Effect of Lactobacillus plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Probiotics
2.2. Volunteers
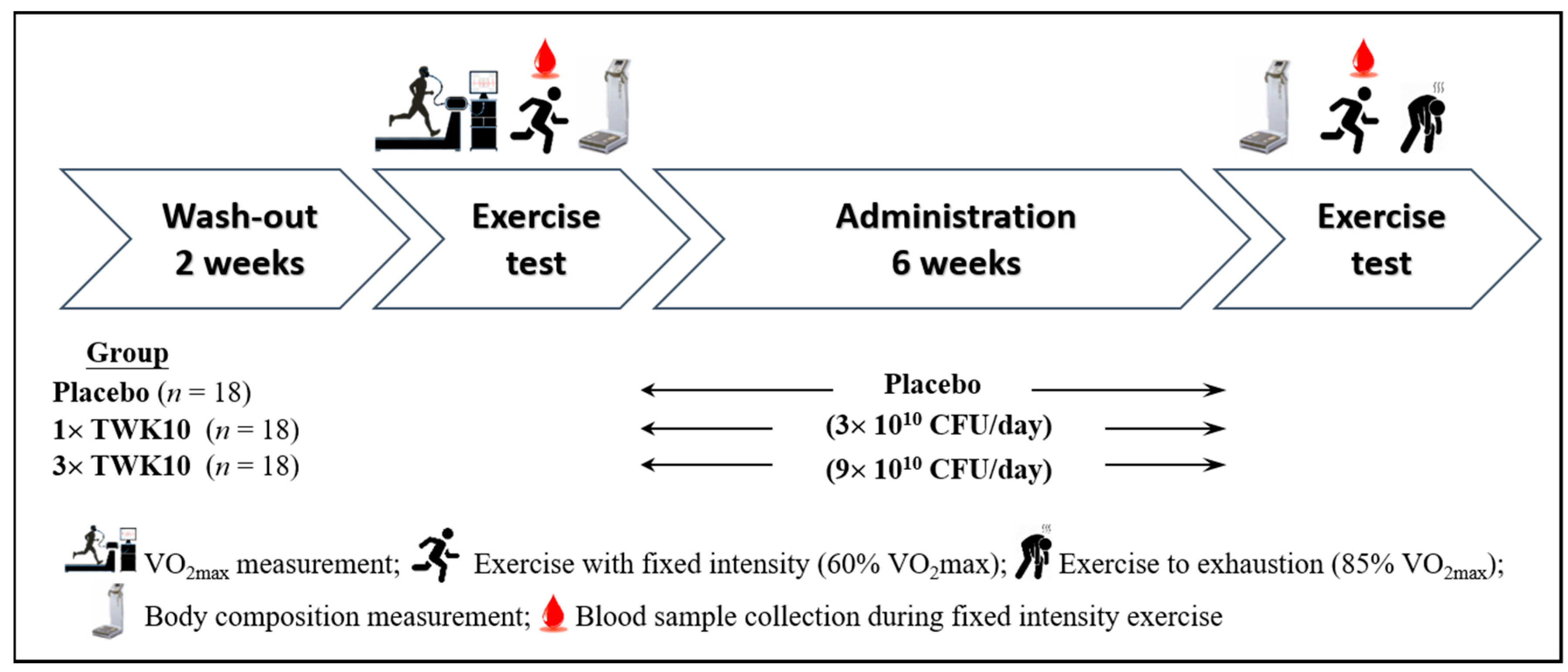
2.3. Experimental Design
2.4. Clinical Biochemistry and Hematology Analysis
2.5. Body Composition
2.6. Statistics
3. Results
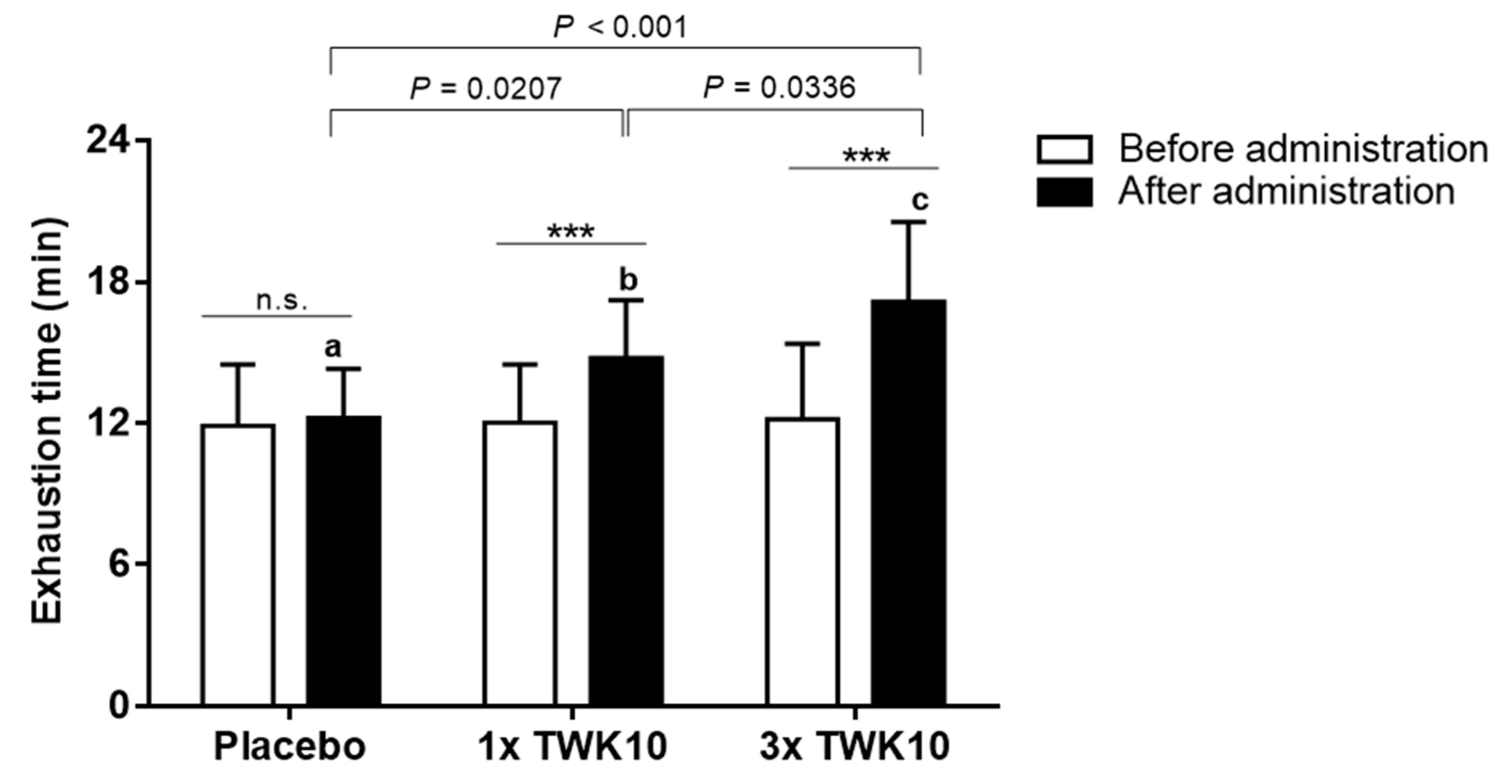
3.1. Effects of TWK10 on Endurance Performance
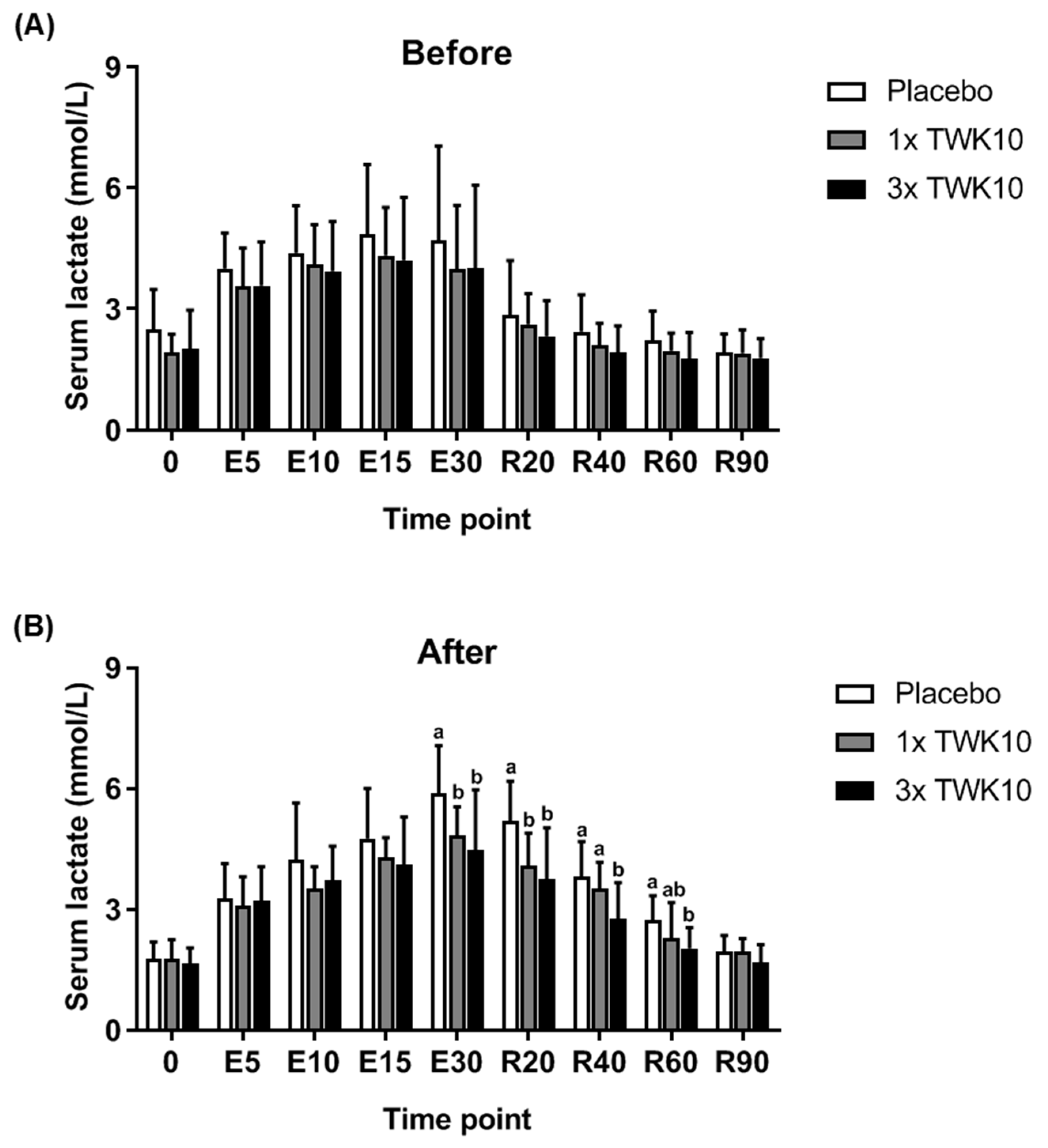
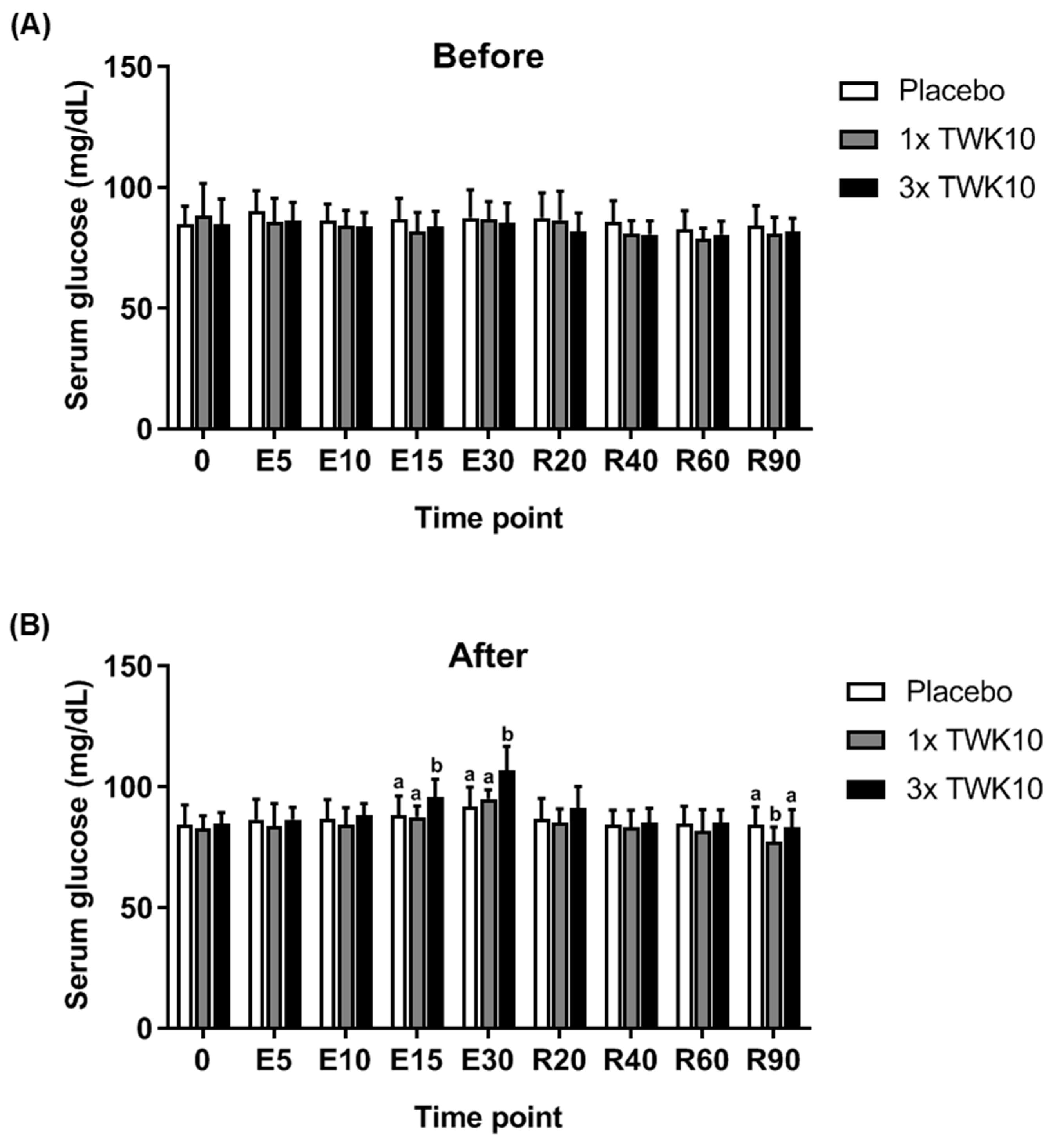
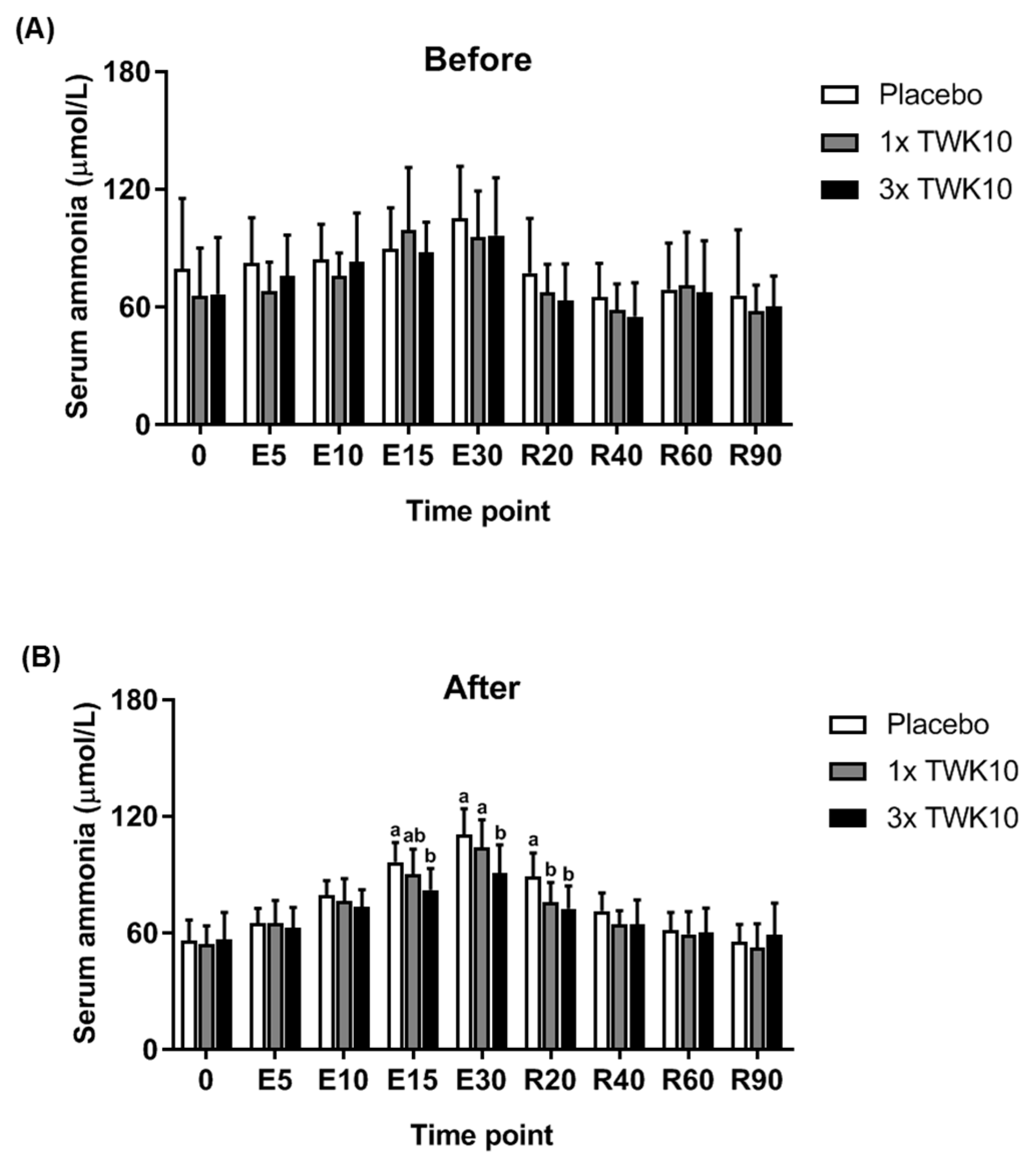
3.2. Effects of TWK10 on Physiological Adaptation and Biochemical Indices
3.3. Effects of TWK10 on Complete Blood Count Profiles
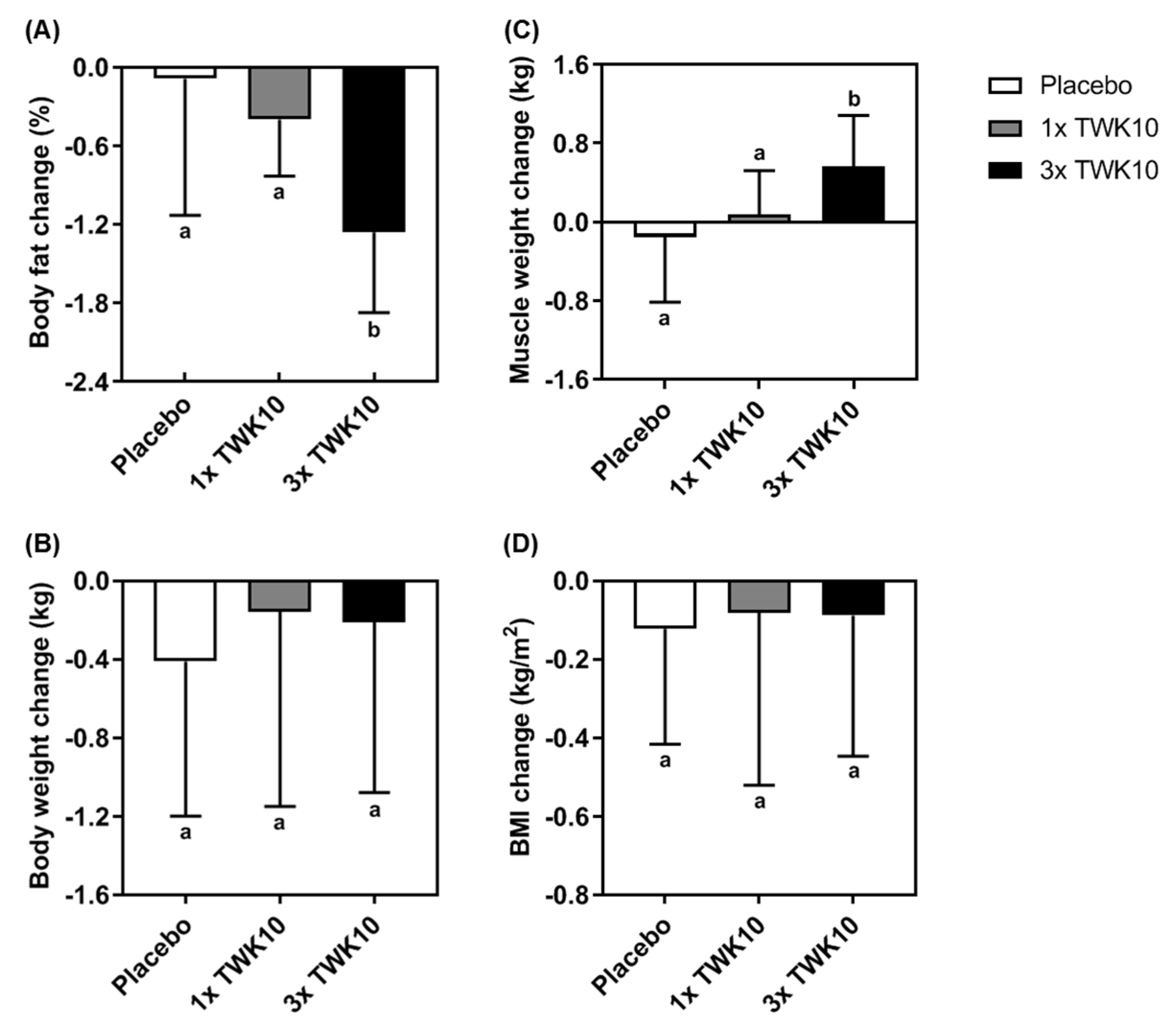
3.4. Effects of TWK10 on Body Composition
3.5. Effects of TWK10 on Safety Assessment
3.6. Effects of TWK10 on Dietary Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jason, L.A.; Evans, M.; Brown, M.; Porter, N. What is Fatigue? Pathological and nonpathological fatigue. PM R 2010, 2, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Cathébras, P.; Bouchou, K.; Charmion, S.; Rousset, H. Chronic fatigue syndrome: A critical review. Rev. Med. Interne 1993, 14, 233–242. [Google Scholar] [CrossRef]
- Davis, J.M. Central and peripheral factors in fatigue. J. Sports Sci. 1995, 13, S49–S53. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Chalimoniuk, M.; Gołaś, A.; Lngfort, J.; Maszczyk, A. Central and peripheral fatigue during resistance exercise—A critical review. J. Hum. Kinet. 2015, 49, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, I.G.; Jamurtas, A.Z. Insights into the molecular etiology of exercise-induced inflammation: Opportunities for optimizing performance. J. Inflamm. Res. 2016, 9, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Harty, P.S.; Cottet, M.L.; Malloy, J.K.; Kerksick, C.M. Nutritional and supplementation strategies to prevent and attenuate exercise-induced muscle damage: A brief review. Sport. Med. Open 2019, 5, 1. [Google Scholar] [CrossRef]
