Administration of Asian Herb Bennet (Geum japonicum) Extract Reverses Depressive-Like Behaviors in Mouse Model of Depression Induced by Corticosterone
Abstract
1. Introduction
2. Materials and Methods
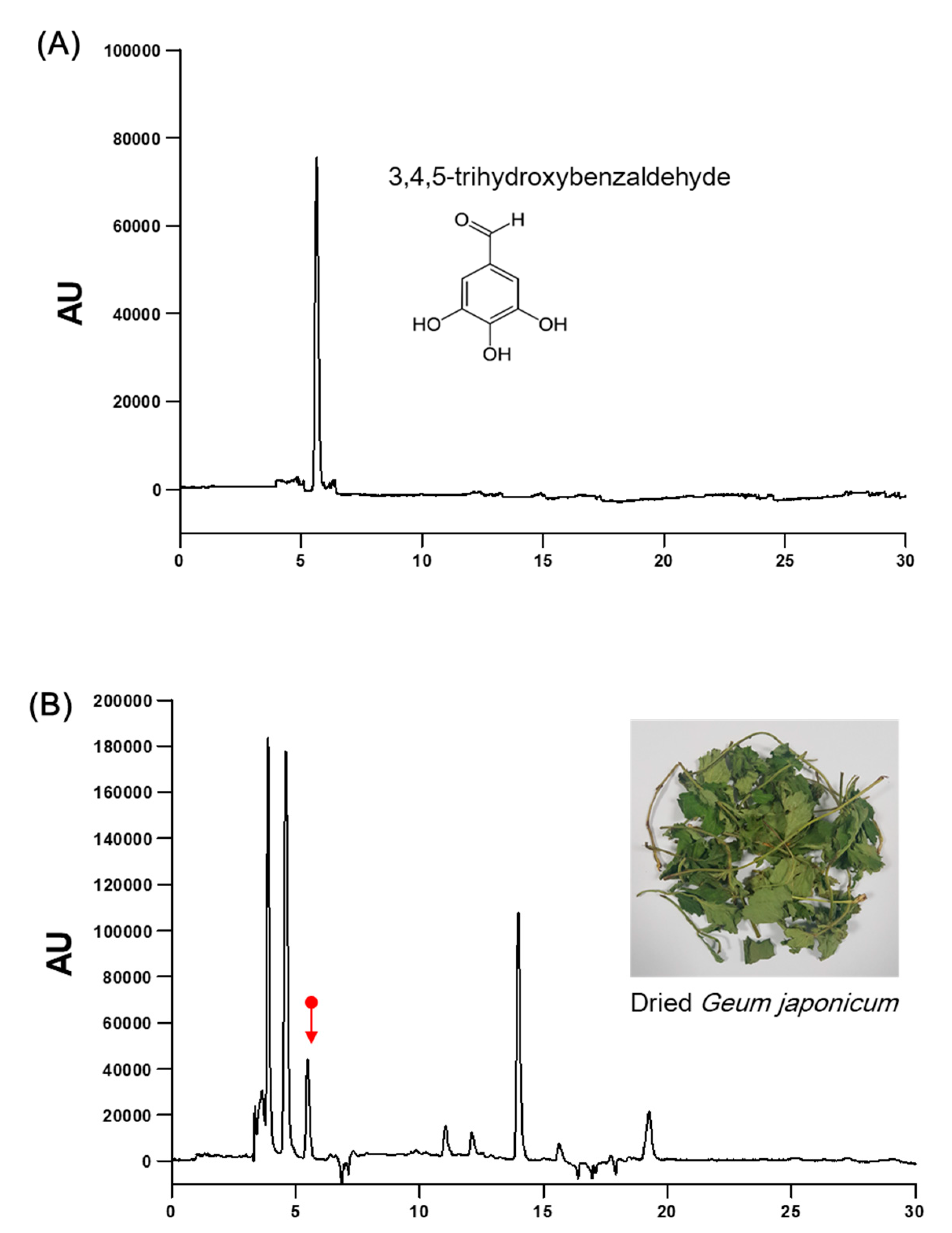
2.1. Preparation of the Standardized Geum japonicum
2.2. Animals
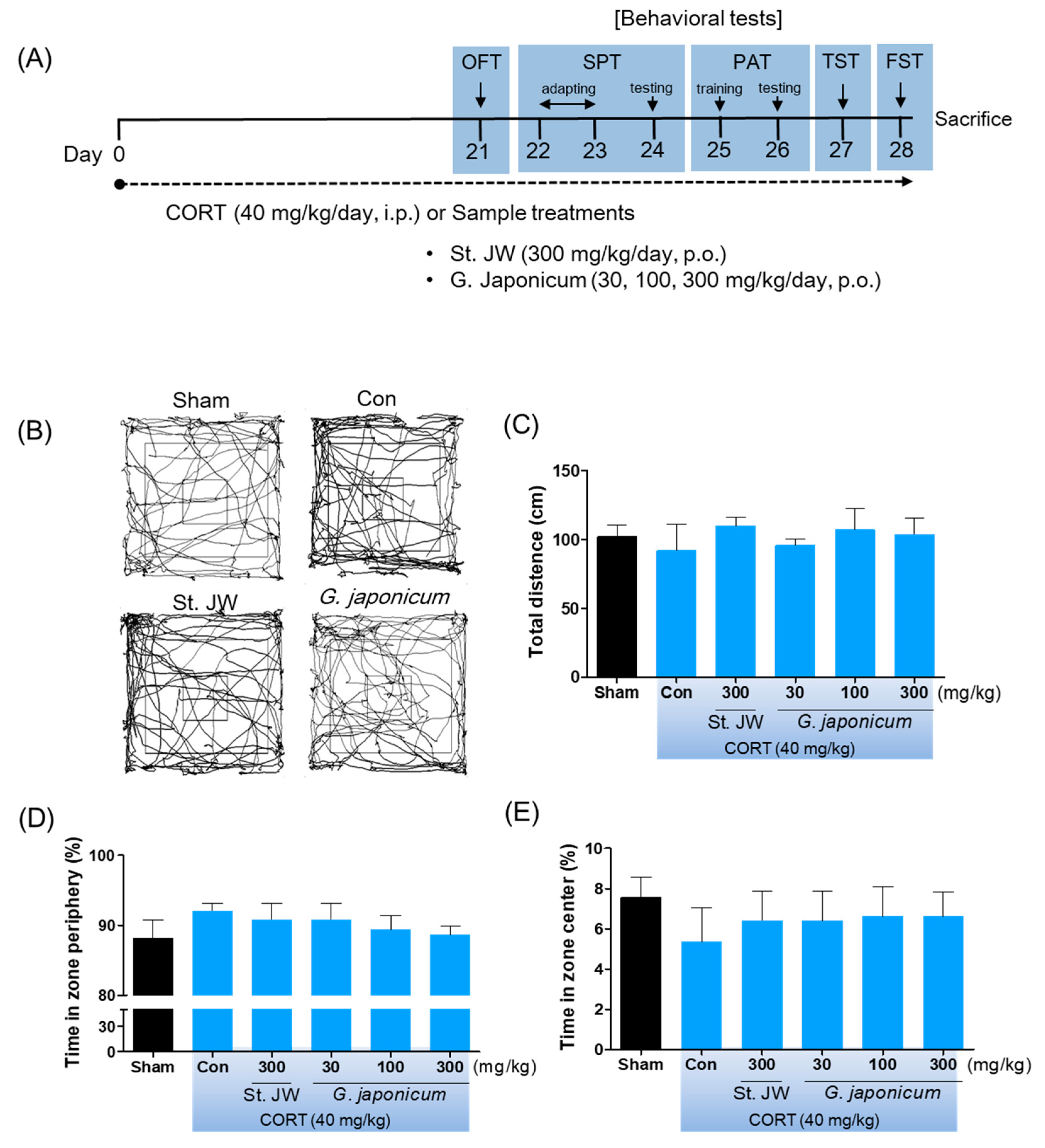
2.3. Corticosterone (CORT) and Extracts Administration
2.4. Open Field Test (OFT)
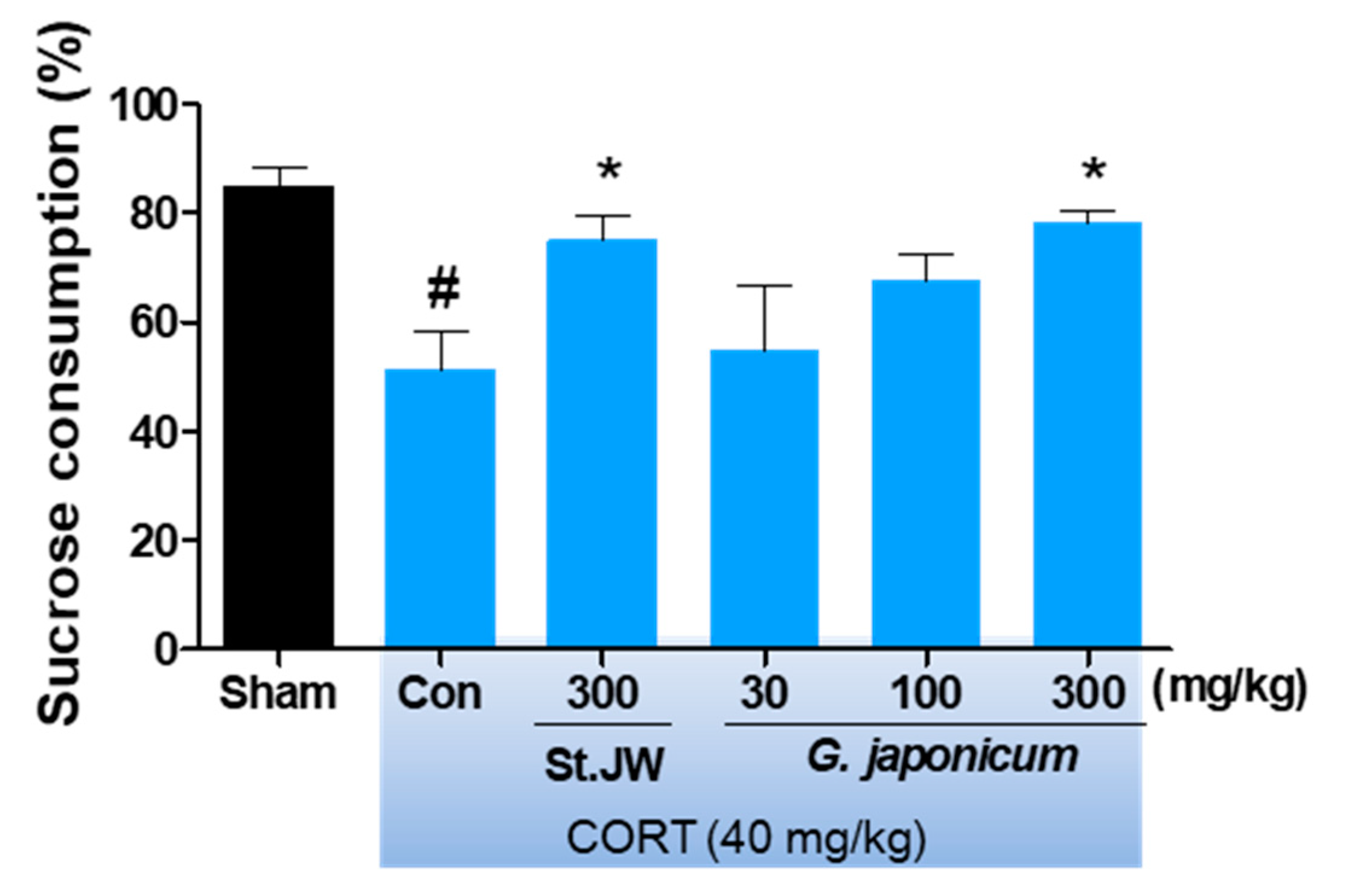
2.5. Sucrose Preference Test (SPT)
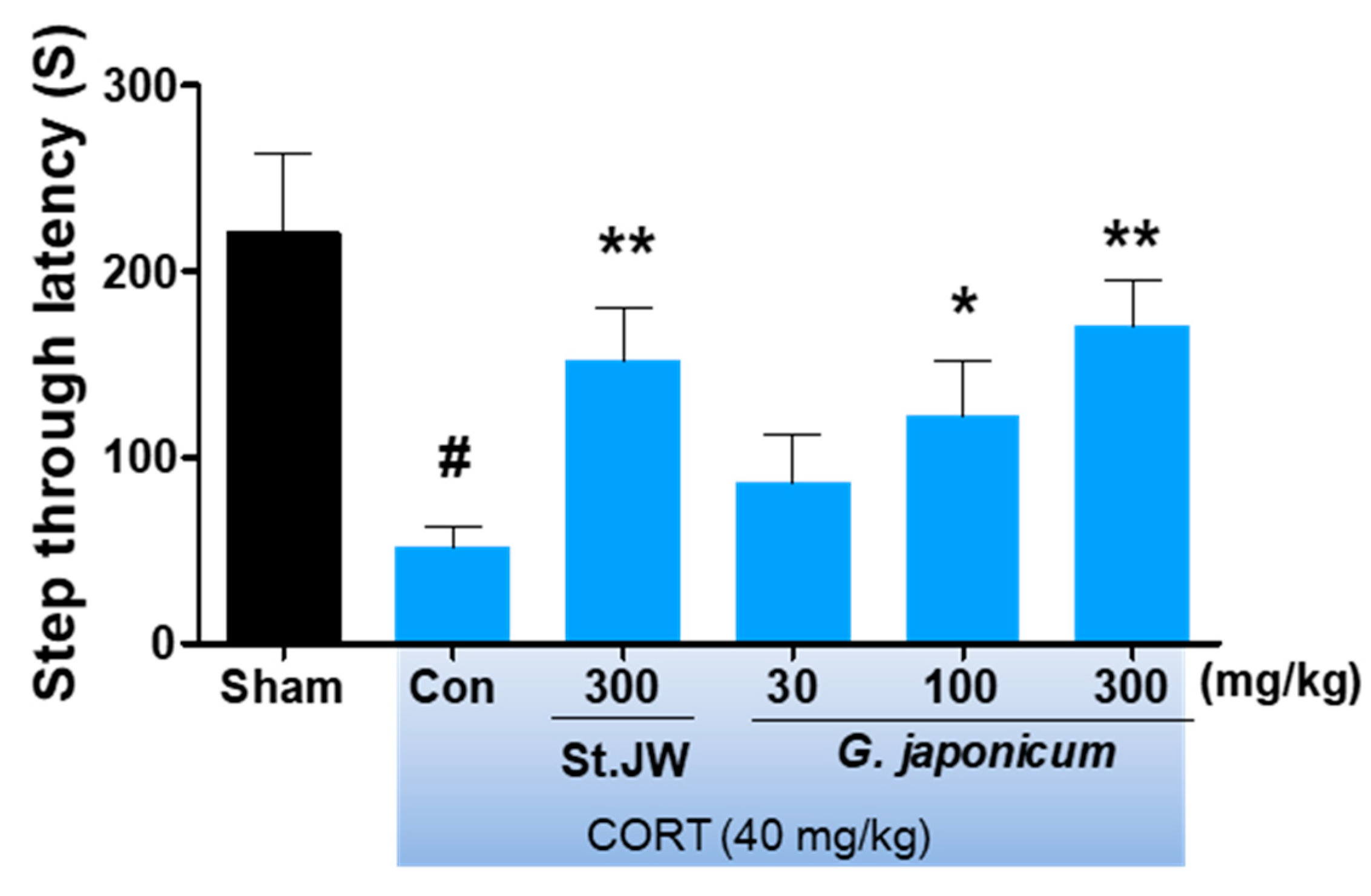
2.6. Passive Avoidance Test (PAT)
2.7. Tail Suspension Test (TST)
2.8. Forced Swim Test (FST)
2.9. Western Blotting
2.10. Cell Viability Assay
2.11. Statistical Analysis
3. Results
3.1. Effect of G. japonicum Extract on OFT
3.2. Effect of G. japonicum Extract on SPT
3.3. Effect of G. japonicum Extract on PAT
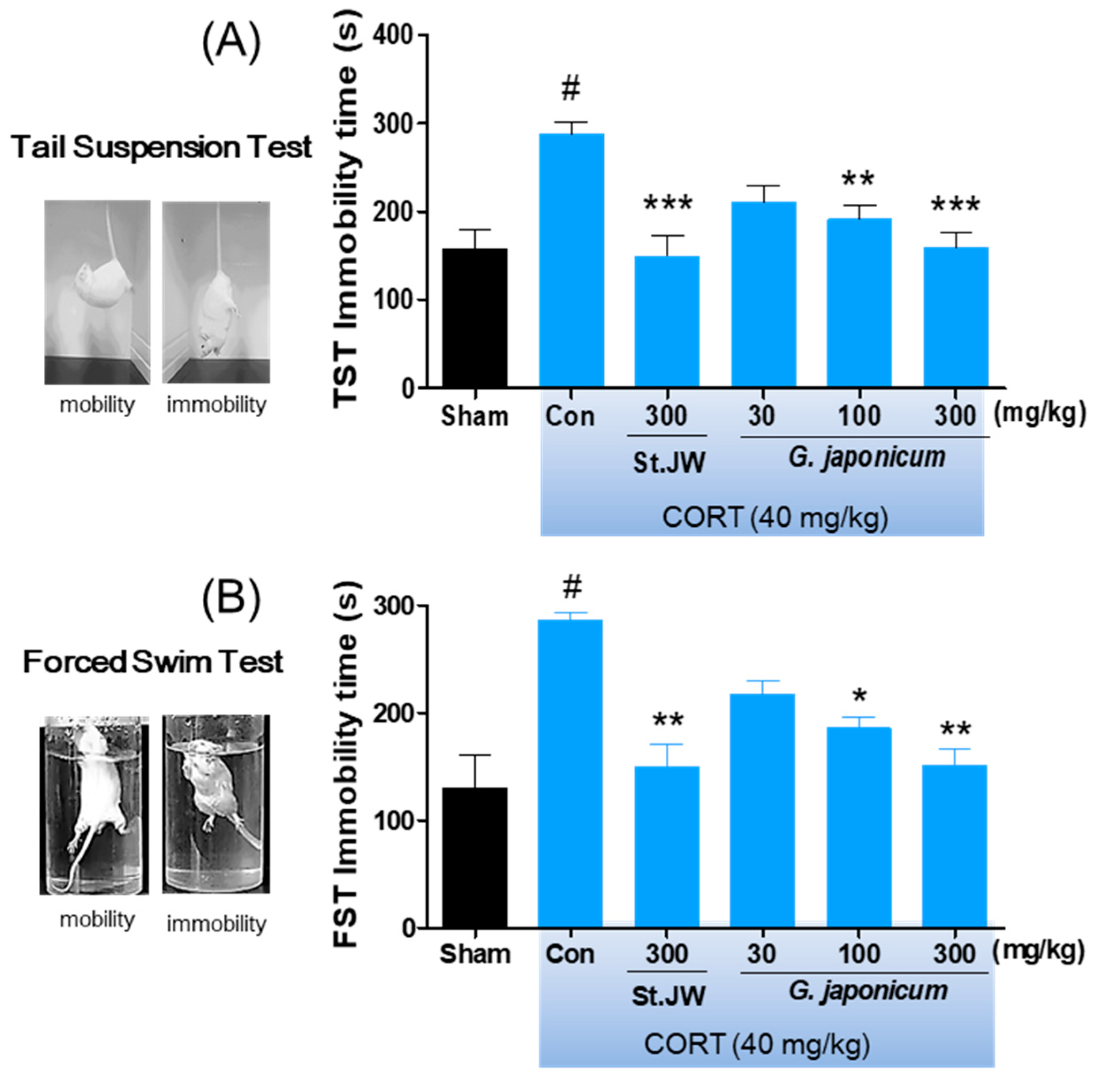
3.4. Effect of G. japonicum Extract on TST and FST
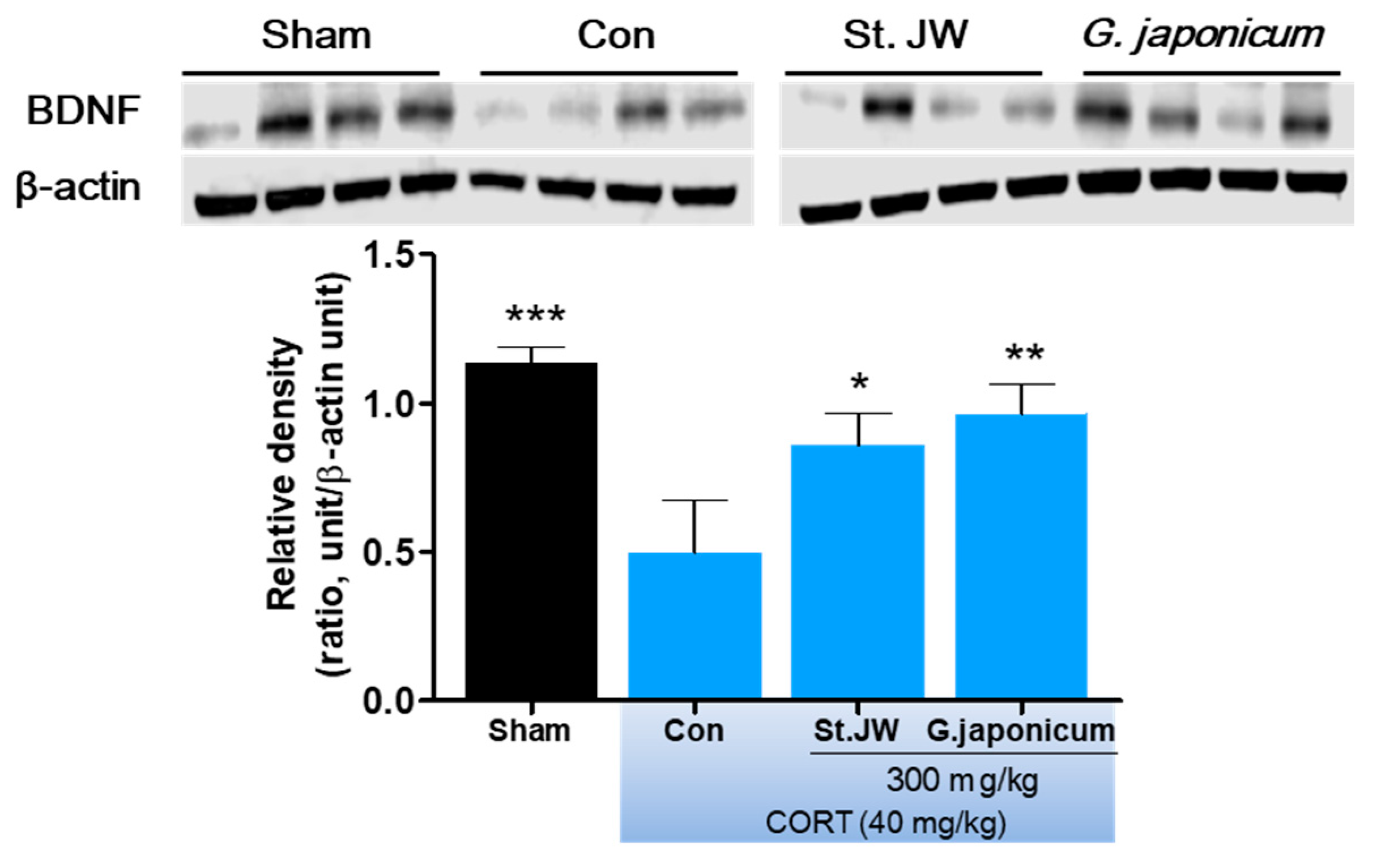
3.5. Effect of G. japonicum Extract on BDNF Expression in the Hippocampus
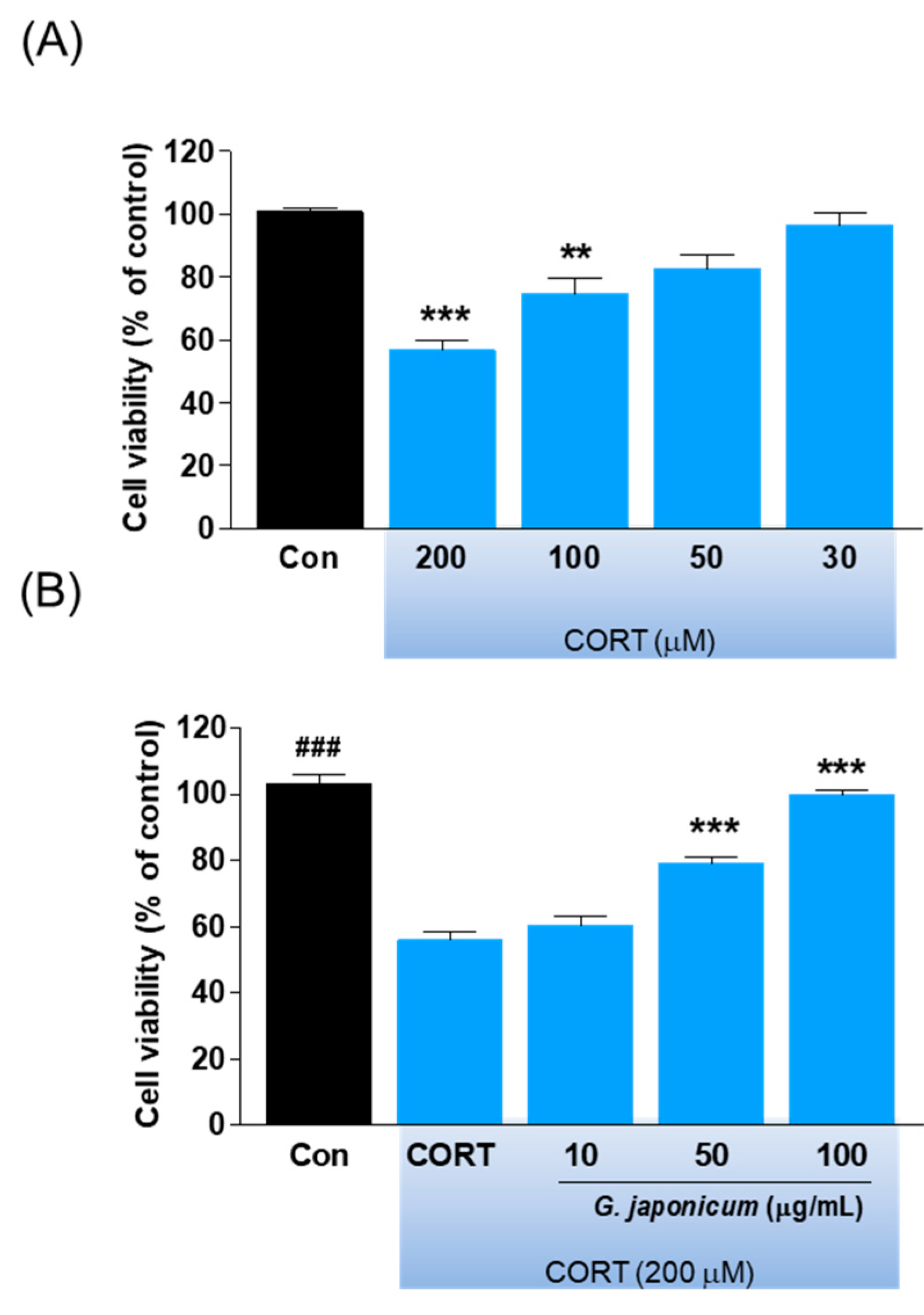
3.6. Effect of G. japonicum Extract on Neurotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, K. Mental health: A world of depression. Nature 2014, 515, 180–181. [Google Scholar] [CrossRef]
- Racagni, G.; Popoli, M. The pharmacological properties of antidepressants. Int. Clin. Psychopharmcol. 