Interactions between DRD2/ANKK1 TaqIA Polymorphism and Dietary Factors Influence Plasma Triglyceride Concentrations in Diabetic Patients from Western Mexico: A Cross-sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Anthropometric Measurements
2.3. Dietary Assessment
2.4. Blood Tests
2.5. DRD2/ANKK1 Genotyping
2.6. Statistical Analyses
3. Results
3.1. Distribution of the DRD2/ANKK1 TaqIA Polymorphism and Characteristics of the Study Population
3.2. Daily Dietary Intake by Genotyes of the DRD2/ANKK1 TaqIA Polymorphism
3.3. Biochemical Profile by Genotyes of the DRD2/ANKK1 TaqIA Polymorphism
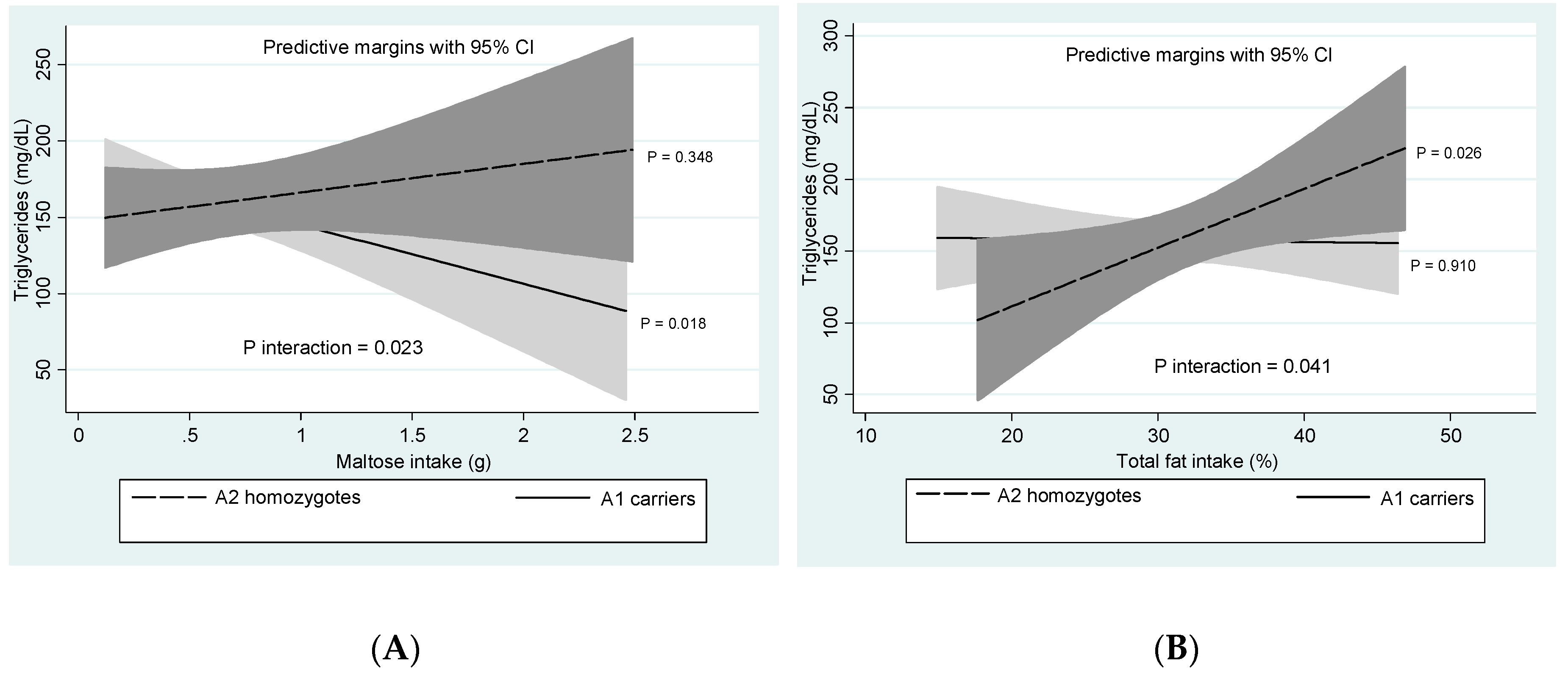
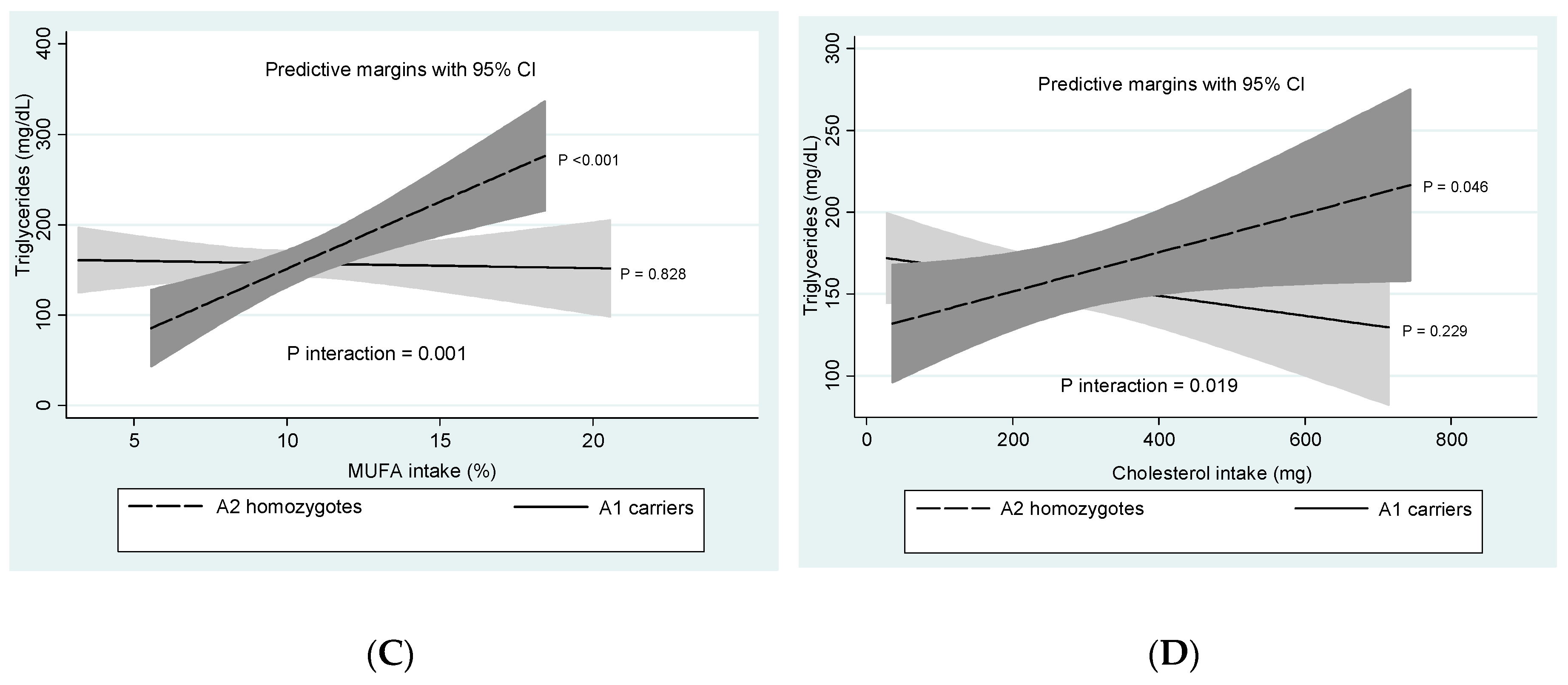
3.4. DRD2/ANKK1-Diet Interactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soto-Estrada, G.; Moreno Altamirano, L.; García-García, J.J.; Ochoa Moreno, I.; Silberman, M. Trends in frequency of type 2 diabetes in Mexico and its relationship to dietary patterns and contextual factors. Gac. Sanit. 2018, 32, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Ramos-López, O.; Román, S.; Ojeda-Granados, C.; Sepúlveda-Villegas, M.; Martínez-López, E.; Torres-Valadez, R.; Trujillo-Trujillo, E.; Panduro, A. Patrón de ingesta alimentaria y actividad física en pacientes hepatópatas en el Occidente de México. Rev. Endocrinol. Nutr. 2013, 21, 7–15. [Google Scholar]
- Campos-Pérez, W.; González-Becerra, K.; Ramos-López, O.; Silva-Gómez, J.A.; Barrón-Cabrera, E.; Roman, S.; Panduro, A.; Martínez-López, E. Same Dietary but different physical activity pattern in normal-weight and overweight Mexican subjects. J. Food Nutr. Res. 2016, 4, 729–735. [Google Scholar]
- Jannasch, F.; Kröger, J.; Schulze, M.B. Dietary patterns and type 2 diabetes: A systematic literature review and meta-analysis of prospective studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Ramos-López, O.; Ojeda-Granados, C.; Román, S.; Panduro, A. Influencia genética en las preferencias alimentarias. Rev. Endocrinol. Nutr. 2013, 21, 74–83. [Google Scholar]
- Neville, M.J.; Johnstone, E.C.; Walton, R.T. Identification and characterization of ANKK1: A novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum. Mutat. 2004, 23, 540–545. [Google Scholar] [CrossRef]
- Yeh, J.; Trang, A.; Henning, S.M.; Wilhalme, H.; Carpenter, C.; Heber, D.; Li, Z. Food cravings, food addiction, and a dopamine-resistant (DRD2 A1) receptor polymorphism in Asian American college students. Asia Pac. J. Clin. Nutr. 2016, 25, 424–429. [Google Scholar]
