Sodium, Potassium and Iodine Intake, in a National Adult Population Sample of the Republic of Moldova
Abstract
:1. Introduction
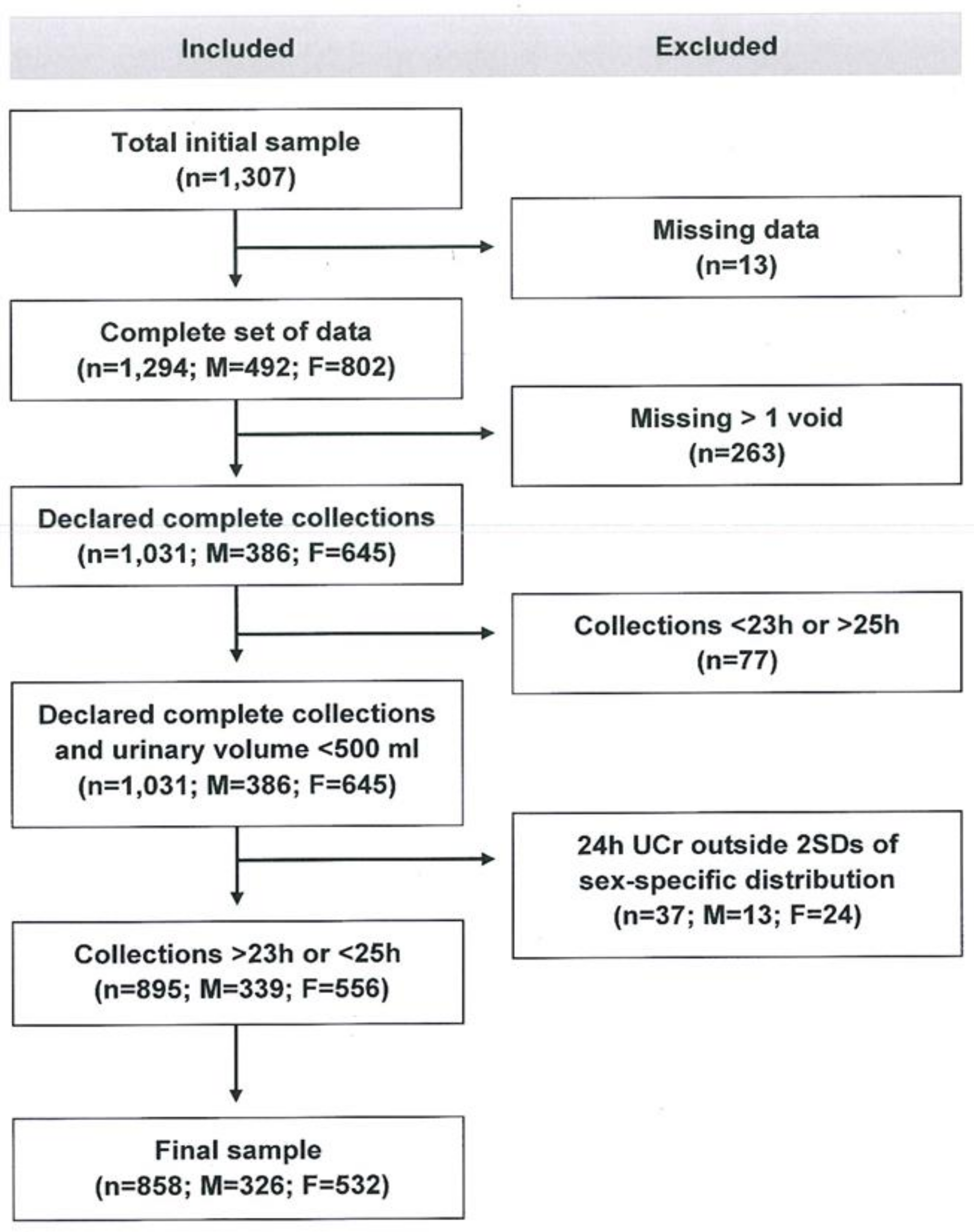
2. Materials and Methods
2.1. Participants and Recruitment
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Daily Urinary Excretions of Volume, Sodium, Potassium and Creatinine and Salt and Potassium Intake
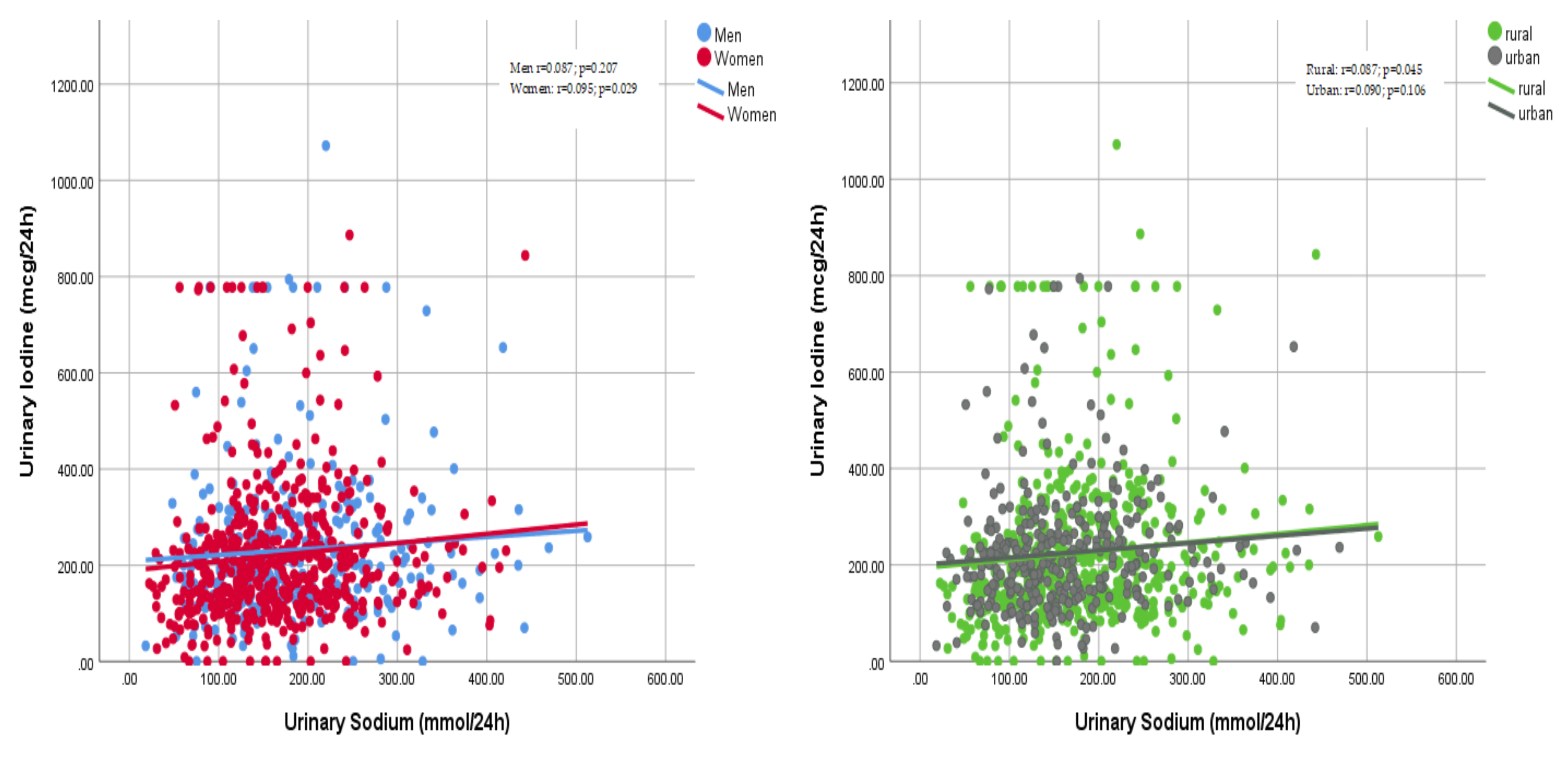
