Efficacy and Safety of New Lactobacilli Probiotics for Unconstipated Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Methods
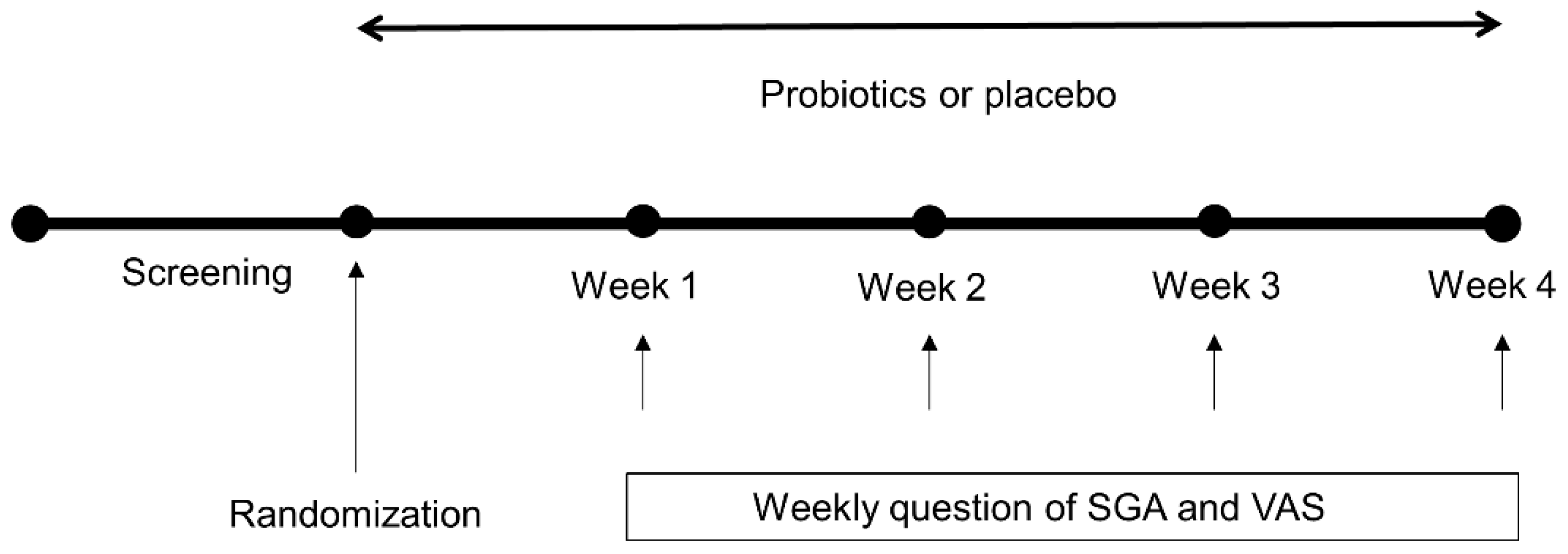
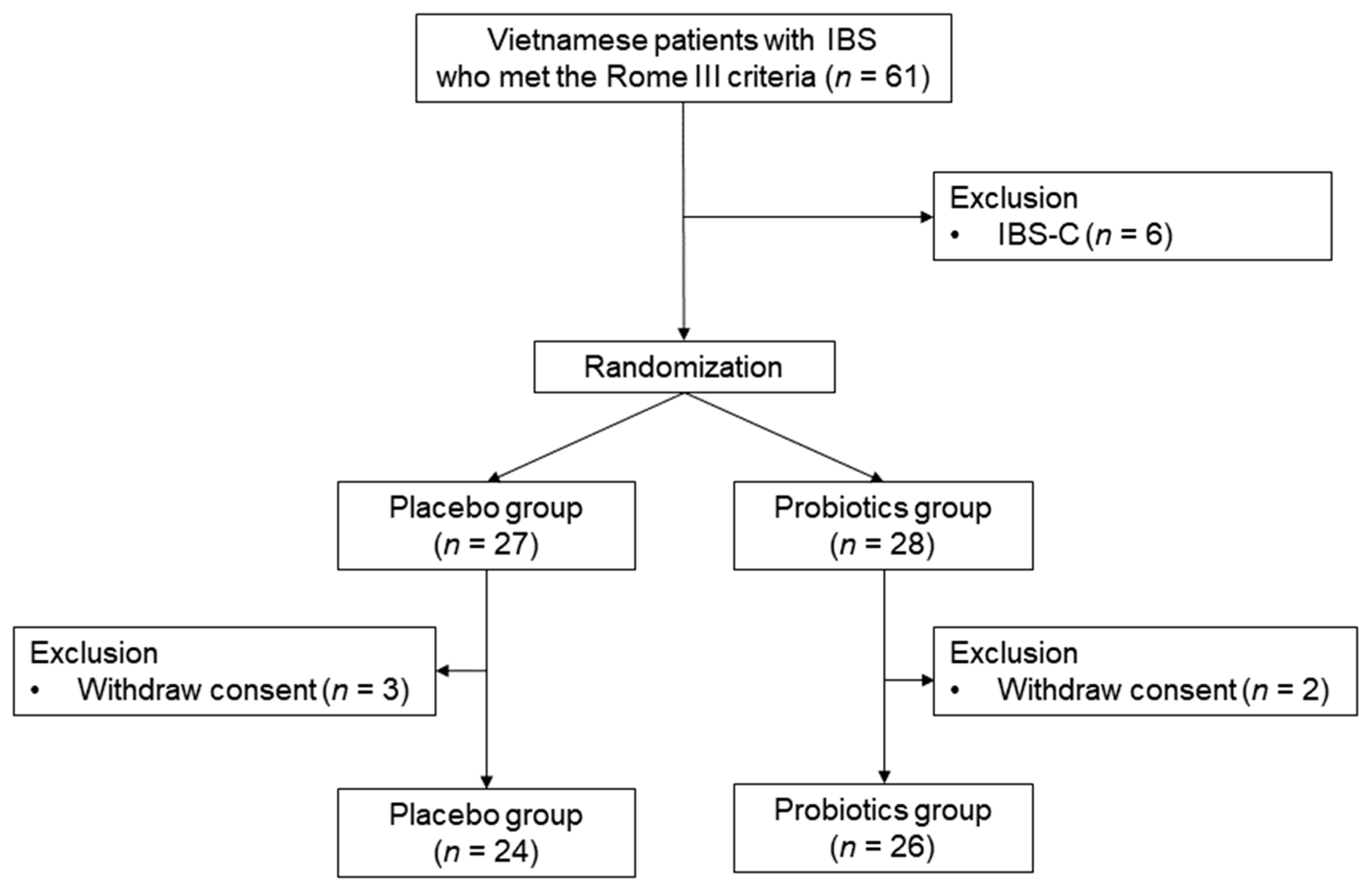
2.1. Study Design, Setting, and Participants
2.2. Probiotic Preparation
2.3. Outcome Measurements
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
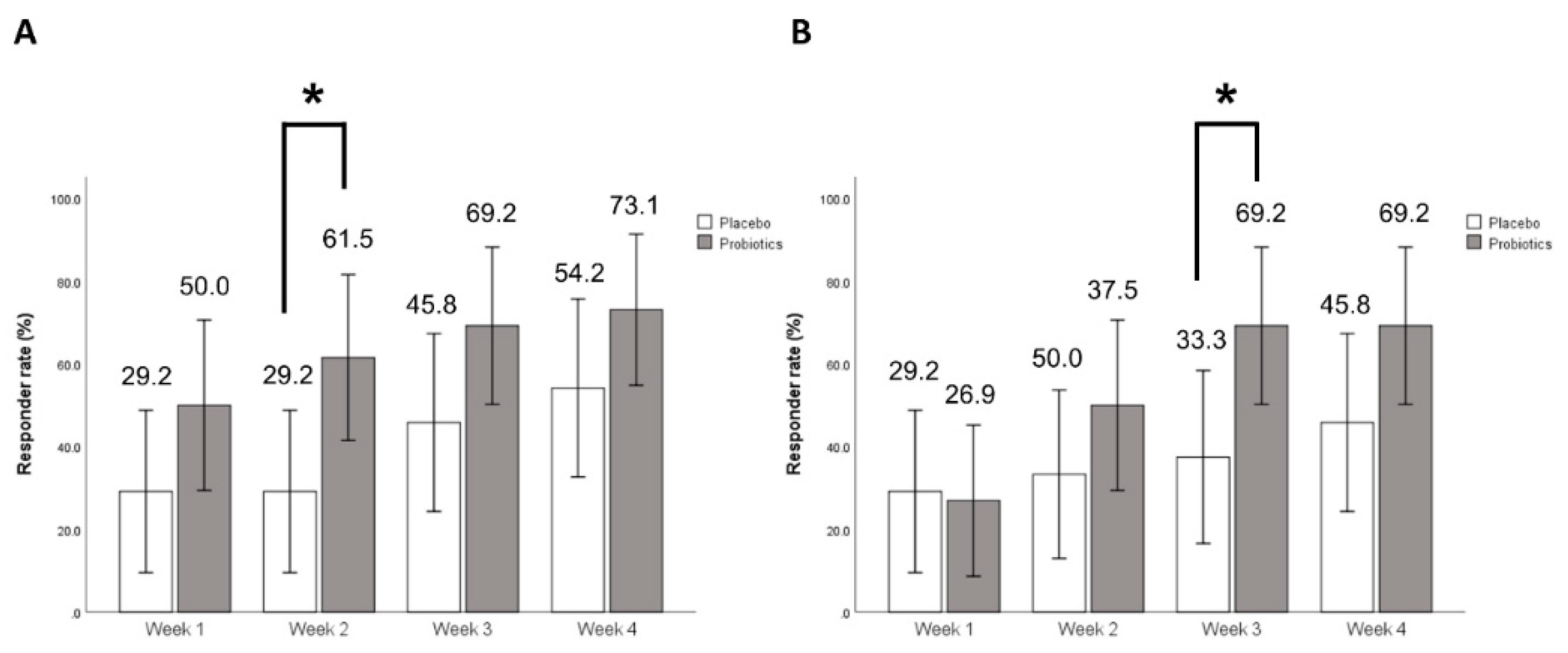
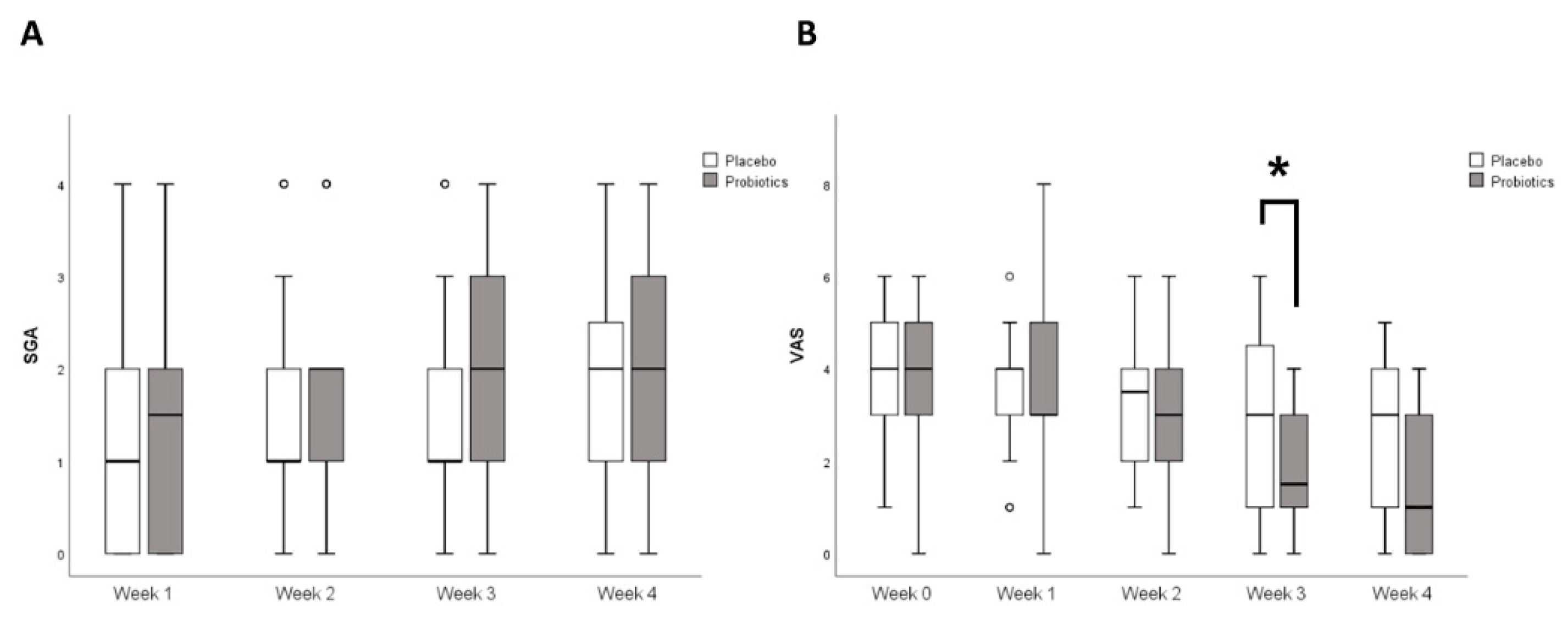
3.2. Primary Outcome
3.3. Secondary Outcome
3.4. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Lembo, A.; Chey, W.D.; Zakko, S.; Ringel, Y.; Yu, J.; Mareya, S.M.; Shaw, A.L.; Bortey, E.; Forbes, W.P.; et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N. Engl. J. Med. 2011, 364, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Parkes, G.C.; Sanderson, J.D.; Whelan, K. Treating irritable bowel syndrome with probiotics: The evidence. Proc. Nutr. Soc. 2010, 69, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Cozzali, R.; Farinelli, E.; Brusaferro, A.; Esposito, S. Gut dysbiosis and irritable bowel syndrome: The potential role of probiotics. J. Infect. 2018, 76, 111–120. [Google Scholar] [CrossRef]
- Parkes, G.C.; Brostoff, J.; Whelan, K.; Sanderson, J.D. Gastrointestinal microbiota in irritable bowel syndrome: Their role in its pathogenesis and treatment. Am. J. Gastroenterol. 2008, 103, 1557–1567. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef]
- Verdu, E.F.; Bercik, P.; Verma-Gandhu, M.; Huang, X.X.; Blennerhassett, P.; Jackson, W.; Mao, Y.; Wang, L.; Rochat, F.; Collins, S.M. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 2006, 55, 182–190. [Google Scholar] [CrossRef]
- Kamiya, T.; Wang, L.; Forsythe, P.; Goettsche, G.; Mao, Y.; Wang, Y.; Tougas, G.; Bienenstock, J. Inhibitory effects of lactobacillus reuteri on visceral pain induced by colorectal distension in sprague-dawley rats. Gut 2006, 55, 191–196. [Google Scholar] [CrossRef]