2010, 25, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Pirotta, M.; Willis, K.; Carter, M.; Forsdike, K.; Newton, D.; Gunn, J. ‘Less like a drug than a drug’: The use of St John’s wort among people who self-identify as having depression and/or anxiety symptoms. Complement. Ther. Med. 2014, 22, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Yan, Y.; Li, F.; Zhang, D.F. Fruit and vegetable consumption and the risk of depression: A meta-analysis. Nutrition 2016, 32, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Wolniczak, I.; Caceres-DelAguila, J.A.; Maguina, J.L.; Bernabe-Ortiz, A. Fruits and vegetables consumption and depressive symptoms: A population-based study in Peru. PLoS ONE 2017, 12, e0186379. [Google Scholar] [CrossRef]
- Liu, H.W.; Li, J.K.; Zhao, W.H.; Bao, L.; Song, X.H.; Xia, Y.; Wang, X.; Zhang, C.; Wang, X.Z.; Yao, X.S.; et al. Fatty acid synthase inhibitors from Geum japonicum Thunb. var. chinense. Chem. Biodivers. 2009, 6, 402–410. [Google Scholar] [CrossRef]
- Dong, H.; Chen, S.X.; Kini, R.M.; Xu, H.X. Effects of tannins from Geum japonicum on the catalytic activity of thrombin and factor Xa of blood coagulation cascade. J. Nat. Prod. 1998, 61, 1356–1360. [Google Scholar] [CrossRef]
- Kim, J.B.; Kim, J.B.; Cho, K.J.; Konig, G.M.; Wright, A.D. Antioxidant activity of 3,4,5-trihydroxybenzaldehyde isolated from Geum japonicum. J. Food Drug Anal. 2006, 14, 190–193. [Google Scholar]
- Xu, H.X.; Ming, D.S.; Dong, H.; But, P.P. A new anti-HIV triterpene from Geum japonicum. Chem. Pharm. Bull. 2000, 48, 1367–1369. [Google Scholar] [CrossRef]
- Heo, J.C.; Son, M.; Woo, S.U.; Kweon, M.A.; Yoon, E.K.; Lee, H.K.; Choi, W.S.; Cho, K.J.; Lee, S.H. A fraction of methylene chloride from Geum japonicum Thunberg inhibits tumor metastatic and angiogenic potential. Oncol. Rep. 2008, 19, 1399–1403. [Google Scholar]