- Sindiani, M.; Eliakim, A.; Segev, D.; Meckel, Y. The effect of two different interval-training programmes on physiological and performance indices. Eur. J. Sport Sci. 2017, 17, 830–837. [Google Scholar] [CrossRef]
- Wiewelhove, T.; Schneider, C.; Döweling, A.; Hanakam, F.; Rasche, C.; Meyer, T.; Kellmann, M.; Pfeiffer, M.; Ferrauti, A. Effects of different recovery strategies following a half-marathon on fatigue markers in recreational runners. PLoS ONE 2018, 13, e0207313. [Google Scholar] [CrossRef]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- de Vries, W.; Stouthamer, A.H. Pathway of glucose fermentation in relation to the taxonomy of bifidobacteria. J. Bacteriol. 1967, 93, 574–576. [Google Scholar] [PubMed]
- Gleeson, M.; Bishop, N.C.; Oliveira, M.; Tauler, P. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Martarelli, D.; Verdenelli, M.C.; Scuri, S.; Cocchioni, M.; Silvi, S.; Cecchini, C.; Pompei, P. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr. Microbiol. 2011, 62, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.; Bender, D.; Vantrease, W.; Sapp, P.; Toy, A.; Woods, C.; Johnson, K. Effects of Probiotic (Bacillus subtilis DE111) supplementation on immune function, hormonal status, and physical performance in division I baseball players. Sports 2018, 6, 70. [Google Scholar] [CrossRef]
- Shing, C.M.; Peake, J.M.; Lim, C.L.; Briskey, D.; Walsh, N.P.; Fortes, M.B.; Ahuja, K.D.K.; Vitetta, L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2014, 114, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sport. Nutr. 2012, 9, 45. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 supplementation improves exercise performance and increases muscle mass in mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef]