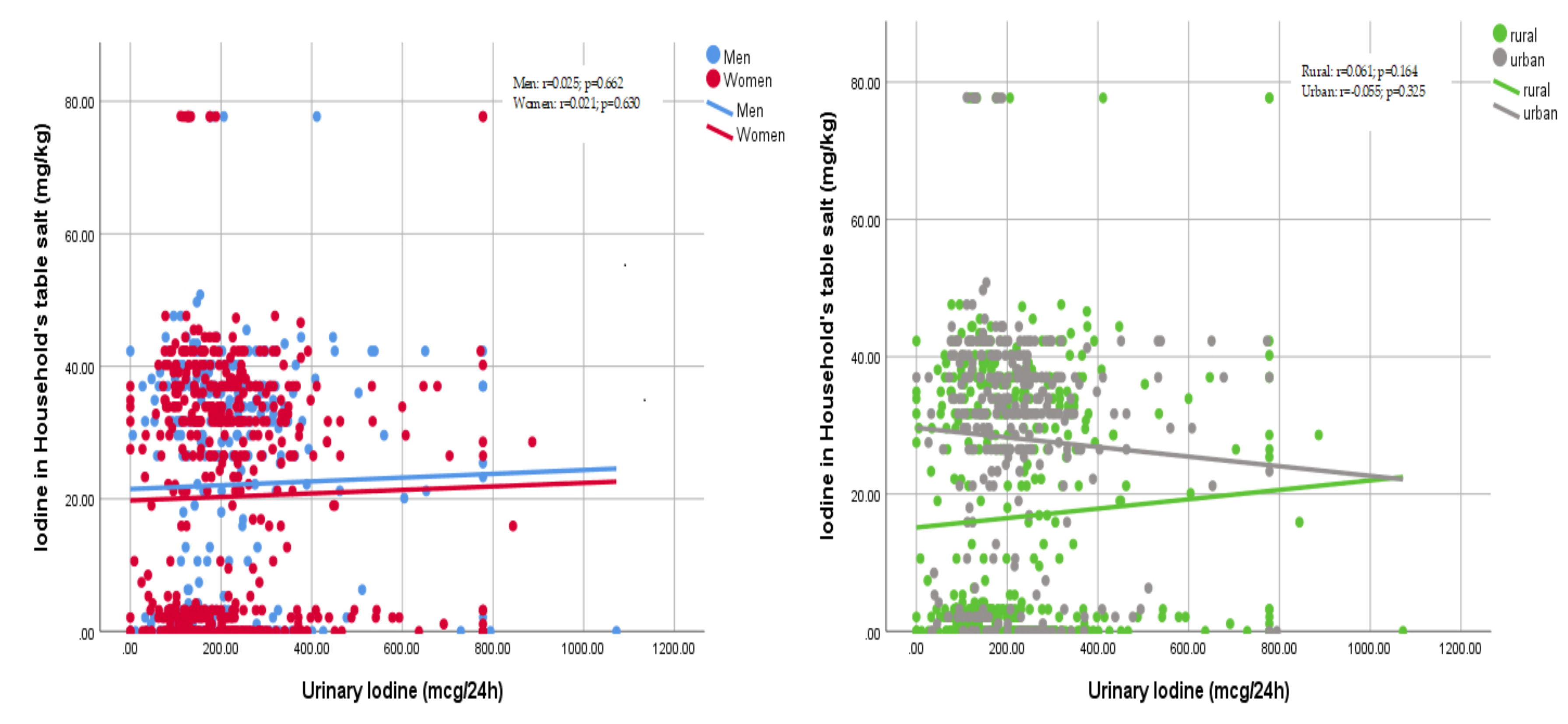
3.3. Daily Intake of Iodine and Use of Iodised Salt
3.4. Knowledge, Attitude and Behaviours Towards Salt Intake
4. Discussion
4.1. Salt Consumption
4.2. Potassium Consumption
4.3. Iodine Consumption
4.4. Comparison with Other European Countries
4.5. Strengths and Limitations
4.6. Impact and Policy Implications
4.7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar]
- World Health Organization. Global Action Plan. for the Prevention and Control of NCDs 2013–2020; World Health Organization: Geneva, Switzerland, 2013; pp. 1–103. [Google Scholar]
- World Health Organization. Tackling NCDs: “Best buys” and Other Recommended Interventions for the Prevention and Control of Noncommunicable Diseases. 2017. Available online: http://www.who.int/ncds/management/best-buys/en/ (accessed on 27 October 2019).
