis measurements in the clinical management of malnutrition. Am. J. Clin. Nutr. 1996, 64, 485S–488S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deurenberg, P.; Wang, W.; Pietrobelli, A.; Baumgartner, R.N.; Heymsfield, S.B. Hydration of fat-free body mass: New physiological modeling approach. Am. J. Physiol. Endocrinol. Metab. 1999, 276, E995–E1003. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H.H.; Hamilton, T.S.; Steggerda, F.R.; Bean, H.W. The chemical composition of the adult human body and its bearing on the biochemistry of growth. J. Biol. Chem. 1945, 158, 625–637. [Google Scholar]
- Widdowson, E.M.; McCance, E.A.; Spray, C.M. The chemical composition of the human body. Clin. Sci. 1951, 10, 113–125. [Google Scholar] [PubMed]
- Forbes, R.M.; Cooper, A.R.; Mitchell, H.H. The composition of the adult human body as determined by chemical analysis. J. Biol. Chem. 1953, 203, 359–366. [Google Scholar] [PubMed]
- Forbes, G.B.; Lewis, A.M. Total sodium, potassium and chloride in adult man. J. Clin. Investig. 1956, 35, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Knight, G.S.; Beddoe, A.H.; Streat, S.J.; Hill, G.L. Body composition of two human cadavers by neutron activation and chemical analysis. Am. J. Physiol. 1986, 250, E179–E185. [Google Scholar] [CrossRef] [PubMed]
- Moore, F.D. Determination of total body water and solids with isotopes. Science 1946, 104, 157–160. [Google Scholar] [CrossRef]
- Ritz, P. Body water spaces and cellular hydration during healthy aging. Ann. N. Y. Acad. Sci. 2000, 904, 474–483. [Google Scholar] [CrossRef]
- Sheng, H.-P.; Huggins, R.A. A review of body composition studies with emphasis on total body water and fat. Am. J. Clin. Nutr. 1979, 32, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Clarys, J.P.; Martin, A.D.; Marfell-Jones, M.J.; Janssens, V.; Caboor, D.; Drinkwater, D.T. Human body composition: A review of adult dissection data. Am. J. Hum. Biol. 1999, 11, 167–174. [Google Scholar] [CrossRef]