- Ford, A.C.; Quigley, E.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Moayyedi, P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561. [Google Scholar] [CrossRef]
- Didari, T.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J. Gastroenterol. 2015, 21, 3072–3084. [Google Scholar] [CrossRef] [PubMed]
- Kajander, K.; Hatakka, K.; Poussa, T.; Farkkila, M.; Korpela, R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: A controlled 6-month intervention. Aliment. Pharmacol. Ther. 2005, 22, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Ki Cha, B.; Mun Jung, S.; Hwan Choi, C.; Song, I.D.; Woong Lee, H.; Joon Kim, H.; Hyuk, J.; Kyung Chang, S.; Kim, K.; Chung, W.S.; et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Clin. Gastroenterol. 2012, 46, 220–227. [Google Scholar] [CrossRef]
- Celiberto, L.S.; Pinto, R.A.; Rossi, E.A.; Vallance, B.A.; Cavallini, D.C.U. Isolation and characterization of potentially probiotic bacterial strains from mice: Proof of concept for personalized probiotics. Nutrients 2018, 10, 1684. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.W.; Priya, S.; Blekhman, R.; Bordenstein, S.R. Gut microbiota diversity across ethnicities in the united states. PLoS Biol. 2018, 16, e2006842. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The microbiome and irritable bowel syndrome—A review on the pathophysiology, current research and future therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Chang, F.Y.; Lu, C.L.; Chen, T.S. The current prevalence of irritable bowel syndrome in asia. J. Neurogastroenterol. Motil. 2010, 16, 389–400. [Google Scholar] [CrossRef]
- Zuckerman, M.J.; Nguyen, G.; Ho, H.; Nguyen, L.; Gregory, G.G. A survey of irritable bowel syndrome in vietnam using the rome criteria. Dig. Dis. Sci. 2006, 51, 946–951. [Google Scholar] [CrossRef]
- Drossman, D.A.; Camilleri, M.; Mayer, E.A.; Whitehead, W.E. Aga technical review on irritable bowel syndrome. Gastroenterology 2002, 123, 2108–2131. [Google Scholar] [CrossRef]