- Huang, W.C.; Hsu, Y.J.; Li, H.; Kan, N.W.; Chen, Y.M.; Lin, J.S.; Hsu, T.K.; Tsai, T.Y.; Chiu, Y.S.; Huang, C.C. Effect of Lactobacillus plantarum TWK10 on improving endurance performance in humans. Chin. J. Physiol. 2018, 61, 163–170. [Google Scholar] [CrossRef]
- Bruce, R.A.; Kusumi, F.; Hosmer, D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am. Heart J. 1973, 85, 546–562. [Google Scholar] [CrossRef]
- Jin, C.-H.; Paik, I.-Y.; Kwak, Y.-S.; Jee, Y.-S.; Kim, J.-Y. Exhaustive submaximal endurance and resistance exercises induce temporary immunosuppression via physical and oxidative stress. J. Exerc. Rehabil. 2015, 11, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Choi, W.J.; Lee, H.S.; Lee, J.W. Relationship between inflammatory markers and visceral obesity in obese and overweight Korean adults: An observational study. Medicine (Baltimore) 2019, 98, e14740. [Google Scholar] [CrossRef] [PubMed]
- Coqueiro, A.Y.; de-Oliveira-Garcia, A.B.; Rogero, M.M.; Tirapegui, J. Probiotic supplementation in sports and physical exercise: Does it present any ergogenic effect? Nutr. Health 2017, 23, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Basset, F.A.; Boulay, M.R. Treadmill and cycle ergometer tests are interchangeable to monitor triathletes annual training. J. Sport. Sci. Med. 2003, 2, 110–116. [Google Scholar]
- Smarkusz, J.; Ostrowska, L.; Witczak-Sawczuk, K. Probiotic strains as the element of nutritional profile in physical activity—New trend or better sports results? Rocz. Panstw. Zakl. Hig. 2017, 68, 229–235. [Google Scholar] [PubMed]