- National Center for Health Management. Health Yearbook: Public Health in Moldova. 2015. Available online: http://www.cnms.md/ro/rapoarteo (accessed on 27 October 2019).
- World Health Organization. Prevalence of Noncommunicable Disease Risk Factors in the Republic of Moldova (STEPS 2013); Regional Office for Europe, World Health Organization: Copenhagen, Denmark, 2014; pp. 1–221. [Google Scholar]
- European Heart Network. Transforming European Food and Drink Policies for Cardiovascular Health; European Heart Network: Brussels, Belgium, 2017; pp. 1–137. [Google Scholar]
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J. Effect of lower sodium intake on health: Systematic review and meta-analysis. Br. Med. J. 2013, 346, f1326. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Li, J.; MacGregor, G.A. Effect of longer-term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. Br. Med. J. 2013, 346, f1325. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; D’Elia, L.; Kandala, N.-B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-Analysis of prospective studies. Br. Med. J. 2009, 339, b4567. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Cutler, J.A.; Obarzanek, E.; Buring, J.E.; Rexrode, K.M.; Kumanyika, S.K.; Appel, L.J.; Whelton, P.K. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: Observational follow-up of the Trials of Hypertension Prevention (TOHP). Br. Med. J. 2007, 334, 885–888. [Google Scholar] [CrossRef]
- Cappuccio, F.P. Cardiovascular and other effects of salt consumption. Kidney Int. 2013, 3, 312–315. [Google Scholar] [CrossRef]
- D’Elia, L.; Rossi, G.; Schiano di Cola, M.; Savino, I.; Galletti, F.; Strazzullo, P. Meta-Analysis of the effect of dietary sodium restriction with or without concomitant renin–angiotensin–aldosterone system-inhibiting treatment on albuminuria. Clin. J. Am. Soc. Nephrol. 2015, 10, 1542–1552. [Google Scholar] [CrossRef]
- D’Elia, L.; Galletti, F.; La Fata, E.; Sabino, P.; Strazzullo, P. Effect of dietary sodium restriction on arterial stiffness: Systematic review and meta-analysis of the randomized controlled trials. J. Hypertens. 2018, 36, 734–743. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012. [Google Scholar]
- Brown, I.J.; Tzoulaki, I.; Candeias, V.; Elliott, P. Salt intakes around the world: Implications for public health. Int. J. Epidemiol. 2009, 38, 791–813. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Capewell, S. Facts, issues, and controversies in salt reduction for the prevention of cardiovascular disease. Funct. Food Rev. 2015, 7, 41–61. [Google Scholar]
- WHO Regional Office for Europe. Mapping Salt Reduction Initiatives in the WHO European Region; WHO Regional Office for Europe: Copenhagen, Denmark, 2013. [Google Scholar]
- Mattes, R.D.; Donnelly, D. Relative contributions of dietary sources. J. Am. Coll. Nutr. 1991, 10, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Castillo, C.P.; Warrender, S.; Whitehead, T.P.; James, W.P. An assessment of the sources of dietary salt in a British population. Clin. Sci. 1987, 72, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Jovicic-Bata, J.; Grujicic, M.; Raden, S.; Novakovic, B. Sodium intake and dietary sources of sodium in a sample of undergraduate students from Novi Sad, Serbia. Vojnosanit. Pregl. 2016, 73, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Ribič, C.H.; Zakotnik, J.M.; Vertnik, L.; Vegnuti, M.; Cappuccio, F.P. Salt intake of the Slovene population assessed by 24-hour urinary sodium excretion. Public Health Nutr. 2010, 13, 1803–1809. [Google Scholar] [CrossRef]