- Xie, Y.W.; Xu, H.X.; Dong, H.; Fiscus, R.R.; But, P.P.H. Role of nitric oxide in the vasorelaxant and hypotensive effects of extracts and purified tannins from Geum japonicum. J. Ethnopharmacol. 2007, 109, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.J.; Tao, W.; Yang, S.B.; Feng, J.T.; Wang, J.F.; Yang, T.; Wu, H.Y.; Huang, Y.G.; Tan, L.J.; Huang, W.F.; et al. The antiapoptosis effect of Geum japonicum Thunb. var. chinense extracts on cerebral ischemia reperfusion injury via PI3K/Akt pathway. Evid. Based Complement. Altern. Med. 2018, 2018, 7290170. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.F.; Shao, J.; Zhao, S.Y.; Qiu, Y.Y.; Teng, L.P.; Huang, W.; Wang, S.S.; Cheng, X.R.; Jiang, Y.Y. Niga-ichigoside F1 ameliorates high-fat diet-induced hepatic steatosis in male mice by Nrf2 activation. Food Funct. 2018, 9, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, L.; Lin, X.L.; Zhou, X.P.; Cai, Z.M.; Li, M. Reconstitution of coronary vasculature by an active fraction of Geum japonicum in ischemic hearts. Sci. Rep. 2014, 4, 3962. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, R.; Shen, J.; Su, H.; Xing, D.M.; Du, L.J. A mouse model of depression induced by repeated corticosterone injections. Eur. J. Pharmacol. 2008, 581, 113–120. [Google Scholar] [CrossRef]
