Dietary Omega-3 Fatty Acid Dampens Allergic Rhinitis via Eosinophilic Production of the Anti-Allergic Lipid Mediator 15-Hydroxyeicosapentaenoic Acid in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Experimental Diet
2.2. Induction of Allergic Rhinitis
2.3. Administration of Reagents to Mice
2.4. Tissue Collection and Preparation of a Single Cell Suspension
2.5. Flow Cytometry
2.6. OVA-Specific IgE ELISA
2.7. Mast Cell Degranulation Assay
2.8. Culture of Bone Marrow-Derived Neutrophils and Eosinophils
2.9. Lipid Metabolism of Cultured Neutrophils and Eosinophils
2.10. Lipid Extraction from Cells, Culture Supernatant, and Plasma
2.11. LC–MS/MS Analysis of Free FAs and Their Metabolites
2.12. Reverse Transcription and Quantitative PCR
2.13. Statistical Analysis
3. Results
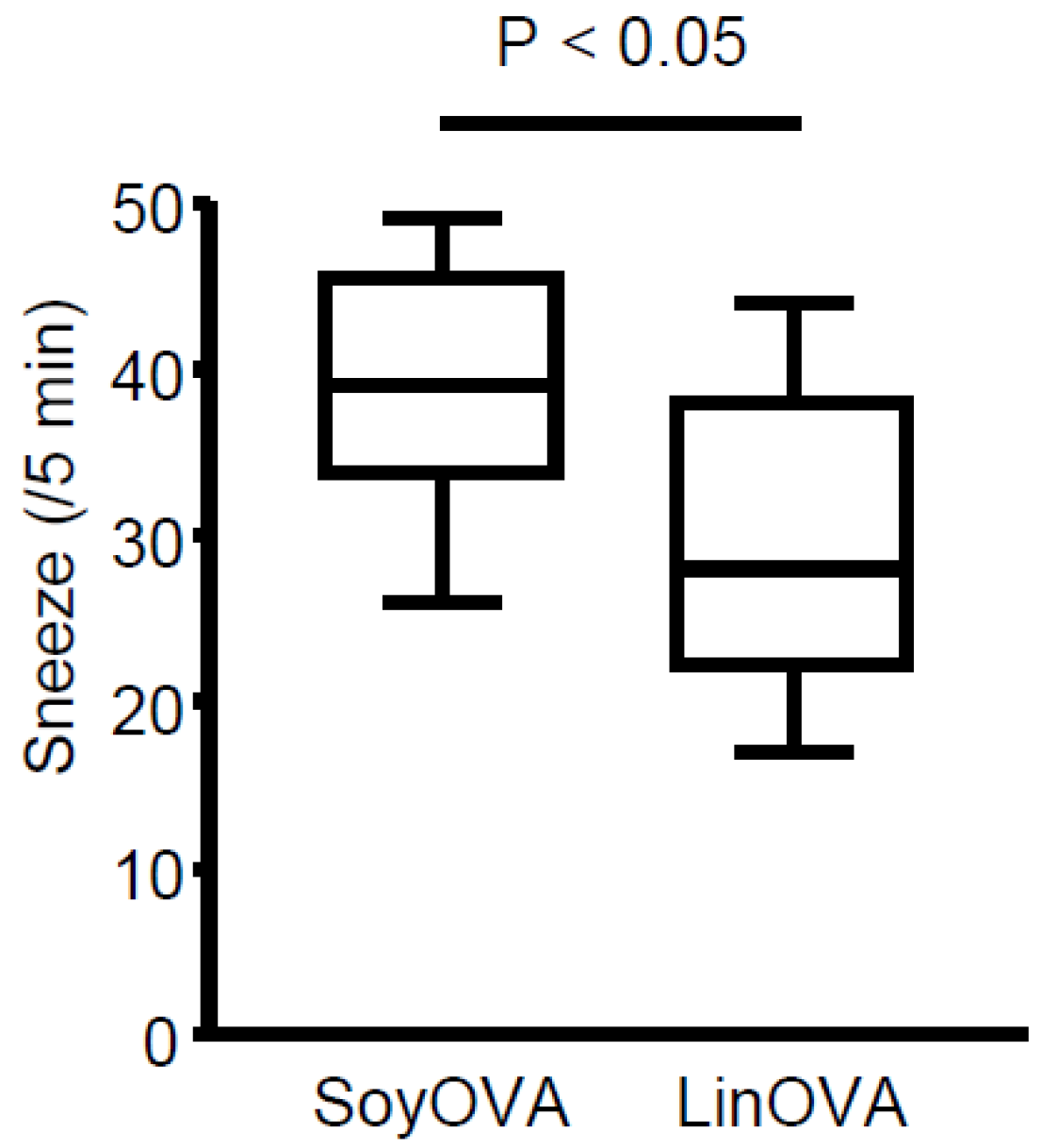
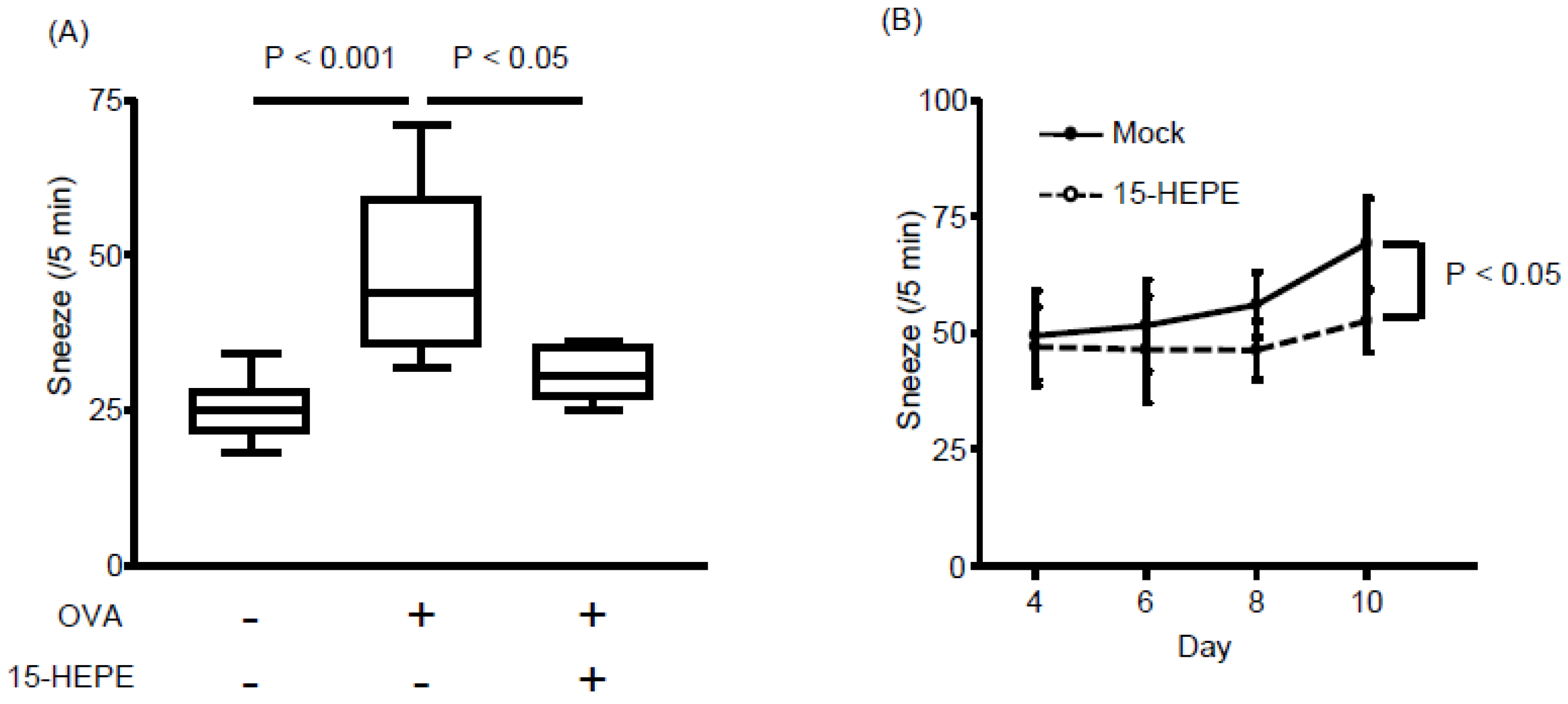
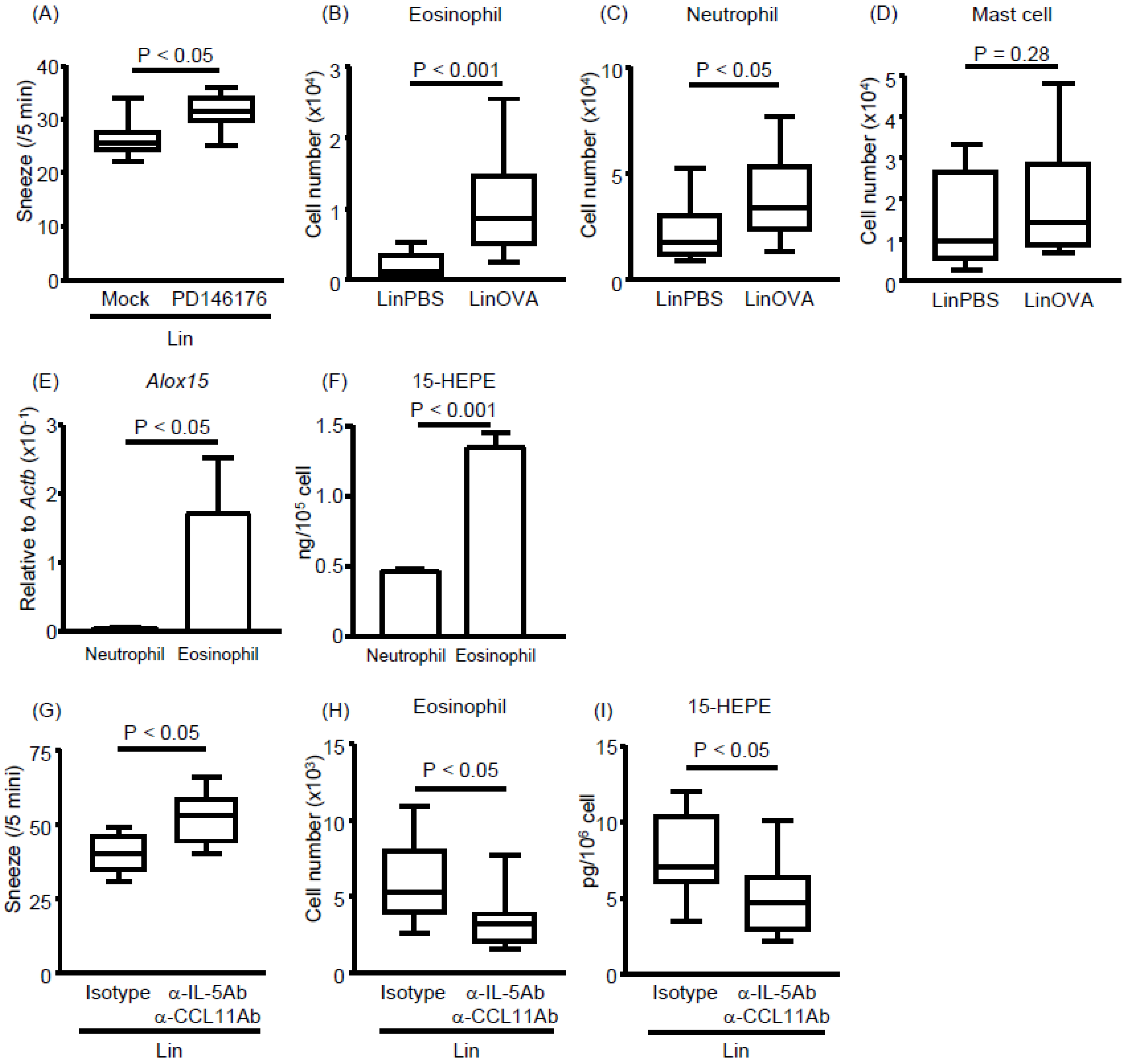
3.1. Dietary Linseed Oil Reduces Allergic Rhinitis Responses
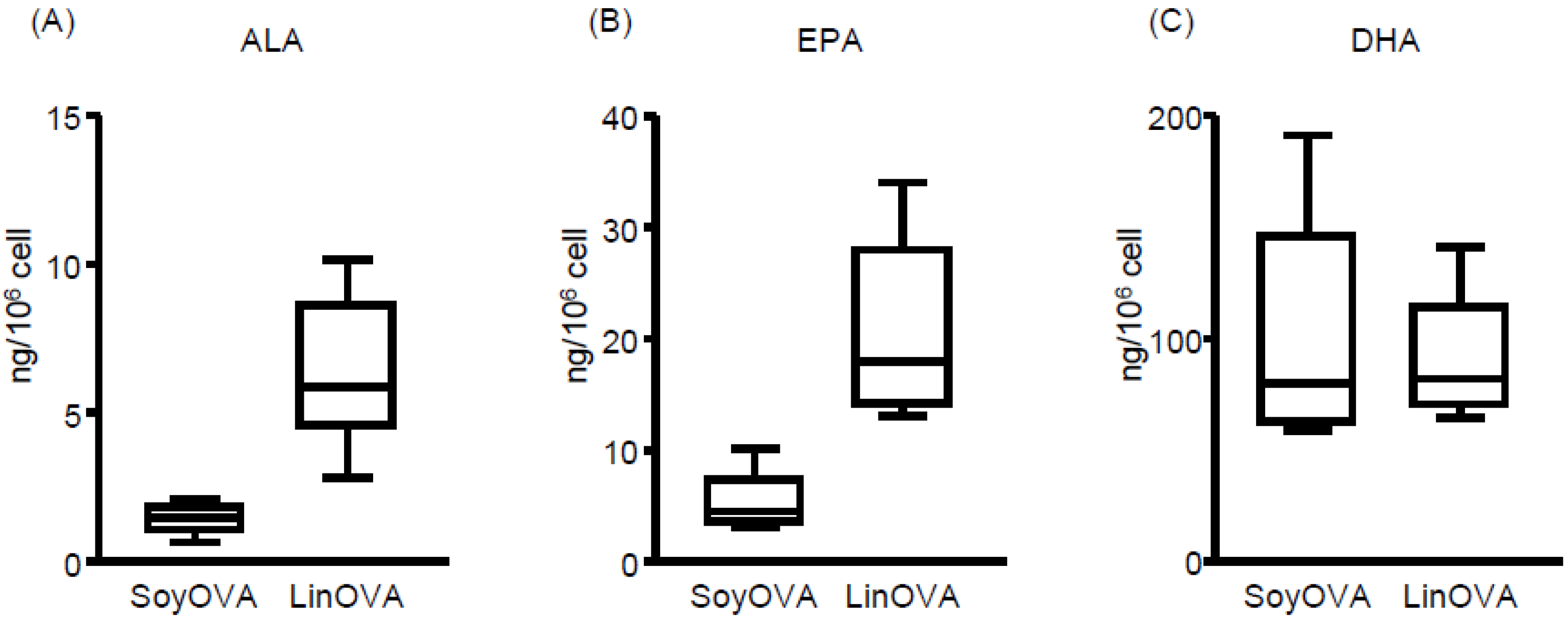
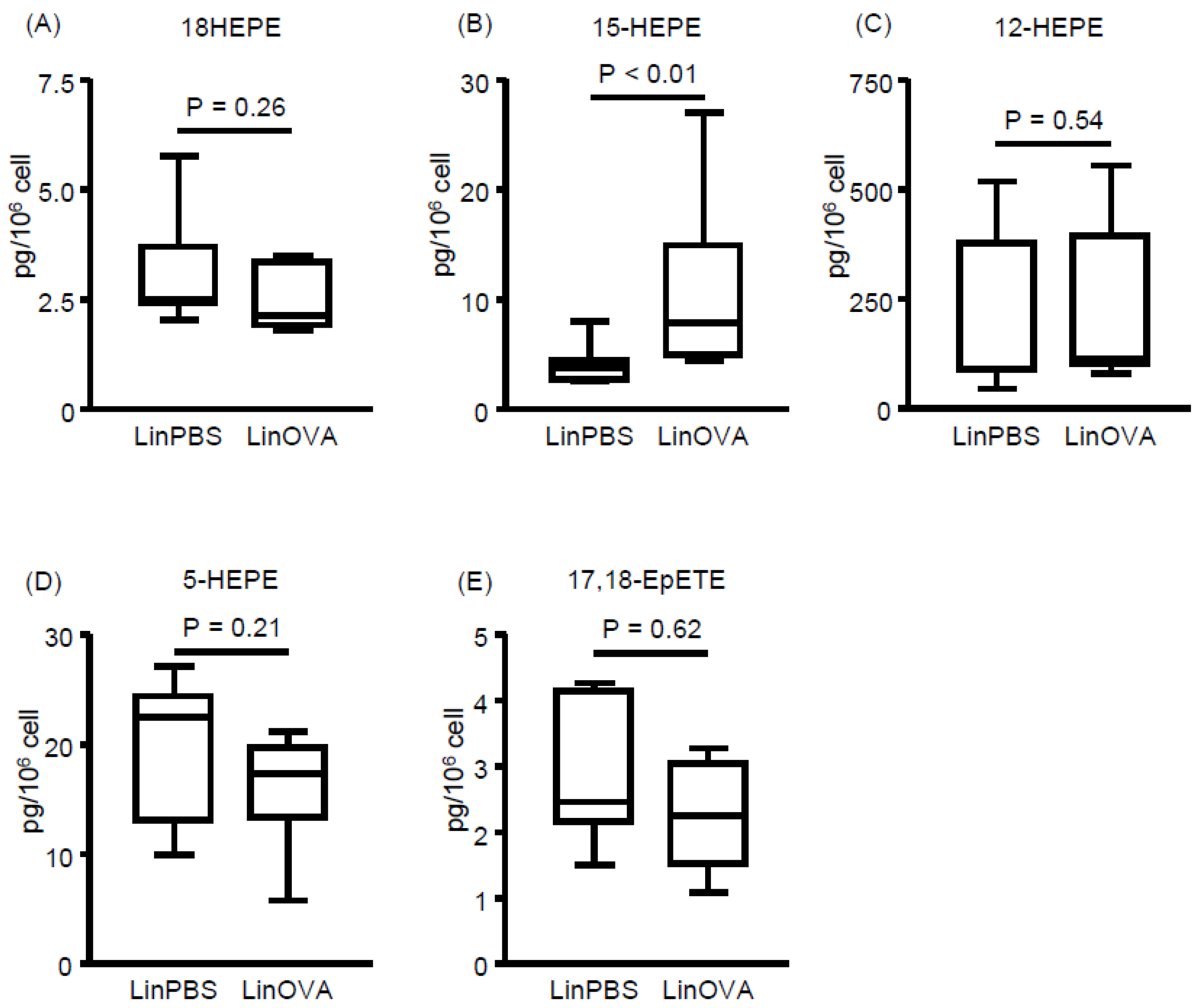
3.2. Dietary FA Changes the FA Composition and Lipid Metabolites in NP and Plasma
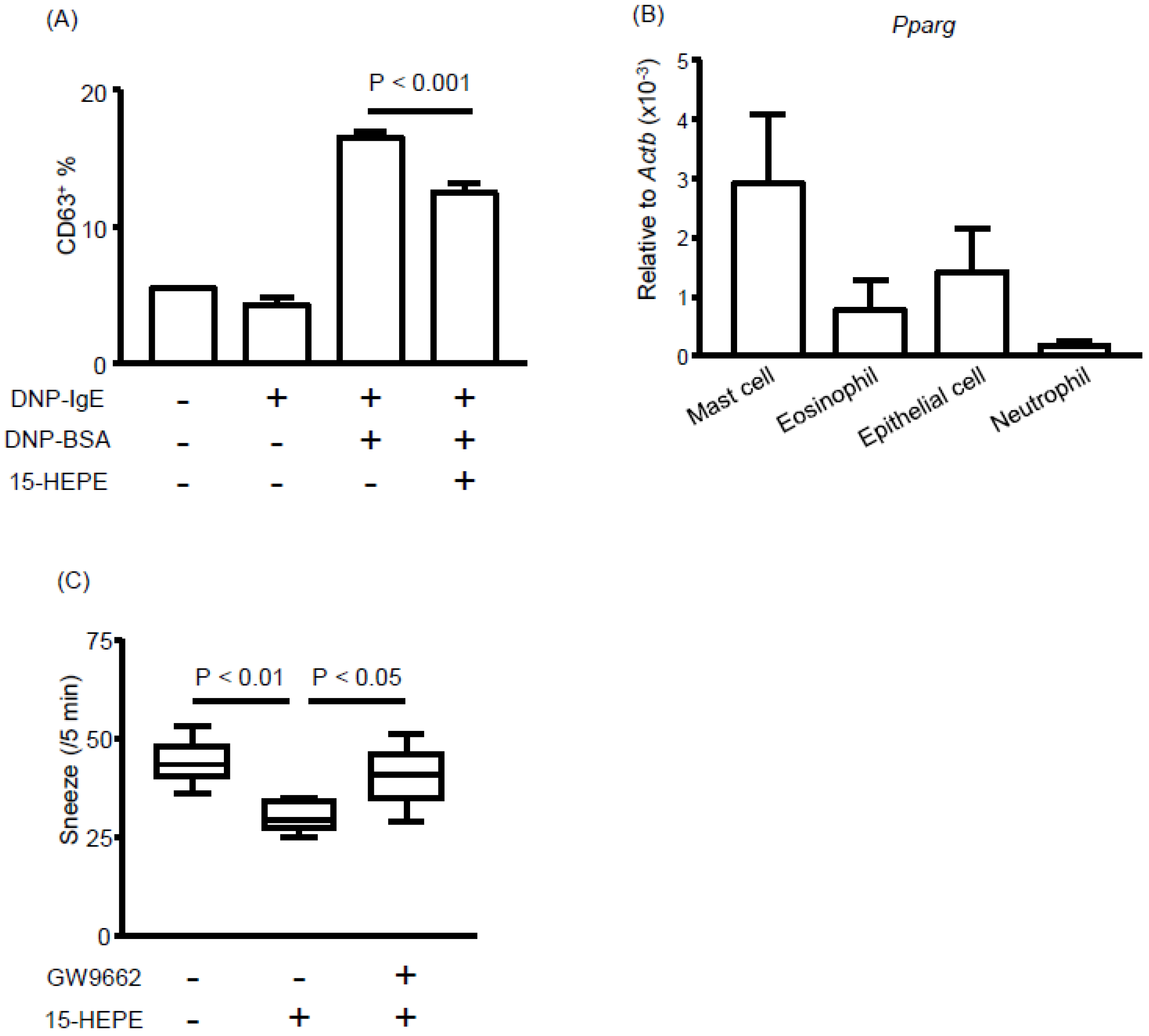
3.3. Nasal Administration of 15-HEPE Inhibits Allergic Rhinitis
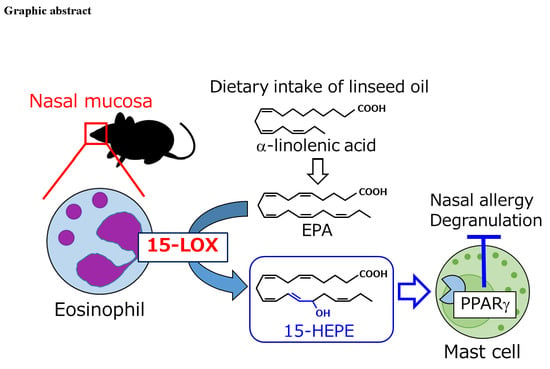
3.4. Eosinophils Express High Levels of 15-lipoxygenase (15-LOX) and Preferentially Produce 15-HEPE from EPA
3.5. The 15-HEPE Interacts with PPARγ and Inhibits Mast Cell Degranulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALA | alpha-linolenic acid |
| ARA | Arachidonic acid |
| CD | Cluster of differentiation |
| COX | Cyclooxygenase |
| CYP | Cytochrome P450 |
| DHA | Docosahexaenoic acid |
| DMSO | Dimethyl sulfoxide |
| EPA | Eicosapentaenoic acid |
| EpETE | Epoxyeicosatetraenoic acid |
| FA | Fatty acid |
| LA | Linoleic acid |
| LC–MS/MS | Liquid chromatography coupled with tandem mass spectrometry |