- D’Elia, L.; Brajovic, M.; Klisic, A.; Breda, J.; Jewell, J.; Cadjenovic, V.; Cappuccio, F.P.; on behalf of Salt Consumption Survey in Montenegro Study Group. Sodium and potassium intake, knowledge attitudes and behaviour towards salt consumption, amongst adults in Podgorica, Montenegro. Nutrients 2019, 11, 160. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Capewell, S.; Lincoln, P.; McPherson, K. Policy options to reduce population salt intake. Br. Med. J. 2011, 343, 402–405. [Google Scholar] [CrossRef]
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of increased potassium intake on cardiovascular risk factors and disease: Systematic review and meta-analyses. Br. Med. J. 2013, 346, f1378. [Google Scholar] [CrossRef]
- D’Elia, L.; Barba, G.; Cappuccio, F.P.; Strazzullo, P. Potassium Intake, Stroke, and Cardiovascular Disease: A meta-analysis of Prospective Studies. J. Am. Coll. Cardiol. 2011, 57, 1210–1219. [Google Scholar] [CrossRef]
- D’Elia, L.; Iannotta, C.; Sabino, P.; Ippolito, R. Potassium rich-diet and risk of stroke: Updated meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 585–587. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Potassium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012; pp. 1–52. [Google Scholar]
- Obreja, G.; Raevschi, E.; Penina, O. Informing national salt reduction strategy. Mold. Med. J. 2018, 61, 9–16. [Google Scholar]
- Nath, S.K.; Moinier, B.; Thullier, F.; Rongier, M.; Desjeux, J.F. Urinary excretion of iodide and fluoride from supplemented food grade salt. Int. J. Vitam. Nutr. Res. 1992, 62, 66–72. [Google Scholar] [PubMed]
- Jahreis, G.; Hausmann, W.; Kiessling, G.; Franke, K.; Leiterer, M. Bioavailability of iodine from normal diets rich in dairy products: Results of balance studies in women. Exp. Clin. Endocrinol. Diabetes 2001, 109, 163–167. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Fortification of Food-Grade Salt with Iodine for the Prevention and Control of Iodine Deficiency Disorders; World Health Organization: Geneva, Switzerland, 2014; pp. 1–54. [Google Scholar]
- Pan American Health Organization/World Health Organization. Salt-Smart Americas: A Guide for Country-Level Action; PAHO: Washington, DC, USA, 2013; pp. 1–159. [Google Scholar]
- World Health Organization. How to Obtain Measures of Population-Level Sodium Intake in 24-Hour Urine Samples; WHO-EM/NUT/279/E; World Health Organization/Regional Office of the Eastern Mediterranean: Cairo, Egypt, 2018; pp. 1–51. [Google Scholar]
- World Medical Association. Declaration of Helsinki: Recommendations guiding doctors in clinical research. Bull. World Health Org. 2008, 86, 650–651. [Google Scholar]
- World Health Organization. The WHO STEPwise Approach to Chronic Disease Risk Factor Surveillance (STEPS); World Health Organization: Geneva, Switzerland, 2014; Available online: https://www.who.int/ncds/surveillance/steps/STEPS_Instrument_v2.1.pdf (accessed on 27 October 2019).