- Lu, C.L.; Chen, C.Y.; Lang, H.C.; Luo, J.C.; Wang, S.S.; Chang, F.Y.; Lee, S.D. Current patterns of irritable bowel syndrome in taiwan: The rome ii questionnaire on a chinese population. Aliment. Pharmacol. Ther. 2003, 18, 1159–1169. [Google Scholar] [CrossRef]
- Cho, Y.H.; Hong, S.M.; Kim, C.H. Isolation and characterization of lactic acid bacteria from kimchi, korean traditional fermented food to apply into fermented dairy products. Korean J. Food Sci. Anim. Resour. 2013, 33, 75–82. [Google Scholar] [CrossRef]
- Shin, H.S.; Yoo, S.H.; Jang, J.A.; Won, J.Y.; Kim, C.H. Probiotic properties of lactic acid bacteria isolated from feces and kimchi. J. Milk Sci. Biotechnol. 2017, 35, 255–261. [Google Scholar] [CrossRef]
- Kim, H.J.; Vazquez Roque, M.I.; Camilleri, M.; Stephens, D.; Burton, D.D.; Baxter, K.; Thomforde, G.; Zinsmeister, A.R. A randomized controlled trial of a probiotic combination vsl# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol. Motil. 2005, 17, 687–696. [Google Scholar] [PubMed]
- Sen, S.; Mullan, M.M.; Parker, T.J.; Woolner, J.T.; Tarry, S.A.; Hunter, J.O. Effect of lactobacillus plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome. Dig. Dis. Sci. 2002, 47, 2615–2620. [Google Scholar] [CrossRef]
- Nobaek, S.; Johansson, M.L.; Molin, G.; Ahrne, S.; Jeppsson, B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2000, 95, 1231–1238. [Google Scholar] [CrossRef]
- Andriulli, A.; Neri, M.; Loguercio, C.; Terreni, N.; Merla, A.; Cardarella, M.P.; Federico, A.; Chilovi, F.; Milandri, G.L.; De Bona, M.; et al. Clinical trial on the efficacy of a new symbiotic formulation, flortec, in patients with irritable bowel syndrome: A multicenter, randomized study. J. Clin. Gastroenterol. 2008, 42 Pt 2 (Suppl. 3), S218–S223. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef]