| LOX | Lipoxygenase |
| NP | Nasal passage |
| OVA | Ovalbumin |
| PPAR | Peroxisome proliferator-activated receptor gamma |
References
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K. Japanese guidelines for allergic rhinitis 2017. Allergol. Int. 2017, 66, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Eckl-Dorna, J.; Villazala-Merino, S.; Linhart, B.; Karaulov, A.V.; Zhernov, Y.; Khaitov, M.; Niederberger-Leppin, V.; Valenta, R. Allergen-specific antibodies regulate secondary allergen-specific immune responses. Front. Immunol. 2018, 9, 3131. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Valent, P.; Akin, C. Mast cells, mastocytosis, and related disorders. N. Engl. J. Med. 2015, 373, 1885–1886. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Meyer, R.W.; Nwaru, B.I.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; Akdis, C.A.; Bischoff, S.; et al. EAACI position paper: Influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy 2019. [Google Scholar] [CrossRef]
- Cazzoletti, L.; Zanolin, M.E.; Spelta, F.; Bono, R.; Chamitava, L.; Cerveri, I.; Garcia-Larsen, V.; Grosso, A.; Mattioli, V.; Pirina, P.; et al. Dietary fats, olive oil and respiratory diseases in Italian adults: A population-based study. Clin. Exp. Allergy 2019, 49, 799–807. [Google Scholar] [CrossRef]
- Nagatake, T.; Kunisawa, J. Emerging roles of metabolites of ω3 and ω6 essential fatty acids in the control of intestinal inflammation. Int. Immunol. 2019. [Google Scholar] [CrossRef]
- Wendell, S.G.; Baffi, C.; Holguin, F. Fatty acids, inflammation, and asthma. J. Allergy Clin. Immunol. 2014, 133, 1255–1264. [Google Scholar] [CrossRef]
- Willemsen, L.E.M. Dietary n-3 long chain polyunsaturated fatty acids in allergy prevention and asthma treatment. Eur. J. Pharmacol. 2016, 785, 174–186. [Google Scholar] [CrossRef]