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef] [PubMed]
- Oesch, U.; Ammann, D.; Simon, W. Ion-Selective membrane electrodes for clinical use. Clin. Chem. 1986, 32, 1448–1459. [Google Scholar] [PubMed]
- Junge, W.; Wilke, B.; Halabi, A.; Klein, G. Determination of reference intervals for serum creatinine, creatinine excretion and creatinine clearance with an enzymatic and a modified Jaffé method. Clin. Chim. Acta 2004, 344, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, T.; Yamaki, M.; Pandav, C.S.; Karmarkar, M.G.; Irie, M. Simple microplate method for determination of urinary iodine. Clin. Chem. 2000, 46, 529–536. [Google Scholar]
- Jooste, P.L.; Strydom, E. Methods for determination of iodine in urine and salt. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 77–88. [Google Scholar] [CrossRef]
- Lucko, A.M.; Doktorchik, C.; Woodward, M.; Cogswell, M.; Neal, B.; Rabi, D.; Anderson, C.; He, F.J.; MacGregor, G.A.; L’Abbe, M.; et al. Percentage of ingested sodium excreted in 24-hour urine collections: A systematic review and meta-analysis. J. Clin. Hypertens. 2018, 20, 1220–1229. [Google Scholar] [CrossRef]
- Stamler, J.; Elliott, P.; Chan, Q.; for the INTERMAP Research group. INTERMAP Appendix tables. J. Hum. Hypertens. 2003, 17, 665–758. [Google Scholar] [CrossRef]
- Donfrancesco, C.; Ippolito, R.; Lo Noce, C.; Palmieri, L.; Iacone, R.; Russo, O.; Vanuzzo, D.; Galletti, F.; Galeone, D.; Giampaoli, S.; et al. Excess dietary sodium and inadequate potassium intake in Italy: Results of the MINISAL study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 850–856. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Ji, C.; Donfrancesco, C.; Palmieri, L.; Ippolito, R.; Vanuzzo, D.; Giampaoli, S.; Strazzullo, P. Geographic and socio-economic variation of sodium and potassium intake in Italy. Results from the MINISAL-GIRCSI programme. BMJ Open 2015, 5, e007467. [Google Scholar] [CrossRef] [PubMed]
- Vasara, E.; Marakis, G.; Breda, J.; Skepastianos, P.; Hassapidou, M.; Kafatos, A.; Rodopaios, N.; Koulouri, A.; Cappuccio, F.P. Sodium and Potassium Intake in Healthy Adults in Thessaloniki Greater Metropolitan Area-The Salt Intake in Northern Greece (SING) Study. Nutrients 2017, 22, 417. [Google Scholar] [CrossRef] [PubMed]
- Erdem, Y.; Akpolat, T.; Derici, Ü.; Şengül, Ş.; Ertürk, Ş.; Ulusoy, Ş.; Altun, B.; Arici, M. Dietary sources of high sodium intake in Turkey: SALTURK II. Nutrients 2017, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Polonia, J.; Martins, L.; Pinto, F.; Nazare, J. Prevalence, awareness, treatment and control of hypertension and salt intake in Portugal: Changes over a decade. The PHYSA study. J. Hypertens. 2014, 32, 1211–1221. [Google Scholar] [CrossRef]
- Lerchl, K.; Rakova, N.; Dahlmann, A.; Rauh, M.; Goller, U.; Basner, M.; Dinges, D.F.; Beck, L.; Agureev, A.; Larina, I.; et al. Agreement between 24-hour salt ingestion and sodium excretion in a controlled environment. Hypertension 2015, 66, 850–857. [Google Scholar] [CrossRef] [Green Version]
- National Centre for Health Management. Statistical Yearbook of the Republic of Moldova; Ministry of Health, Labour and Social Protection of the Republic of Moldova: Chişinău, Moldova, 2017.