- Lim, D.W.; Han, T.; Jung, J.; Song, Y.; Um, M.Y.; Yoon, M.; Kim, Y.T.; Cho, S.; Kim, I.H.; Han, D.; et al. Chlorogenic acid from hawthorn berry (Crataegus pinnatifida Fruit) prevents stress hormone-induced depressive behavior, through monoamine oxidase B-reactive oxygen species signaling in hippocampal astrocytes of mice. Mol. Nutr. Food Res. 2018, 62, 1800029. [Google Scholar] [CrossRef]
- Lim, D.W.; Um, M.Y.; Han, T.; Lee, J.; Kim, Y.T.; Cho, S.; Kim, I.H.; Han, D.; Lee, C. Standardized Citrus unshiu peel extract ameliorates dexamethasone-induced neurotoxicity and depressive-like behaviors in mice. Metab. Brain Dis. 2018, 33, 1877–1886. [Google Scholar] [CrossRef]
- Um, M.Y.; Lim, D.W.; Son, H.J.; Cho, S.; Lee, C. Phlorotannin-rich fraction from Ishige foliacea brown seaweed prevents the scopolamine-induced memory impairment via regulation of ERK-CREB-BDNF pathway. J. Funct. Foods 2018, 40, 110–116. [Google Scholar] [CrossRef]
- Ali, S.H.; Madhana, R.M.; Athira, K.V.; Kasala, E.R.; Bodduluru, L.N.; Pitta, S.; Mahareddy, J.R.; Lahkar, M. Resveratrol ameliorates depressive-like behavior in repeated corticosterone-induced depression in mice. Steroids 2015, 101, 37–42. [Google Scholar] [CrossRef]
- Mello, A.F.; Mello, M.F.; Carpenter, L.L.; Price, L.H. Update on stress and depression: The role of the hypothalamic-pituitary-adrenal (HPA) axis. Braz. J. Psychiatry 2003, 25, 231–238. [Google Scholar] [CrossRef]
- Schule, C.; Baghai, T.C.; Eser, D.; Rupprecht, R. Hypothalamic-pituitary-adrenocortical system dysregulation and new treatment strategies in depression. Expert Rev. Neurother. 2009, 9, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.L.; Pariante, C.M. The effects of antidepressants on the hypothalamic-pituitary-adrenal axis. Drug News Perspect. 