- Gluskin, B.S.; Mickey, B.J. Genetic variation and dopamine D2 receptor availability: A systematic review and meta-analysis of human in vivo molecular imaging studies. Transl. Psychiatry 2016, 6, e747. [Google Scholar] [CrossRef]
- Eisenstein, S.A.; Bogdan, R.; Love-Gregory, L.; Corral-Frías, N.S.; Koller, J.M.; Black, K.J.; Moerlein, S.M.; Perlmutter, J.S.; Barch, D.M.; Hershey, T. Prediction of striatal D2 receptor binding by DRD2/ANKK1 TaqIA allele status. Synapse 2016, 70, 418–431. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Panduro, A.; Martinez-Lopez, E.; Roman, S. Sweet taste receptor TAS1R2 polymorphism (Val191Val) is associated with a higher carbohydrate intake and hypertriglyceridemia among the population of west Mexico. Nutrients 2016, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Roman, S.; Martinez-Lopez, E.; Fierro, N.A.; Gonzalez-Aldaco, K.; Jose-Abrego, A.; Panduro, A. CD36 genetic variation, fat intake and liver fibrosis in chronic hepatitis C virus infection. World J. Hepatol. 2016, 8, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Panduro, A.; Rivera-Iñiguez, I.; Roman, S. Dopamine D2 receptor polymorphism (C957T) is associated with sugar consumption and triglyceride levels in West Mexicans. Physiol. Behav. 2018, 194, 532–537. [Google Scholar] [CrossRef]
- Torres-Castillo, N.; Silva-Gómez, J.A.; Campos-Perez, W.; Barron-Cabrera, E.; Hernandez-Cañaveral, I.; Garcia-Cazarin, M.; Marquez-Sandoval, Y.; Gonzalez-Becerra, K.; Barron-Gallardo, C.; Martinez-Lopez, E. High Dietary ω-6:ω-3 PUFA ratio is positively associated with excessive adiposity and waist circumference. Obes. Facts 2018, 11, 344–353. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Rev. Esp. Cardiol. 2016, 69, 939. [Google Scholar]
- Navarro-González, D.; Sánchez-Íñigo, L.; Pastrana-Delgado, J.; Fernández-Montero, A.; Martinez, J.A. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The Vascular-Metabolic CUN cohort. Prev. Med. 2016, 86, 99–105. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Haffner, S.M.; American Diabetes Association. Dyslipidemia management in adults with diabetes. Diabetes Care 2004, 27 (Suppl. 1), S68–S71. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