- Huang, W.C.; Wei, C.C.; Huang, C.C.; Chen, W.L.; Huang, H.Y. The beneficial effects of Lactobacillus plantarum PS128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The science and translation of lactate shuttle theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J. The fuels for exercise. Aust. J. Nutr. Diet 2001, 58, S19–S22. [Google Scholar]
- Komano, Y.; Shimada, K.; Naito, H.; Fukao, K.; Ishihara, Y.; Fujii, T.; Kokubo, T.; Daida, H. Efficacy of heat-killed Lactococcus lactis JCM 5805 on immunity and fatigue during consecutive high intensity exercise in male athletes: A randomized, placebo-controlled, double-blinded trial. J. Int. Soc. Sport. Nutr. 2018, 15, 39. [Google Scholar] [CrossRef]
- Wilson, J.M.; Loenneke, J.P.; Jo, E.; Wilson, G.J.; Zourdos, M.C.; Kim, J.S. The effects of endurance, strength, and power training on muscle fiber type shifting. J. Strength Cond. Res. 2012, 26, 1724–1729. [Google Scholar] [CrossRef]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.A.; Terjung, R.L. Differences in ammonia and adenylate metabolism in contracting fast and slow muscle. Am. J. Physiol. Cell Physiol. 1979, 237, C111–C118. [Google Scholar] [CrossRef] [PubMed]
- Raggi, A.; Ronca-Testoni, S.; Ronca, G. Muscle AMP aminohydrolase. II. Distribution of AMP aminohydrolase, myokinase and creatine kinase activities in skeletal muscle. Biochim. Biophys. Acta 1969, 178, 619–622. [Google Scholar] [CrossRef]
- Winder, W.W.; Terjung, R.L.; Baldwin, K.M.; Holloszy, J.O. Effect of exercise on AMP deaminase and adenylosuccinase in rat skeletal muscle. Am. J. Physiol. 1974, 272, 1411–1414. [Google Scholar] [CrossRef]
- Coggan, A.R. Plasma glucose metabolism during exercise in humans. Sport. Med. 1991, 11, 101–124. [Google Scholar] [CrossRef]
- Gollnick, P.D.; Pernow, B.; Essen, B.; Jansson, E.; Saltin, B. Availability of glycogen and plasma FFA for substrate utilization in leg muscle of man during exercise. Clin. Physiol. 1981, 1, 27–42. [Google Scholar] [CrossRef]