- IAEA. Introduction to Body Composition Assessment Using the Deuterium Dilution Technique with Analysis of Saliva Samples by Fourier Transform Infrared Spectrometry; International Atomic Energy Agency: Vienna, Austria, 2013; Volume 12. [Google Scholar]
- Cohn, S.H.; Vaswani, A.N.; Yasumura, S.; Yuen, K.; Ellis, K.J. Improved models for determination of body fat by in vivo neutron activation. Am. J. Clin. Nutr. 1984, 40, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, D.A. Changes in total body water with age. Am. J. Clin. Nutr. 1989, 50, 1176–1181. [Google Scholar] [CrossRef]
- Fuller, N.J.; Sawyer, M.B.; Elia, M. Comparative evaluation of body composition methods and predictions, and calculation of density and hydration fraction of fat-free mass, in obese women. Int. J. Obes. Relat. Metab. Disord. 1994, 18, 503. [Google Scholar]
- Das, S.K. Body composition measurement in severe obesity. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 602–606. [Google Scholar] [CrossRef]
- Haroun, D.; Wells, J.C.K.; Williams, J.E.; Fuller, N.J.; Fewtrell, M.S.; Lawson, M.S. Composition of the fat-free mass in obese and nonobese children: Matched case–control analyses. Int. J. Obes. 2005, 29, 29–36. [Google Scholar] [CrossRef]
- Ritz, P.; Vol, S.; Berrut, G.; Tack, I.; Arnaud, M.J.; Tichet, J. Influence of gender and body composition on hydration and body water spaces. Clin. Nutr. 2008, 27, 740–746. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Thomas, R.J.; Collazo-Clavell, M.L.; Korinek, J.; Allison, T.G.; Batsis, J.A.; Sert-Kuniyoshi, F.H.; Lopez-Jimenez, F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int. J. Obes. 2008, 32, 959–966. [Google Scholar] [CrossRef]