- Fussbroich, D.; Zimmermann, K.; Göpel, A.; Eickmeier, O.; Trischler, J.; Zielen, S.; Schubert, R.; Beermann, C. A specific combined long-chain polyunsaturated fatty acid supplementation reverses fatty acid profile alterations in a mouse model of chronic asthma. Lipids Health Dis. 2019, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Arita, M.; Hayasaka, T.; Harada, T.; Iwamoto, R.; Nagasawa, R.; Shikata, S.; Nagatake, T.; Suzuki, H.; Hashimoto, E.; et al. Dietary ω3 fatty acid exerts anti-allergic effect through the conversion to 17,18-epoxyeicosatetraenoic acid in the gut. Sci. Rep. 2015, 5, 9750. [Google Scholar] [CrossRef] [PubMed]
- Hirakata, T.; Lee, H.C.; Ohba, M.; Saeki, K.; Okuno, T.; Murakami, A.; Matsuda, A.; Yokomizo, T. Dietary omega-3 fatty acids alter the lipid mediator profile and alleviate allergic conjunctivitis without modulating T. FASEB J. 2018. [Google Scholar] [CrossRef]
- Turner, N.; Cooney, G.J.; Kraegen, E.W.; Bruce, C.R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 2014, 220, T61–T79. [Google Scholar] [CrossRef] [PubMed]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Yoshida, M.; Arita, M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int. Immunol. 2019. [Google Scholar] [CrossRef]
- Haworth, O.; Cernadas, M.; Yang, R.; Serhan, C.N.; Levy, B.D. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008, 9, 873–879. [Google Scholar] [CrossRef]
- Li, D.; Hodges, R.R.; Jiao, J.; Carozza, R.B.; Shatos, M.A.; Chiang, N.; Serhan, C.N.; Dartt, D.A. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol. 2013, 6, 1119–1130. [Google Scholar] [CrossRef]
- Takamura, K.; Fukuyama, S.; Nagatake, T.; Kim, D.Y.; Kawamura, A.; Kawauchi, H.; Kiyono, H. Regulatory role of lymphoid chemokine CCL19 and CCL21 in the control of allergic rhinitis. J. Immunol. 2007, 179, 5897–5906. [Google Scholar] [CrossRef]