2006, 19, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Huyghens, L.; Zhang, H.B.; Schiettecatte, J.; Smitz, J.; Vincent, J.L. Cortisol is an associated-risk factor of brain dysfunction in patients with severe sepsis and septic shock. Biomed. Res. Int. 2014, 2014, 712742. [Google Scholar] [CrossRef] [PubMed]
- Gonul, A.S.; Kitis, O.; Eker, M.C.; Eker, O.D.; Ozan, E.; Coburn, K. Association of the brain-derived neurotrophic factor Val66Met polymorphism with hippocampus volumes in drug-free depressed patients. World J. Biol. Psychiatry 2011, 12, 110–118. [Google Scholar] [CrossRef]
- Colle, R.; Cury, C.; Chupin, M.; Deflesselle, E.; Hardy, P.; Nasser, G.; Falissard, B.; Ducreux, D.; Colliot, O.; Corruble, E. Hippocampal volume predicts antidepressant efficacy in depressed patients without incomplete hippocampal inversion. Neuroimage Clin. 2016, 12, 949–955. [Google Scholar] [CrossRef]
- Raone, A.; Cassanelli, A.; Scheggi, S.; Rauggi, R.; Danielli, B.; de Montis, M.G. Hypothalamus-pituitary-adrenal modifications consequent to chronic stress exposure in an experimental model of depression in rats. Neuroscience 2007, 146, 1734–1742. [Google Scholar] [CrossRef]
- Szymanska, M.; Budziszewska, B.; Basta-Kaim, A.; Jaworska-Feil, L.; Kubera, M.; Regulska, M.; Leskiewicz, M.; Lason, W. The effect of antidepressant drugs on the hypothalamic-pituitary-adrenal axis regulation in an animal model of depression. Bipolar Disord. 2008, 10, 88. [Google Scholar]