- Kelly, T.L.; Wilson, K.E.; Heymsfield, S.B. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009, 4, e7038. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Marfell-Jones, M.J.; Stewart, A.D.; De Ridder, J.H. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Wellington, New Zealand, 2012. [Google Scholar]
- Jennings, G.; Bluck, L.; Wright, A.; Elia, M. The use of infrared spectrophotometry for measuring body water spaces. Clin. Chem. 1999, 45, 1077–1081. [Google Scholar] [PubMed]
- Aleman-Mateo, H.; Huerta, R.H.; Esparza-Romero, J.; Méndez, R.O.; Urquidez, R.; Valencia, M.E. Body composition by the four-compartment model: Validity of the BOD POD for assessing body fat in Mexican elderly. Eur. J. Clin. Nutr. 2007, 61, 830. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baumgartner, R.N.; Heymsfield, S.B.; Lichtman, S.; Wang, J.; Pierson, R.N., Jr. Body composition in elderly people: Effect of criterion estimates on predictive equations. Am. J. Clin. Nutr. 1991, 53, 1345–1353. [Google Scholar] [CrossRef]
- Alemán-Mateo, H.; Lee, S.Y.; Javed, F.; Thornton, J.; Heymsfield, S.B.; Pierson, R.N.; Pi-Sunyer, F.X.; Wang, Z.M.; Wang, J.; Gallagher, D. Elderly Mexicans have less muscle and greater total and truncal fat compared to African-Americans and Caucasians with the same BMI. J. Nutr. Health Aging 2009, 13, 919–923. [Google Scholar] [CrossRef]
- Goran, M.I.; Toth, M.J.; Poehlman, E.T. Assessment of research-based body composition techniques in healthy elderly men and women using the 4-compartment model as a criterion method. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 135–142. [Google Scholar] [CrossRef][Green Version]
- Yee, A.J.; Fuerst, T.; Salamone, L.; Visser, M.; Dockrell, M.; Van Loan, M.; Kern, M. Calibration and validation of an air-displacement plethysmography method for estimating percentage body fat in an elderly population: A comparison among compartmental models. Am. J. Clin. Nutr. 2001, 74, 637–642. [Google Scholar] [CrossRef][Green Version]