- Muller-Lissner, S.; Koch, G.; Talley, N.J.; Drossman, D.; Rueegg, P.; Dunger-Baldauf, C.; Lefkowitz, M. Subject’s global assessment of relief: An appropriate method to assess the impact of treatment on irritable bowel syndrome-related symptoms in clinical trials. J. Clin. Epidemiol. 2003, 56, 310–316. [Google Scholar] [CrossRef]
- Gallagher, E.J.; Bijur, P.E.; Latimer, C.; Silver, W. Reliability and validity of a visual analog scale for acute abdominal pain in the ed. Am. J. Emerg. Med. 2002, 20, 287–290. [Google Scholar] [CrossRef]
- Sinn, D.H.; Song, J.H.; Kim, H.J.; Lee, J.H.; Son, H.J.; Chang, D.K.; Kim, Y.H.; Kim, J.J.; Rhee, J.C.; Rhee, P.L. Therapeutic effect of lactobacillus acidophilus-sdc 2012, 2013 in patients with irritable bowel syndrome. Dig. Dis. Sci. 2008, 53, 2714–2718. [Google Scholar] [CrossRef]
- Kim, H.J.; Camilleri, M.; McKinzie, S.; Lempke, M.B.; Burton, D.D.; Thomforde, G.M.; Zinsmeister, A.R. A randomized controlled trial of a probiotic, vsl#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2003, 17, 895–904. [Google Scholar]
- Guyonnet, D.; Chassany, O.; Ducrotte, P.; Picard, C.; Mouret, M.; Mercier, C.H.; Matuchansky, C. Effect of a fermented milk containing bifidobacterium animalis dn-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: A multicentre, randomized, double-blind, controlled trial. Aliment. Pharmacol. Ther. 2007, 26, 475–486. [Google Scholar] [CrossRef]
- Niv, E.; Naftali, T.; Hallak, R.; Vaisman, N. The efficacy of lactobacillus reuteri atcc 55730 in the treatment of patients with irritable bowel syndrome--a double blind, placebo-controlled, randomized study. Clin. Nutr. 2005, 24, 925–931. [Google Scholar] [CrossRef]
- Moayyedi, P.; Ford, A.C.; Talley, N.J.; Cremonini, F.; Foxx-Orenstein, A.E.; Brandt, L.J.; Quigley, E.M. The efficacy of probiotics in the treatment of irritable bowel syndrome: A systematic review. Gut 2010, 59, 325–332. [Google Scholar] [CrossRef]