- Richter, E.A.; Galbo, H. High glycogen levels enhance glycogen breakdown in isolated contracting skeletal muscle. J. Appl. Physiol. 1986, 61, 827–831. [Google Scholar] [CrossRef]
- Flynn, M.G.; Mcfarlin, B.K.; Markofski, M.M. State of the art reviews: The anti-inflammatory actions of exercise training. Am. J. Lifestyle Med. 2007, 1, 220–235. [Google Scholar] [CrossRef]
- Krüger, K.; Seimetz, M.; Ringseis, R.; Wilhelm, J.; Pichl, A.; Couturier, A.; Eder, K.; Weissmann, N.; Mooren, F.C. Exercise training reverses inflammation and muscle wasting after tobacco smoke exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R366–R376. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Mousavi, S.M.; Sadeghi, A.; Salari-Moghaddam, A.; Parohan, M.; Larijani, B.; Esmaillzadeh, A. The effect of probiotics on inflammatory biomarkers: A meta-analysis of randomized clinical trials. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Agha, M.; Agha, R. The rising prevalence of obesity: Part A: Impact on public health. Int. J. Surg. Oncol. 2017, 2, e17. [Google Scholar] [CrossRef] [PubMed]
- Quist, J.S.; Rosenkilde, M.; Petersen, M.B.; Gram, A.S.; Sjödin, A.; Stallknecht, B. Effects of active commuting and leisure-time exercise on fat loss in women and men with overweight and obesity: A randomized controlled trial. Int. J. Obes. 2018, 42, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.H.; Slentz, C.A.; Bateman, L.A.; Shields, A.T.; Piner, L.W.; Bales, C.W.; Houmard, J.A.; Kraus, W.E. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J. Appl. Physiol. 2012, 113, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kwon, J.; Kim, M.S.; Park, H.; Ji, Y.; Holzapfel, W.; Hyun, C.K. Protective effects of Bacillus probiotics against high-fat diet-induced metabolic disorders in mice. PLoS ONE 2018, 13, e0210120. [Google Scholar] [CrossRef]