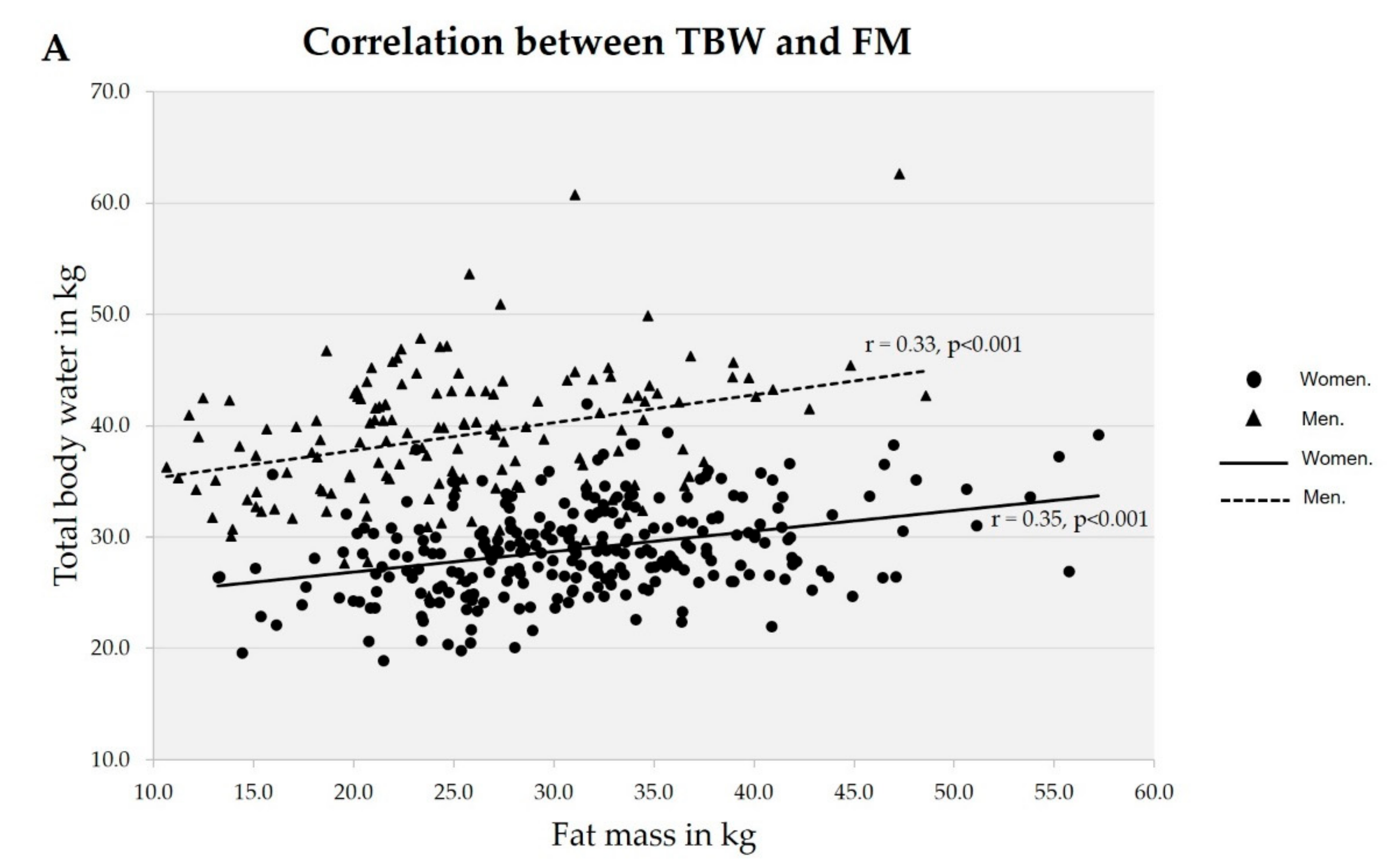
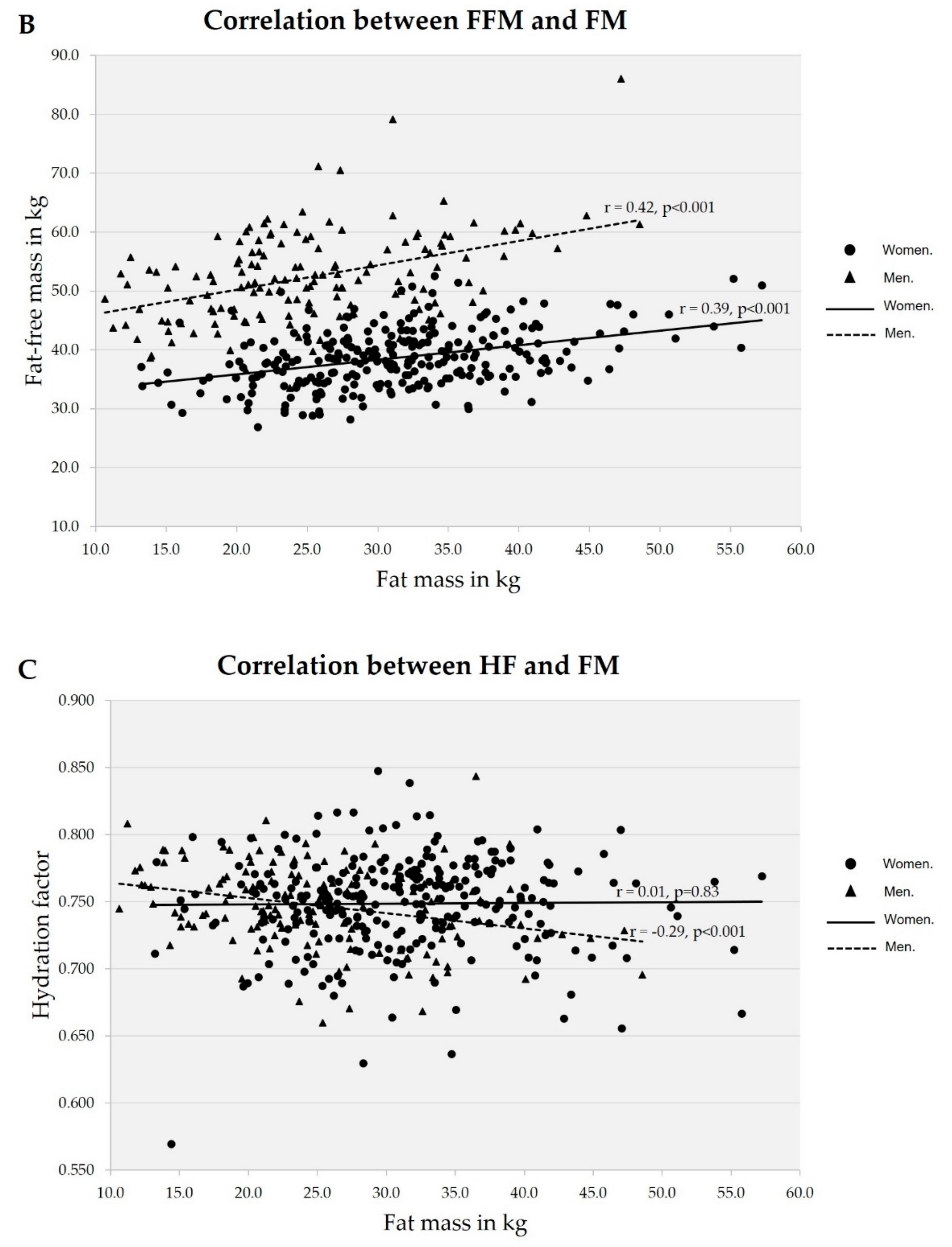
| Variable | Normal | Excess fat | Obesity | Total |
|---|---|---|---|---|
| Men | ||||
| Age, years | 71.3 ± 5.5 a | 67.9 ± 6.1 a | 67.7 ± 5.8 a | 68.3 ± 5.9 |
| Weight, kg | 60.1 ± 5.3 a,* | 75.2 ± 9.5 b,* | 85.7 ± 11.9 c,* | 77.7 ± 13.1 * |
| Height, m | 1.7 ± 0.04 a,* | 1.7 ± 0.07 a,* | 1.7 ± 0.06 a,* | 1.7 ± 0.06 * |
| BMI, kg/m2 | 21.8 ± 1.9 a | 26.3 ± 2.1 b | 29.7 ± 3.2 c | 27.1 ± 3.7 |
| FFM, kg | 47.1 ± 5.3 a,* | 53.1 ± 7.8 b,* | 53.3 ± 7.7 b,* | 52.4 ± 7.7 * |
| TBW, kg | 35.7 ± 3.73 a,* | 39.9 ± 6.0 b,* | 39.3 ± 5.9 b,* | 39.1 ± 5.8 * |
| FM, kg | 13.8 ± 1.7 a | 22.1 ± 2.9 b | 32.4 ± 5.8 c | 25.3 ± 7.9 |
| FMI, kg/m2 | 4.9 ± 0.7 a | 7.7 ± 0.8 b | 11.2 ± 1.6 c | 8.8 ± 2.5 |
| HF | 0.759 ± 0.025 a | 0.752 ± 0.027 a | 0.737 ± 0.033 b | 0.746 ± 0.030 |
| Women | ||||
| Age, years | 69.1 ± 8.3 a | 67.9 ± 6.7 a | 69.2 ± 6.4 a | 68.5 ± 6.8 |
| Weight, kg | 55.2 ± 6.7 a | 65.9 ± 7.5 b | 77.5 ± 8.9 c | 69.9 ± 10.8 |
| Height, m | 1.6 ± 0.07 a | 1.5 ± 0.07 a | 1.5 ± 0.06 a | 1.6 ± 0.06 |
| BMI, kg/m2 | 22.9 ± 1.8 a | 27.2 ± 2.1 b,* | 32.4 ± 3.1 c,* | 29.0 ± 4.1 * |
| FFM, kg | 36.6 ± 5.1 a | 38.3 ± 5.1 a,b | 39.5 ± 4.6 b | 38.6 ± 4.9 |
| TBW, kg | 27.2 ± 4.3 a | 28.6 ± 4.2 a,b | 29.7 ± 3.9 b | 28.9 ± 4.1 |
| FM, kg | 18.7 ± 2.9 a,* | 27.7 ± 3.6 b,* | 37.9 ± 5.9 c,* | 31.3 ± 7.9 * |
| FMI, kg/m2 | 7.8 ± 1.0 a,* | 11.4 ± 1.1 b,* | 15.9 ± 2.3 c,* | 12.9 ± 3.2 * |
| HF | 0.743 ± 0.048 a | 0.746 ± 0.033 a | 0.753 ± 0.034 a,* | 0.748 ± 0.035 |
| FMI Category | Mean | 0.732 Value from Chemical Analysis | 0.725 Value from “Grand Mean” | 0.734 Value from Older French Adults |
|---|---|---|---|---|
| Normal (n = 44) | 0.750 ± 0.039 | p = 0.003 | p ≤ 0.001 | p = 0.008 |