- Grandy, D.K.; Zhang, Y.; Civelli, O. PCR detection of the TaqA RFLP at the DRD2 locus. Hum. Mol. Genet. 1993, 2, 2197. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Cuervo, M.; Goni, L.; Martinez, J.A. Prediction of blood lipid phenotypes using obesity-related genetic polymorphisms and lifestyle data in subjects with excessive body weight. Int. J. Genom. 2018, 2018, 4283078. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.; Zepeda-Carrillo, E.A.; Moreno-Luna, L.E.; Panduro, A. Alcoholism and liver disease in Mexico: Genetic and environmental factors. World J. Gastroenterol. 2013, 19, 7972–7982. [Google Scholar] [CrossRef] [PubMed]
- Panduro, A.; Ramos-Lopez, O.; Campollo, O.; Zepeda-Carrillo, E.A.; Gonzalez-Aldaco, K.; Torres-Valadez, R.; Roman, S. High frequency of the DRD2/ANKK1 A1 allele in Mexican Native Amerindians and Mestizos and its association with alcohol consumption. Drug Alcohol Depend. 2017, 172, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.P.; Hanson, R.; Cray, K.; Wiedrich, C.; Knowler, W.C.; Bogardus, C.; Baier, L. Association of dopamine D2 receptor polymorphisms Ser311Cys and TaqIA with obesity or type 2 diabetes mellitus in Pima Indians. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1233–1238. [Google Scholar] [CrossRef]
- Barr, C.L.; Kidd, K.K. Population frequencies of the A1 allele at the dopamine D2 receptor locus. Biol. Psychiatry 1993, 34, 204–209. [Google Scholar] [CrossRef]
- International HapMap Consortium. The International HapMap Project. Nature 2003, 426, 789–796. [Google Scholar] [CrossRef]
- 1000 Genomes Project Consortium; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar]
- Stice, E.; Dagher, A. Genetic variation in dopaminergic reward in humans. Forum Nutr. 2010, 63, 176–185. [Google Scholar] [PubMed]
- Obregón, A.M.; Valladares, M.; Goldfield, G. Association of the dopamine D2 receptor rs1800497 polymorphism and eating behavior in Chilean children. Nutrition 2017, 35, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.; Ojeda-Granados, C.; Ramos-Lopez, O.; Panduro, A. Genome-based nutrition: An intervention strategy for the prevention and treatment of obesity and nonalcoholic steatohepatitis. World J. Gastroenterol. 2015, 21, 3449–3461. [Google Scholar] [CrossRef] [PubMed]
- Beigrezaei, S.; Ghiasvand, R.; Feizi, A.; Iraj, B. Relationship between dietary patterns and incidence of type 2 diabetes. Int. J. Prev. Med. 2019, 10, 122. [Google Scholar]