- Crovesy, L.; Ostrowski, M.; Ferreira, D.M.T.P.; Rosado, E.L.; Soares-Mota, M. Effect of Lactobacillus on body weight and body fat in overweight subjects: A systematic review of randomized controlled clinical trials. Int. J. Obes. 2017, 41, 1607–1614. [Google Scholar] [CrossRef]
- Szulińska, M.; Łoniewski, I.; van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-dependent effects of multispecies probiotic supplementation on the lipopolysaccharide (LPS) level and cardiometabolic profile in obese postmenopausal women: A 12-week randomized clinical trial. Nutrients 2018, 10, 776. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Suganthy, N.; Chaiyasut, C. A Review on role of microbiome in obesity and antiobesity properties of probiotic supplements. Biomed. Res. Int. 2019, 2019, 3291367. [Google Scholar] [CrossRef]
- Borgeraas, H.; Johnson, L.K.; Skattebu, J.; Hertel, J.K.; Hjelmesaeth, J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2018, 19, 219–232. [Google Scholar] [CrossRef]
| Characteristic | Placebo | 1× TWK10 | 3× TWK10 |
|---|---|---|---|
| Male | n = 9 | n = 9 | n = 9 |
| Height (cm) | 173.3 ± 3.6 | 175.6 ± 4.2 | 174.2 ± 7.3 |
| Weight (kg) | 70.6 ± 6.6 | 73.2 ± 10.1 | 77.6 ± 12.0 |
| Age (y) | 22.4 ± 1.8 | 22.8 ± 2.1 | 22.0 ± 1.7 |
| BMI (m2/kg) | 23.5 ± 2.2 | 23.8 ± 3.6 | 25.4 ± 2.8 |
| VO2max (mL/kg/min) | 49.7 ± 7.1 | 49.8 ± 7.4 | 49.9 ± 8.3 |
| Female | n = 9 | n = 9 | n = 9 |
| Height (cm) | 158.4 ± 6.2 | 160.2 ± 3.4 | 157.3 ± 5.1 |
| Weight (kg) | 50.3 ± 2.1 | 55.2 ± 5.2 | 49.7 ± 6.5 |
| Age (y) | 20.8 ± 1.0 | 21.2 ± 0.8 | 23.0 ± 5.2 |
| BMI (m2/kg) | 20.0 ± 1.2 | 21.5 ± 2.1 | 20.0 ± 2.0 |
| VO2max (mL/kg/min) | 44.9 ± 8.6 | 44.8 ± 7.8 | 44.9 ± 9.5 |