- Lucassen, P.J.; Muller, M.B.; Holsboer, F.; Bauer, J.; Holtrop, A.; Wouda, J.; Hoogendijk, W.J.G.; de Kloet, E.R.; Swaab, D.F. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am. J. Pathol. 2001, 158, 453–468. [Google Scholar] [CrossRef]
- Iijima, M.; Ito, A.; Kurosu, S.; Chaki, S. Pharmacological characterization of repeated corticosterone injection-induced depression model in rats. Brain Res. 2010, 1359, 75–80. [Google Scholar] [CrossRef]
- Renard, C.E.; Dailly, E.; David, D.J.P.; Hascoet, M.; Bourin, M. Monoamine metabolism changes following the mouse forced swimming test but not the tail suspension test. Fundam. Clin. Pharmacol. 2003, 17, 449–455. [Google Scholar] [CrossRef]
- Liu, M.Y.; Yin, C.Y.; Zhu, L.J.; Zhu, X.H.; Xu, C.; Luo, C.X.; Chen, H.S.; Zhu, D.Y.; Zhou, Q.G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Lussier, A.L.; Lebedeva, K.; Fenton, E.Y.; Guskjolen, A.; Caruncho, H.J.; Kalynchuk, L.E. The progressive development of depression-like behavior in corticosterone-treated rats is paralleled by slowed granule cell maturation and decreased reelin expression in the adult dentate gyrus. Neuropharmacology 2013, 71, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.; Shim, I.; Lee, H.; Hahm, D.H. Baicalin improves chronic corticosterone-induced learning and memory deficits via the enhancement of impaired hippocampal brain-derived neurotrophic factor and cAMP response element-binding protein expression in the rat. J. Nat. Med. 2014, 68, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S. Neurotrophic factors and regulation of mood: Role of exercise, diet and metabolism. Neurobiol. Aging 2005, 26, S88–S93. [Google Scholar] [CrossRef]