- Kim, D.Y.; Fukuyama, S.; Nagatake, T.; Takamura, K.; Kong, I.G.; Yokota, Y.; Lee, C.H.; Kiyono, H. Implications of nasopharynx-associated lymphoid tissue (NALT) in the development of allergic responses in an allergic rhinitis mouse model. Allergy 2012, 67, 502–509. [Google Scholar] [CrossRef]
- Nishijima, H.; Kondo, K.; Toma-Hirano, M.; Iwasaki, S.; Kikuta, S.; Fujimoto, C.; Ueha, R.; Kagoya, R.; Yamasoba, T. Denervation of nasal mucosa induced by posterior nasal neurectomy suppresses nasal secretion, not hypersensitivity, in an allergic rhinitis rat model. Lab. Investig. 2016, 96, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Nakagawa, Y.; Shimizu, M.; Wakabayashi, A.; Negishi, Y.; Hiroi, T.; Okubo, K.; Takahashi, H. Effects of dendritic cell subset manipulation on airway allergy in a mouse model. Int. Arch. Allergy Immunol. 2015, 168, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Eum, S.Y.; Maghni, K.; Hamid, Q.; Eidelman, D.H.; Campbell, H.; Isogai, S.; Martin, J.G. Inhibition of allergic airways inflammation and airway hyperresponsiveness in mice by dexamethasone: Role of eosinophils, IL-5, eotaxin, and IL-13. J. Allergy Clin. Immunol. 2003, 111, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Sato, A.; Fukuyama, S.; Sagara, H.; Nagatake, T.; Kong, I.G.; Goda, K.; Nochi, T.; Kunisawa, J.; Sato, S.; et al. The airway antigen sampling system: Respiratory M cells as an alternative gateway for inhaled antigens. J. Immunol. 2011, 186, 4253–4262. [Google Scholar] [CrossRef]
- Kurashima, Y.; Amiya, T.; Nochi, T.; Fujisawa, K.; Haraguchi, T.; Iba, H.; Tsutsui, H.; Sato, S.; Nakajima, S.; Iijima, H.; et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat. Commun. 2012, 3, 1034. [Google Scholar] [CrossRef]