- Hoveyda, N.; Heneghan, C.; Mahtani, K.R.; Perera, R.; Roberts, N.; Glasziou, P. A systematic review and meta-analysis: Probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef] [Green Version]
- Schachtsiek, M.; Hammes, W.P.; Hertel, C. Characterization of lactobacillus coryniformis dsm 20001t surface protein cpf mediating coaggregation with and aggregation among pathogens. Appl. Environ. Microbiol. 2004, 70, 7078–7085. [Google Scholar] [CrossRef] [Green Version]
- Oelschlaeger, T.A. Mechanisms of probiotic actions—A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef]
- Markowiak, P.; Slizewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Isolauri, E.; Sutas, Y.; Kankaanpaa, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on immunity. Am. J. Clin. Nutr. 2001, 73, 444S–450S. [Google Scholar] [CrossRef] [Green Version]
- Rajilic-Stojanovic, M.; Jonkers, D.M.; Salonen, A.; Hanevik, K.; Raes, J.; Jalanka, J.; de Vos, W.M.; Manichanh, C.; Golic, N.; Enck, P.; et al. Intestinal microbiota and diet in ibs: Causes, consequences, or epiphenomena? Am. J. Gastroenterol. 2015, 110, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damodharan, K.; Palaniyandi, S.A.; Suh, J.W.; Yang, S.H. Probiotic characterization of lactobacillus paracasei subsp. Paracasei kni9 inhibiting adherence of yersinia enterocolitica on caco-2 cells in vitro. Probiotics Antimicrob. Proteins 2019. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, L.; O’Callaghan, L.; McCarthy, J.; Shilling, D.; Scully, P.; Sibartie, S.; Kavanagh, E.; Kirwan, W.O.; Redmond, H.P.; Collins, J.K.; et al. Differential cytokine response from dendritic cells to commensal and pathogenic bacteria in different lymphoid compartments in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G839–G845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, J.; O’Mahony, L.; O’Callaghan, L.; Sheil, B.; Vaughan, E.E.; Fitzsimons, N.; Fitzgibbon, J.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut 2003, 52, 975–980. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Lundberg, J.M.; Alving, K. Intragastric nitric oxide production in humans: Measurements in expelled air. Gut 1994, 35, 1543–1546. [Google Scholar] [CrossRef] [Green Version]