- Barnard, N.D.; Noble, E.P.; Ritchie, T.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Green, A.A.; Ferdowsian, H. D2 dopamine receptor Taq1A polymorphism, body weight, and dietary intake in type 2 diabetes. Nutrition 2009, 25, 58–65. [Google Scholar] [CrossRef]
- Huang, T.; Hu, F.B. Gene-environment interactions and obesity: Recent developments and future directions. BMC Med. Genom. 2015, 8 (Suppl. 1), S2. [Google Scholar] [CrossRef]
- Rivera-Iñiguez, I.; Panduro, A.; Ramos-Lopez, O.; Villaseñor-Bayardo, S.J.; Roman, S. DRD2/ANKK1 TaqI A1 polymorphism associates with overconsumption of unhealthy foods and biochemical abnormalities in a Mexican population. Eat. Weight Disord. 2018. [Google Scholar] [CrossRef]
- Cardel, M.I.; Lemas, D.J.; Lee, A.M.; Miller, D.R.; Huo, T.; Klimentidis, Y.C.; Fernandez, J.R. Taq1a polymorphism (rs1800497) is associated with obesity-related outcomes and dietary intake in a multi-ethnic sample of children. Pediatr. Obes. 2019, 14, e12470. [Google Scholar] [CrossRef]
- Heni, M.; Kullmann, S.; Ahlqvist, E.; Wagner, R.; Machicao, F.; Staiger, H.; Häring, H.U.; Almgren, P.; Groop, L.C.; Small, D.M.; et al. Interaction between the obesity-risk gene FTO and the dopamine D2 receptor gene ANKK1/TaqIA on insulin sensitivity. Diabetologia 2016, 59, 2622–2631. [Google Scholar] [CrossRef]
- Rivas-Gomez, B.; Almeda-Valdés, P.; Tussié-Luna, M.T.; Aguilar-Salinas, C.A. Dyslipidemia in Mexico, a call for action. Rev. Invest. Clin. 2018, 70, 211–216. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Martinez-Lopez, E.; Roman, S.; Fierro, N.A.; Panduro, A. Genetic, metabolic and environmental factors involved in the development of liver cirrhosis in Mexico. World J. Gastroenterol. 2015, 21, 11552–11566. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Cuervo, M.; Goni, L.; Martinez, J.A. models integrating genetic and lifestyle interactions on two adiposity phenotypes for personalized prescription of energy-restricted diets with different macronutrient distribution. Front. Genet. 2019, 10, 686. [Google Scholar] [CrossRef] [PubMed]
| Variable | A1 Carriers n = 120 | A2 Homozygotes n = 55 | p Value |
|---|---|---|---|
| Age (years) | 58.4 ± 11.0 | 57.7 ± 11.1 | 0.699 |
| Sex (F/M) | 77/43 | 34/21 | 0.866 |
| Years with T2D | 7.7 ± 8.79 | 7.7 ± 7.54 | 0.847 |
| BMI (kg/m2) | 29.8 ± 5.7 | 29.5 ± 5.2 | 0.711 |
| Normal weight, n (%) | 21 (18.4%) | 12 (22.6%) | 0.804 |