- Government of the Republic of Moldova. Decision no. 403 of 6 April 2016 on the approval of the National Action Plan for 2016–2020 on the implementation of the National Strategy on Prevention and Control of Noncommunicable Diseases. Monitorul Oficial Republicii Moldova 2016, 100, 464. (In Romanian) [Google Scholar]
- Collins, D.; Ciobanu, A.; Laatikainen, T.; Curocichin, G.; Salaru, V.; Zatic, T.; Anisei, A.; Farrington, J. Protocol for the evaluation of a pilot implementation of essential interventions for the prevention of cardiovascular diseases in primary healthcare in the Republic of Moldova. BMJ Open 2019, 9, e025705. [Google Scholar] [CrossRef]
- Pastorelli, A.A.; Stacchini, P.; Olivieri, A. Daily iodine intake and the impact of salt reduction on iodine prophylaxis in the Italian population. Eur. J. Clin. Nutr. 2015, 69, 211–215. [Google Scholar] [CrossRef]
- He, F.J.; Ma, Y.; Feng, X.; Zhang, W.; Lin, L.; Guo, X.; Zhang, J.; Niu, W.; Wu, Y.; MacGregor, G.A. Effect of salt reduction on iodine status assessed by 24 hours urinary iodine excretion in children and their families in northern China: A substudy of a cluster randomised controlled trial. BMJ Open 2016, 6, e011168. [Google Scholar] [CrossRef] [Green Version]

| Variable | All | Men | Women |
|---|---|---|---|
| (n = 858) | (n = 326) | (n = 532) | |
| Age (years) | 48.5 (13.8) | 47.3 (13.6) | 49.2 (13.9) |
| Height (cm) | 166.7 (8.8) | 172.8 (8.1) | 162.9 (7.0) † |
| Weight (kg) | 78.2 (15.8) | 82.0 (15.8) | 75.8 (15.3) † |
| B.M.I. (kg/m2) | 28.1 (5.4) | 27.4 (4.9) | 28.6 (5.7) ‡ |
| Waist circumference (cm) | – | 93.8 (15.5) | 91.8 (15.1) |
| Hip circumference (cm) | – | 100.5 (12.3) | 106.5 (14.0) |
| Systolic BP (mmHg) | 134.3 (21.2) | 136.1 (18.5) | 133.1 (22.6) * |
| Diastolic BP (mmHg) | 86.8 (11.9) | 87.1 (10.8) | 86.6 (12.6) |
| Pulse rate (b/min) | 76.2 (9.5) | 78.0 (10.3) | 75.2 (8.8) |
| Hypertension # n (%) | 385 (45.5) | 148 (45.8) | 237 (45.2) |
| Variables | All | Men | Women | Rural | Urban |
|---|---|---|---|---|---|
| (n = 858) | (n = 326) | (n = 532) | (n = 531) | (n = 327) | |
| Volume (mL/24 h) | 1441 (529) | 1505 (536) | 1401 (521) ^ | 1333 (427) | 1616 (624) # |
| Sodium (mmoL/24 h) | 172.7 (79.3) | 183.9 (86.0) | 165.8 (74.1) † | 180.4 (80.2) | 160.1 (76.2) # |
| Salt intake (g/day) | 10.8 (4.9) | 11.5 (5.4) | 10.3 (4.6) # | 11.3 (5.0) | 10.0 (4.8) # |
| Potassium (mmoL/24 h) | 72.7 (31.5) | 76.0 (33.4) | 70.7 (30.1) * | 73.8 (31.6) | 71.0 (31.2) |
| Potassium intake (g/day) | 3.40 (1.47) | 3.55 (1.56) | 3.31 (1.41) * | 3.45 (1.47) | 3.32 (1.46) |
| Creatinine (mmol/24h) | 11.7 (5.0) | 13.3 (5.6) | 10.7 (4.2) # | 12.3 (4.8) | 11.4 (5.0) † |
| Group (mcg/L) | All | Men | Women | Rural | Urban |
|---|---|---|---|---|---|
| (n = 858) | (n = 326) | (n = 532) | (n = 531) | (n = 327) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Insufficient (<100) | 245 (28.6) | 95 (29.1) | 150 (28.2) | 104 (31.8) | 141 (26.6) |