- Tiwari, P.; Nagatake, T.; Hirata, S.I.; Sawane, K.; Saika, A.; Shibata, Y.; Morimoto, S.; Honda, T.; Adachi, J.; Abe, Y.; et al. Dietary coconut oil ameliorates skin contact hypersensitivity through mead acid production in mice. Allergy 2019. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. Bone marrow derived eosinophil cultures. Bio Protoc. 2014, 4, e1161. [Google Scholar] [CrossRef]
- Miyata, J.; Fukunaga, K.; Iwamoto, R.; Isobe, Y.; Niimi, K.; Takamiya, R.; Takihara, T.; Tomomatsu, K.; Suzuki, Y.; Oguma, T.; et al. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J. Allergy Clin. Immunol. 2013, 131, 353–360. [Google Scholar] [CrossRef]
- Isobe, Y.; Itagaki, M.; Ito, Y.; Naoe, S.; Kojima, K.; Ikeguchi, M.; Arita, M. Comprehensive analysis of the mouse cytochrome P450 family responsible for omega-3 epoxidation of eicosapentaenoic acid. Sci. Rep. 2018, 8, 7954. [Google Scholar] [CrossRef]
- Nagatake, T.; Fujita, H.; Minato, N.; Hamazaki, Y. Enteroendocrine cells are specifically marked by cell surface expression of claudin-4 in mouse small intestine. PLoS ONE 2014, 9, e90638. [Google Scholar] [CrossRef]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Mochimaru, T.; Fukunaga, K.; Miyata, J.; Matsusaka, M.; Masaki, K.; Kabata, H.; Ueda, S.; Suzuki, Y.; Goto, T.; Urabe, D.; et al. 12-OH-17,18-Epoxyeicosatetraenoic acid alleviates eosinophilic airway inflammation in murine lungs. Allergy 2018, 73, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Fukui, N.; Honda, K.; Ito, E.; Ishikawa, K. Peroxisome proliferator-activated receptor gamma negatively regulates allergic rhinitis in mice. Allergol. Int. 2009, 58, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Wada, K.; Katayama, K.; Kamisaki, Y.; Maeyama, K.; Kadowaki, T.; Blumberg, R.S.; Nakajima, A. Activation of peroxisome proliferator-activated receptor gamma suppresses mast cell maturation involved in allergic diseases. Allergy 2008, 63, 1136–1147. [Google Scholar] [CrossRef]