| Indicator | Placebo | 1× TWK10 | 3× TWK10 | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| WBC (cumm) | 7382 ± 1961 | 7357 ± 2350 | 8447 ± 2226 | 7924 ± 1898 | 7358 ± 1549 | 7063 ± 2043 |
| Neutrophil (%) | 69.0 ± 6.7 | 66.9 ± 9.6 | 70.9 ± 6.9 | 67.3 ± 8.2 | 63.2 ± 6.5 | 60.9 ± 8.4 |
| Lymphocyte (%) | 23.7 ± 6.0 | 25.6 ± 8.9 | 22.0 ± 5.4 | 24.7 ± 6.3 | 28.7 ± 6.6 | 29.7 ± 7.2 |
| Monocyte (%) | 5.35 ± 1.03 | 5.49 ± 1.25 | 5.17 ± 1.30 | 5.53 ± 1.64 | 5.80 ± 1.07 | 6.37 ± 2.51 |
| Eosinophil (%) | 1.42 ± 1.06 | 1.42 ± 0.96 | 1.40 ± 1.06 | 1.98 ± 1.77 | 1.67 ± 0.93 | 2.40 ± 1.30 |
| Basophil (%) | 0.58 ± 0.32 | 0.54 ± 0.25 | 0.47 ± 0.23 | 0.50 ± 0.23 | 0.63 ± 0.32 | 0.63 ± 0.31 |
| RBC (MIL/cumm) | 4.99 ± 0.54 | 5.02 ± 0.56 | 5.09 ± 0.55 | 5.07 ± 0.66 | 4.97 ± 0.61 | 5.04 ± 0.64 |
| Hemoglobin (g/dL) | 14.2 ± 1.9 | 14.2 ± 2.1 | 14.4 ± 1.3 | 14.4 ± 1.6 | 13.8 ± 1.4 | 13.8 ± 1.7 |
| Hematocrit (%) | 43.9 ± 5.1 | 44.1 ± 4.9 | 44.6 ± 3.5 | 44.4 ± 4.1 | 42.7 ± 3.4 | 43.4 ± 4.3 |
| MCV (fl) | 88.3 ± 8.0 | 88.1 ± 8.7 | 88.0 ± 6.6 | 88.1 ± 7.1 | 86.7 ± 8.9 | 86.8 ± 7.9 |
| MCH (pg) | 28.5 ± 3.2 | 28.3 ± 3.4 | 28.4 ± 2.5 | 28.5 ± 2.5 | 28.0 ± 3.3 | 27.6 ± 3.3 |
| MCHC (%) | 32.2 ± 1.3 | 32.0 ± 1.4 | 32.2 ± 0.9 | 32.3 ± 1.1 | 32.2 ± 1.1 | 31.8 ± 1.3 |
| Platelet (103/cumm) | 258 ± 61 | 258 ± 70 | 280 ± 57 | 274 ± 58 | 243 ± 62 | 238 ± 66 |
| NLR | 3.14 ± 0.98 a | 3.12 ± 1.62 | 3.50 ± 1.28 a | 2.99 ± 1.11 | 2.34 ± 0.68 b | 2.25 ± 0.89 |
| PLR | 165 ± 62 a,b | 149 ± 60 | 159 ± 27 a | 150 ± 34 | 121 ± 34 b | 122 ± 36 |
| Before Administration | After Administration | |||||
|---|---|---|---|---|---|---|
| Placebo | 1× TWK10 | 3× TWK10 | Placebo | 1× TWK10 | 3× TWK10 | |
| Men | n = 9 | n = 9 | n = 9 | n = 9 | n = 9 | n = 9 |
| Carbohydrates (g/day) | 298 ± 29 | 290 ± 40 | 292 ± 54 | 281 ± 45 | 288 ± 36 | 285 ± 43 |
| Protein (g/day) | 110.3 ± 16.2 | 98.9 ± 11.2 | 102.9 ± 20.0 | 95.1 ± 17.1 | 92.5 ± 19.3 | 90.9 ± 22.9 |