| Severe (<20) | 20 (2.3) | 6 (1.8) | 14 (2.6) | 4 (1.2) | 16 (3.0) |
| Moderate (20–49) | 60 (7.0) | 24 (7.4) | 36 (6.8) | 28 (8.6) | 32 (6.0) |
| Mild (50–99) | 165 (19.2) | 65 (19.9) | 100 (18.8) | 72 (22.0) | 93 (17.5) |
| Adequate (100–199) | 351 (40.9) | 132 (40.5) | 219 (41.2) | 131 (40.1) | 220 (41.4) |
| Above requirement (200–299) | 152 (17.7) | 59 (18.1) | 93 (17.5) | 58 (17.7) | 94 (17.7) |
| Excessive (≥300) | 108 (12.6) | 40 (12.3) | 68 (12.8) | 34 (10.4) | 74 (13.9) |
| Variables | All | Men | Women | Rural | Urban |
|---|---|---|---|---|---|
| (n = 858) | (n = 326) | (n = 532) | (n = 531) | (n = 327) | |
| Iodine (mcg/24 h) | 225 (152) | 232 (154) | 221 (150) | 225 (145) | 224 (128) |
| Iodine in table salt (mg/kg) | 21.0 (18.6) | 22.1 (18.2) | 20.3 (18.9) | 16.7 (18.6) | 28.1 (16.5) # |
| Participants Who: | All | Men | Women | Rural | Urban |
|---|---|---|---|---|---|
| (n = 858) | (n = 326) | (n = 532) | (n = 531) | (n = 327) | |
| Limit their consumption of processed food | 81.7 | 79.7 | 82.4 | 80.4 | 83.9 |
| Look at salt/sodium content in foods | 8.8 | 10.1 | 8.2 | 3.8 | 17.2 * |
| Buy low salt/sodium alternatives | 14.3 | 17.4 | 13.2 | 3.8 | 24.7 * |
| Do not add salt at the table | 77.3 | 69.6 | 80.2 | 75.9 | 79.6 |
| Do not add salt when cooking | 31.1 | 24.6 | 33.5 | 13.3 | 61.3 * |
| Use spices instead of salt when cooking | 22.3 | 15.9 | 24.7 | 25.3 | 17.2 |
| Avoid eating food prepared outside a home | 33.1 | 27.5 | 35.2 | 43.7 | 15.1 * |
| Take other measures to control salt intake | 0.8 | 1.4 | 0.5 | 1.3 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Elia, L.; Obreja, G.; Ciobanu, A.; Breda, J.; Jewell, J.; Cappuccio, F.P., the Salt Consumption Survey in the Republic of Moldova Study Group. Sodium, Potassium and Iodine Intake, in a National Adult Population Sample of the Republic of Moldova. Nutrients 2019, 11, 2896. https://doi.org/10.3390/nu11122896
D’Elia L, Obreja G, Ciobanu A, Breda J, Jewell J, Cappuccio FP the Salt Consumption Survey in the Republic of Moldova Study Group. Sodium, Potassium and Iodine Intake, in a National Adult Population Sample of the Republic of Moldova. Nutrients. 2019; 11(12):2896. https://doi.org/10.3390/nu11122896
Chicago/Turabian StyleD’Elia, Lanfranco, Galina Obreja, Angela Ciobanu, Joao Breda, Jo Jewell, and Francesco P. Cappuccio the Salt Consumption Survey in the Republic of Moldova Study Group. 2019. "Sodium, Potassium and Iodine Intake, in a National Adult Population Sample of the Republic of Moldova" Nutrients 11, no. 12: 2896. https://doi.org/10.3390/nu11122896
APA StyleD’Elia, L., Obreja, G., Ciobanu, A., Breda, J., Jewell, J., & Cappuccio, F. P., the Salt Consumption Survey in the Republic of Moldova Study Group. (2019). Sodium, Potassium and Iodine Intake, in a National Adult Population Sample of the Republic of Moldova. Nutrients, 11(12), 2896. https://doi.org/10.3390/nu11122896