- Caligiuri, S.P.; Aukema, H.M.; Ravandi, A.; Guzman, R.; Dibrov, E.; Pierce, G.N. Flaxseed consumption reduces blood pressure in patients with hypertension by altering circulating oxylipins via an α-linolenic acid-induced inhibition of soluble epoxide hydrolase. Hypertension 2014, 64, 53–59. [Google Scholar] [CrossRef]
- Fukumitsu, S.; Villareal, M.O.; Onaga, S.; Aida, K.; Han, J.; Isoda, H. α-Linolenic acid suppresses cholesterol and triacylglycerol biosynthesis pathway by suppressing SREBP-2, SREBP-1a and -1c expression. Cytotechnology 2013, 65, 899–907. [Google Scholar] [CrossRef]
- Nagatake, T.; Shiogama, Y.; Inoue, A.; Kikuta, J.; Honda, T.; Tiwari, P.; Kishi, T.; Yanagisawa, A.; Isobe, Y.; Matsumoto, N.; et al. The 17,18-epoxyeicosatetraenoic acid-G protein-coupled receptor 40 axis ameliorates contact hypersensitivity by inhibiting neutrophil mobility in mice and cynomolgus macaques. J. Allergy Clin. Immunol. 2017. [Google Scholar] [CrossRef]
- Lin, H.; Zheng, C.; Li, J.; Yang, C.; Hu, L. Lentiviral shRNA against KCa3.1 inhibits allergic response in allergic rhinitis and suppresses mast cell activity via PI3K/AKT signaling pathway. Sci. Rep. 2015, 5, 13127. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Fang, S.; Zhu, Z.; Yao, M.; Ying, L.; Zhu, L.; Ma, Z.; Wang, W. Peroxisome proliferator-activated receptor γ agonist suppresses mast cell maturation and induces apoptosis. Mol. Med. Rep. 2017, 16, 1793–1800. [Google Scholar] [CrossRef]
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Leishangthem, G.D.; Singh, S.; Dinda, A.K.; Biswal, S.; Agrawal, A.; Ghosh, B. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci. Rep. 2013, 3, 1540. [Google Scholar] [CrossRef]