- Aicardi, G.; Argilli, E.; Cappello, S.; Santi, S.; Riccio, M.; Thoenen, H.; Canossa, M. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA 2004, 101, 15788–15792. [Google Scholar] [CrossRef]
- Matrisciano, F.; Bonaccorso, S.; Ricciardi, A.; Scaccianoce, S.; Panaccione, I.; Wang, L.; Ruberto, A.; Tatarelli, R.; Nicoletti, F.; Girardi, P.; et al. Changes in BDNF serum levels in patients with major depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. J. Psychiatr. Res. 2008, 43, 247–254. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Gama, C.S.; Cereser, K.M.; Yatham, L.N.; Fries, G.R.; Colpo, G.; de Lucena, D.; Kunz, M.; Gomes, F.A.; Kapczinski, F. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: A systematic review and meta-regression analysis. J. Psychiatr Res. 2011, 45, 995–1004. [Google Scholar] [CrossRef]
- Sen, S.; Duman, R.S.; Sanacora, G. Meta-analysis of serum brain-derived neurotrophic factor (BDNF) levels in depression and antidepressant treatment. In Biological Psychiatry; Elsevier Science Inc.: New York, NY, USA, 2008; Volume 63, p. 98s. [Google Scholar]
- Shen, J.D.; Ma, L.G.; Hu, C.Y.; Pei, Y.Y.; Jin, S.L.; Fang, X.Y.; Li, Y.C. Berberine up-regulates the BDNF expression in hippocampus and attenuates corticosterone-induced depressive-like behavior in mice. Neurosci. Lett. 2016, 614, 77–82. [Google Scholar] [CrossRef]
- Nitta, A.; Ohmiya, M.; Sometani, A.; Itoh, M.; Nomoto, H.; Furukawa, Y.; Furukawa, S. Brain-derived neurotrophic factor prevents neuronal cell death induced by corticosterone. J. Neurosci. Res. 1999, 57, 227–235. [Google Scholar] [CrossRef]