| Overweight, n (%) | 45 (39.4%) | 20 (37.7%) | |
| Obesity, n (%) | 48 (42.1%) | 21 (39.6%) | |
| WHR | 0.95 ± 0.104 | 0.94 ± 0.074 | 0.405 |
| Total body fat % | 33.8 ± 8.55 | 34.0 ± 7.78 | 0.894 |
| Nutrient | Reference Values | A1 Carriers | A2 Homozygotes | p Value |
|---|---|---|---|---|
| Total energy (Kcal/d) | - | 1556.8 ± 401.7 | 1554.7 ± 450.8 | 0.975 |
| Total carbohydrates (%E/d) | 50–60 | 50.6 ± 10.2 | 47.9 ± 9.8 | 0.091 |
| Glucose (g/d) | - | 10.8 ± 9.3 | 9.7 ± 6.8 | 0.457 |
| Galactose (g/d) | - | 0.05 ± 0.27 | 0.02 ± 0.07 | 0.459 |
| Fructose (g/d) | - | 14.85 ± 11.82 | 13.4 ± 10.1 | 0.442 |
| Sacarose (g/d) | - | 10.8 ± 8.1 | 8.9 ± 6.9 | 0.120 |
| Lactose (g/d) | - | 1.2 ± 3.5 | 1.2 ± 3.0 | 0.924 |
| Maltose (g/d) | - | 0.68 ± 0.42 | 0.73 ± 0.56 | 0.486 |
| Sugar (g/d) | - | 54.5 ± 31.6 | 50.7 ± 24.5 | 0.440 |
| Fiber (g/d) | 30 | 20.8 ± 6.9 | 18.7 ± 6.4 | 0.060 |
| Total protein (%E/d) | 15 | 17.7 ± 3.1 | 18.0 ± 2.8 | 0.657 |
| Total fat (%E/d) | <30 | 30.7 ± 6.6 | 32.2 ± 6.0 | 0.178 |
| SFA (%E/d) | <7 | 8.4 ± 2.6 | 8.5 ± 2.4 | 0.838 |
| MUFA (%E/d) | 10–15 | 10.0 ± 2.8 | 10.7 ± 2.7 | 0.137 |
| PUFA (%E/d) | 10 | 7.6 ± 2.7 | 8.1 ± 2.4 | 0.221 |
| Cholesterol (mg/d) | <200 | 264.2 ± 136.1 | 280.6 ± 184.4 | 0.511 |
| Nutrient | Reference Values | A1 Carriers | A2 Homozygotes | p Value |
|---|---|---|---|---|
| Vitamin A (µg/d) | 900 | 389.7 ± 247.0 | 301.4 ± 194.4 | 0.020 |
| Vitamin B1 (mg/d) | 1.5 | 1.02 ± 0.35 | 1.02 ± 0.33 | 0.891 |
| Vitamin B2 (mg/d) | 1.7 | 1.29 ± 0.48 | 1.26 ± 0.46 | 0.701 |
| Vitamin B3 (mg/d) | 20 | 15.8 ± 5.8 | 15.7 ± 5.3 | 0.888 |
| Vitamin B5 (mg/d) | 10 | 3.55 ± 1.86 | 3.31 ± 1.59 | 0.404 |
| Vitamin B6 (mg/d) | 2 | 1.79 ± 0.73 | 1.68 ± 0.59 | 0.303 |
| Vitamin B8 (µg/d) | 200 | 8.29 ± 5.98 | 8.24 ± 6.66 | 0.963 |
| Vitamin B9 (µg/d) | 200 | 255.3 ± 118.4 | 228.3 ± 82.4 | 0.128 |
| Vitamin B12 (µg/d) | 2 | 3.05 ± 3.76 | 2.52 ± 1.33 | 0.306 |
| Vitamin C (mg/d) | 60 | 83.0 ± 75.9 | 72.3 ± 83.7 | 0.402 |
| Vitamin D (IU/d) | 400 | 136.4 ± 83.4 | 139.7 ± 95.2 | 0.820 |
| Vitamin E (IU/d) | 10 | 5.96 ± 5.90 | 6.05 ± 5.60 | 0.924 |
| Ca (mg/d) | 800 | 609.9 ± 238.4 | 591.4 ± 300.9 | 0.661 |
| K (mg/d) | 1800 | 2503.6 ± 656.7 | 2353.9 ± 765.1 | 0.186 |
| Se (µg/d) | 55–70 | 68.9 ± 27.0 | 71.2 ± 29.0 | 0.615 |
| Mg (mg/d) | 350 | 295.9 ± 85.5 | 286.2 ± 91.4 | 0.499 |
| Zn (mg/d) | 15 | 8.57 ± 3.99 | 8.5 ± 2.7 | 0.867 |