- Endo, J.; Sano, M.; Isobe, Y.; Fukuda, K.; Kang, J.X.; Arai, H.; Arita, M. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. J. Exp. Med. 2014, 211, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Bianchini, F.; Aliberti, J.; Sher, A.; Chiang, N.; Hong, S.; Yang, R.; Petasis, N.A.; Serhan, C.N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005, 201, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.F.; Spencer, L.A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017, 17, 746–760. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.C.; Ackerman, S.J.; Gleich, G.J.; Thomas, L.L. Activation of basophil and mast cell histamine release by eosinophil granule major basic protein. J. Exp. Med. 1983, 157, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Elishmereni, M.; Bachelet, I.; Ben-Efraim, A.H.N.; Mankuta, D.; Levi-Schaffer, F. Interacting mast cells and eosinophils acquire an enhanced activation state in vitro. Allergy 2013, 68, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Arnold, I.C.; Artola-Borán, M.; de Lara, P.T.; Kyburz, A.; Taube, C.; Ottemann, K.; van den Broek, M.; Yousefi, S.; Simon, H.U.; Müller, A. Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J. Exp. Med. 2018, 215, 2055–2072. [Google Scholar] [CrossRef] [PubMed]
- Mesnil, C.; Raulier, S.; Paulissen, G.; Xiao, X.; Birrell, M.A.; Pirottin, D.; Janss, T.; Starkl, P.; Ramery, E.; Henket, M.; et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J. Clin. Investig. 2016, 126, 3279–3295. [Google Scholar] [CrossRef] [PubMed]
- Arita, M. Eosinophil polyunsaturated fatty acid metabolism and its potential control of inflammation and allergy. Allergol. Int. 2016, 65 (Suppl.), S2–S5. [Google Scholar] [CrossRef]
- Isobe, Y.; Arita, M.; Matsueda, S.; Iwamoto, R.; Fujihara, T.; Nakanishi, H.; Taguchi, R.; Masuda, K.; Sasaki, K.; Urabe, D.; et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 2012, 287, 10525–10534. [Google Scholar] [CrossRef]