- Zheng, M.Z.; Liu, C.M.; Pan, F.G.; Shi, D.F.; Zhang, Y.C. Antidepressant-like effect of hyperoside isolated from Apocynum venetum leaves: Possible cellular mechanisms. Phytomedicine 2012, 19, 145–149. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, D.W.; Han, T.; Um, M.Y.; Yoon, M.; Kim, T.-E.; Kim, Y.T.; Han, D.; Lee, J.; Lee, C.H. Administration of Asian Herb Bennet (Geum japonicum) Extract Reverses Depressive-Like Behaviors in Mouse Model of Depression Induced by Corticosterone. Nutrients 2019, 11, 2841. https://doi.org/10.3390/nu11122841
Lim DW, Han T, Um MY, Yoon M, Kim T-E, Kim YT, Han D, Lee J, Lee CH. Administration of Asian Herb Bennet (Geum japonicum) Extract Reverses Depressive-Like Behaviors in Mouse Model of Depression Induced by Corticosterone. Nutrients. 2019; 11(12):2841. https://doi.org/10.3390/nu11122841
Chicago/Turabian StyleLim, Dong Wook, Taewon Han, Min Young Um, Minseok Yoon, Tae-Eun Kim, Yun Tai Kim, Daeseok Han, Jaekwang Lee, and Chang Ho Lee. 2019. "Administration of Asian Herb Bennet (Geum japonicum) Extract Reverses Depressive-Like Behaviors in Mouse Model of Depression Induced by Corticosterone" Nutrients 11, no. 12: 2841. https://doi.org/10.3390/nu11122841
APA StyleLim, D. W., Han, T., Um, M. Y., Yoon, M., Kim, T.-E., Kim, Y. T., Han, D., Lee, J., & Lee, C. H. (2019). Administration of Asian Herb Bennet (Geum japonicum) Extract Reverses Depressive-Like Behaviors in Mouse Model of Depression Induced by Corticosterone. Nutrients, 11(12), 2841. https://doi.org/10.3390/nu11122841