| Biochemical Variable | Reference Values | A1 Carriers | A2 Homozygotes | p Value |
|---|---|---|---|---|
| Total cholesterol (mg/dL) | <200 | 179.5 ± 34.5 | 178.5 ± 31.5 | 0.801 |
| Triglycerides (mg/dL) | <150 | 157.3 ± 75.7 | 161.3 ± 81.6 | 0.891 |
| HbA1c (%) | <6.5 | 7.50 ± 2.00 | 7.51 ± 1.96 | 0.701 |
| Glucose (mg/dL) | <110 | 144 ± 57.2 | 152 ± 70.3 | 0.421 |
| TyG index (ratio) | <8.31 | 9.14 ± 0.62 | 9.32 ± 0.78 | 0.097 |
| HDL-c (mg/dL) | >50 | 46 ± 12.1 | 49 ± 14.8 | 0.315 |
| Non-HDL-c (mg/dL) | <130 | 135 ± 34.5 | 130 ± 29.2 | 0.349 |
| LDL-c (mg/dL) | <100 | 104 ± 30.3 | 98 ± 24.5 | 0.214 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Lopez, O.; Mejia-Godoy, R.; Frías-Delgadillo, K.J.; Torres-Valadez, R.; Flores-García, A.; Sánchez-Enríquez, S.; Aguiar-García, P.; Martínez-López, E.; Zepeda-Carrillo, E.A. Interactions between DRD2/ANKK1 TaqIA Polymorphism and Dietary Factors Influence Plasma Triglyceride Concentrations in Diabetic Patients from Western Mexico: A Cross-sectional Study. Nutrients 2019, 11, 2863. https://doi.org/10.3390/nu11122863
Ramos-Lopez O, Mejia-Godoy R, Frías-Delgadillo KJ, Torres-Valadez R, Flores-García A, Sánchez-Enríquez S, Aguiar-García P, Martínez-López E, Zepeda-Carrillo EA. Interactions between DRD2/ANKK1 TaqIA Polymorphism and Dietary Factors Influence Plasma Triglyceride Concentrations in Diabetic Patients from Western Mexico: A Cross-sectional Study. Nutrients. 2019; 11(12):2863. https://doi.org/10.3390/nu11122863
Chicago/Turabian StyleRamos-Lopez, Omar, Roberto Mejia-Godoy, Kevin J. Frías-Delgadillo, Rafael Torres-Valadez, Aurelio Flores-García, Sergio Sánchez-Enríquez, Pedro Aguiar-García, Erika Martínez-López, and Eloy A. Zepeda-Carrillo. 2019. "Interactions between DRD2/ANKK1 TaqIA Polymorphism and Dietary Factors Influence Plasma Triglyceride Concentrations in Diabetic Patients from Western Mexico: A Cross-sectional Study" Nutrients 11, no. 12: 2863. https://doi.org/10.3390/nu11122863
APA StyleRamos-Lopez, O., Mejia-Godoy, R., Frías-Delgadillo, K. J., Torres-Valadez, R., Flores-García, A., Sánchez-Enríquez, S., Aguiar-García, P., Martínez-López, E., & Zepeda-Carrillo, E. A. (2019). Interactions between DRD2/ANKK1 TaqIA Polymorphism and Dietary Factors Influence Plasma Triglyceride Concentrations in Diabetic Patients from Western Mexico: A Cross-sectional Study. Nutrients, 11(12), 2863. https://doi.org/10.3390/nu11122863