- Yamada, T.; Tani, Y.; Nakanishi, H.; Taguchi, R.; Arita, M.; Arai, H. Eosinophils promote resolution of acute peritonitis by producing proresolving mediators in mice. FASEB J. 2011, 25, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Miyata, J.; Fukunaga, K.; Kawashima, Y.; Watanabe, T.; Saitoh, A.; Hirosaki, T.; Araki, Y.; Kikawada, T.; Betsuyaku, T.; Ohara, O.; et al. Dysregulated fatty acid metabolism in nasal polyp-derived eosinophils from patients with chronic rhinosinusitis. Allergy 2019, 74, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawane, K.; Nagatake, T.; Hosomi, K.; Hirata, S.-i.; Adachi, J.; Abe, Y.; Isoyama, J.; Suzuki, H.; Matsunaga, A.; Fukumitsu, S.; et al. Dietary Omega-3 Fatty Acid Dampens Allergic Rhinitis via Eosinophilic Production of the Anti-Allergic Lipid Mediator 15-Hydroxyeicosapentaenoic Acid in Mice. Nutrients 2019, 11, 2868. https://doi.org/10.3390/nu11122868
Sawane K, Nagatake T, Hosomi K, Hirata S-i, Adachi J, Abe Y, Isoyama J, Suzuki H, Matsunaga A, Fukumitsu S, et al. Dietary Omega-3 Fatty Acid Dampens Allergic Rhinitis via Eosinophilic Production of the Anti-Allergic Lipid Mediator 15-Hydroxyeicosapentaenoic Acid in Mice. Nutrients. 2019; 11(12):2868. https://doi.org/10.3390/nu11122868
Chicago/Turabian StyleSawane, Kento, Takahiro Nagatake, Koji Hosomi, So-ichiro Hirata, Jun Adachi, Yuichi Abe, Junko Isoyama, Hidehiko Suzuki, Ayu Matsunaga, Satoshi Fukumitsu, and et al. 2019. "Dietary Omega-3 Fatty Acid Dampens Allergic Rhinitis via Eosinophilic Production of the Anti-Allergic Lipid Mediator 15-Hydroxyeicosapentaenoic Acid in Mice" Nutrients 11, no. 12: 2868. https://doi.org/10.3390/nu11122868
APA StyleSawane, K., Nagatake, T., Hosomi, K., Hirata, S.-i., Adachi, J., Abe, Y., Isoyama, J., Suzuki, H., Matsunaga, A., Fukumitsu, S., Aida, K., Tomonaga, T., Arita, M., & Kunisawa, J. (2019). Dietary Omega-3 Fatty Acid Dampens Allergic Rhinitis via Eosinophilic Production of the Anti-Allergic Lipid Mediator 15-Hydroxyeicosapentaenoic Acid in Mice. Nutrients, 11(12), 2868. https://doi.org/10.3390/nu11122868