| Lipid (g/day) | 117.1 ± 16.0 | 110.6 ± 13.8 | 111.8 ± 16.5 | 105.7 ± 9.3 | 104.5 ± 28.2 | 99.8 ± 15.9 |
| Calories (kcal/day) | 2686 ± 199 | 2268 ± 865 | 2585 ± 388 | 2456 ± 285 | 2464 ± 414 | 2401 ± 278 |
| Women | n = 9 | n = 9 | n = 9 | n = 9 | n = 9 | n = 9 |
| Carbohydrates (g/day) | 204 ± 31 | 200 ± 29 | 195 ± 19 | 200 ± 17 | 206 ± 27 | 206 ± 15 |
| Protein (g/day) | 68.1 ± 13.6 | 69.5 ± 7.7 | 66.1 ± 8.2 | 67.1 ± 14.8 | 68.6 ± 16.8 | 65.0 ± 10.2 |
| Lipid (g/day) | 82.6 ± 14.2 | 83.2 ± 12.4 | 80.8 ± 5.9 | 84.5 ± 14.6 | 85.6 ± 12.6 | 81.2 ± 12.2 |
| Calories (kcal/day) | 1831 ± 254 | 1826 ± 165 | 1770 ± 123 | 1827 ± 166 | 1867 ± 138 | 1814 ± 145 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-C.; Lee, M.-C.; Lee, C.-C.; Ng, K.-S.; Hsu, Y.-J.; Tsai, T.-Y.; Young, S.-L.; Lin, J.-S.; Huang, C.-C. Effect of Lactobacillus plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans. Nutrients 2019, 11, 2836. https://doi.org/10.3390/nu11112836
Huang W-C, Lee M-C, Lee C-C, Ng K-S, Hsu Y-J, Tsai T-Y, Young S-L, Lin J-S, Huang C-C. Effect of Lactobacillus plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans. Nutrients. 2019; 11(11):2836. https://doi.org/10.3390/nu11112836
Chicago/Turabian StyleHuang, Wen-Ching, Mon-Chien Lee, Chia-Chia Lee, Ker-Sin Ng, Yi-Ju Hsu, Tsung-Yu Tsai, San-Land Young, Jin-Seng Lin, and Chi-Chang Huang. 2019. "Effect of Lactobacillus plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans" Nutrients 11, no. 11: 2836. https://doi.org/10.3390/nu11112836
APA StyleHuang, W.-C., Lee, M.-C., Lee, C.-C., Ng, K.-S., Hsu, Y.-J., Tsai, T.-Y., Young, S.-L., Lin, J.-S., & Huang, C.-C. (2019). Effect of Lactobacillus plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans. Nutrients, 11(11), 2836. https://doi.org/10.3390/nu11112836