| Placebo (n = 24) | Probiotics (n = 26) | p-Value | |
|---|---|---|---|
| Age (year) | 33.0 (28.0–44.5) | 32.5 (26.5–39.0) | 0.28 |
| Female | 19 (79.2) | 17 (65.4) | 0.28 |
| BMI (kg/m2) | 20.7 (18.3–22.3) | 22.2 (19.9–23.6) | 0.08 |
| Never smoker | 24 (100) | 24 (92.3) | 0.17 |
| Never drinker | 19 (79.2) | 20 (76.9) | 0.70 |
| Duration of stay in Korea (month) | 31.0 (7.0–67.0) | 36.0 (14.7–102.0) | 0.48 |
| IBS subtypes | 0.75 | ||
| IBS-D | 10 (41.7) | 11 (42.3) | |
| IBS-M | 6 (25.0) | 4 (15.4) | |
| IBS-U | 8 (33.3) | 11 (42.3) | |
| Abdominal pain (VAS) | 4.0 (3.0–5.0) | 4.0 (2.7–5.0) | 0.53 |
| Stool form (BSC) | 5.0 (3.2–5.7) | 4.5 (4.0–6.0) | 0.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.H.; Jang, Y.S.; Kang, D.; Chang, D.K.; Min, Y.W. Efficacy and Safety of New Lactobacilli Probiotics for Unconstipated Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 2887. https://doi.org/10.3390/nu11122887
Oh JH, Jang YS, Kang D, Chang DK, Min YW. Efficacy and Safety of New Lactobacilli Probiotics for Unconstipated Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2019; 11(12):2887. https://doi.org/10.3390/nu11122887
Chicago/Turabian StyleOh, Joo Hyun, Yeon Sil Jang, Danbee Kang, Dong Kyung Chang, and Yang Won Min. 2019. "Efficacy and Safety of New Lactobacilli Probiotics for Unconstipated Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 11, no. 12: 2887. https://doi.org/10.3390/nu11122887
APA StyleOh, J. H., Jang, Y. S., Kang, D., Chang, D. K., & Min, Y. W. (2019). Efficacy and Safety of New Lactobacilli Probiotics for Unconstipated Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 11(12), 2887. https://doi.org/10.3390/nu11122887