| Excess fat (n = 193) | 0.748 ± 0.031 | p ≤ 0.001 | p ≤ 0.001 | p ≤ 0.001 |
| Obesity (n = 175) | 0.747 ± 0.035 | p ≤ 0.001 | p ≤ 0.001 | p ≤ 0.001 |
| Total sample (n = 412) | 0.748 ± 0.033 | p ≤ 0.001 | p ≤ 0.001 | p ≤ 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Arellanes, R.; Urquidez-Romero, R.; Rodríguez-Tadeo, A.; Esparza-Romero, J.; Méndez-Estrada, R.-O.; Ramírez-López, E.; Robles-Sardin, A.-E.; Pacheco-Moreno, B.-I.; Alemán-Mateo, H. High Hydration Factor in Older Hispanic-American Adults: Possible Implications for Accurate Body Composition Estimates. Nutrients 2019, 11, 2897. https://doi.org/10.3390/nu11122897
González-Arellanes R, Urquidez-Romero R, Rodríguez-Tadeo A, Esparza-Romero J, Méndez-Estrada R-O, Ramírez-López E, Robles-Sardin A-E, Pacheco-Moreno B-I, Alemán-Mateo H. High Hydration Factor in Older Hispanic-American Adults: Possible Implications for Accurate Body Composition Estimates. Nutrients. 2019; 11(12):2897. https://doi.org/10.3390/nu11122897
Chicago/Turabian StyleGonzález-Arellanes, Rogelio, Rene Urquidez-Romero, Alejandra Rodríguez-Tadeo, Julián Esparza-Romero, Rosa-Olivia Méndez-Estrada, Erik Ramírez-López, Alma-Elizabeth Robles-Sardin, Bertha-Isabel Pacheco-Moreno, and Heliodoro Alemán-Mateo. 2019. "High Hydration Factor in Older Hispanic-American Adults: Possible Implications for Accurate Body Composition Estimates" Nutrients 11, no. 12: 2897. https://doi.org/10.3390/nu11122897
APA StyleGonzález-Arellanes, R., Urquidez-Romero, R., Rodríguez-Tadeo, A., Esparza-Romero, J., Méndez-Estrada, R.-O., Ramírez-López, E., Robles-Sardin, A.-E., Pacheco-Moreno, B.-I., & Alemán-Mateo, H. (2019). High Hydration Factor in Older Hispanic-American Adults: Possible Implications for Accurate Body Composition Estimates. Nutrients, 11(12), 2897. https://doi.org/10.3390/nu11122897





