Butyric Acid and Leucine Induce α-Defensin Secretion from Small Intestinal Paneth Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reagents
2.3. Crypt Isolation
2.3.1. Crypt Isolation for Sandwich Enzyme-Linked Immunosorbent Assay (ELISA) and Bactericidal Assay
2.3.2. Crypt Isolation for Quantitative Polymerase Chain Reaction (qPCR) and Enteroid Culture
2.4. Stimulation and Collection of Paneth Cell Secretions
2.5. Sandwich ELISA
2.6. Bactericidal Assay
2.7. qPCR Analysis of Receptor and Transporter Gene Expression
2.8. Western Blot
2.9. Immunofluorescence Staining
2.10. Quantification of Paneth Cell Granule Secretion in Enteroids
2.11. Statistical Analysis
3. Results
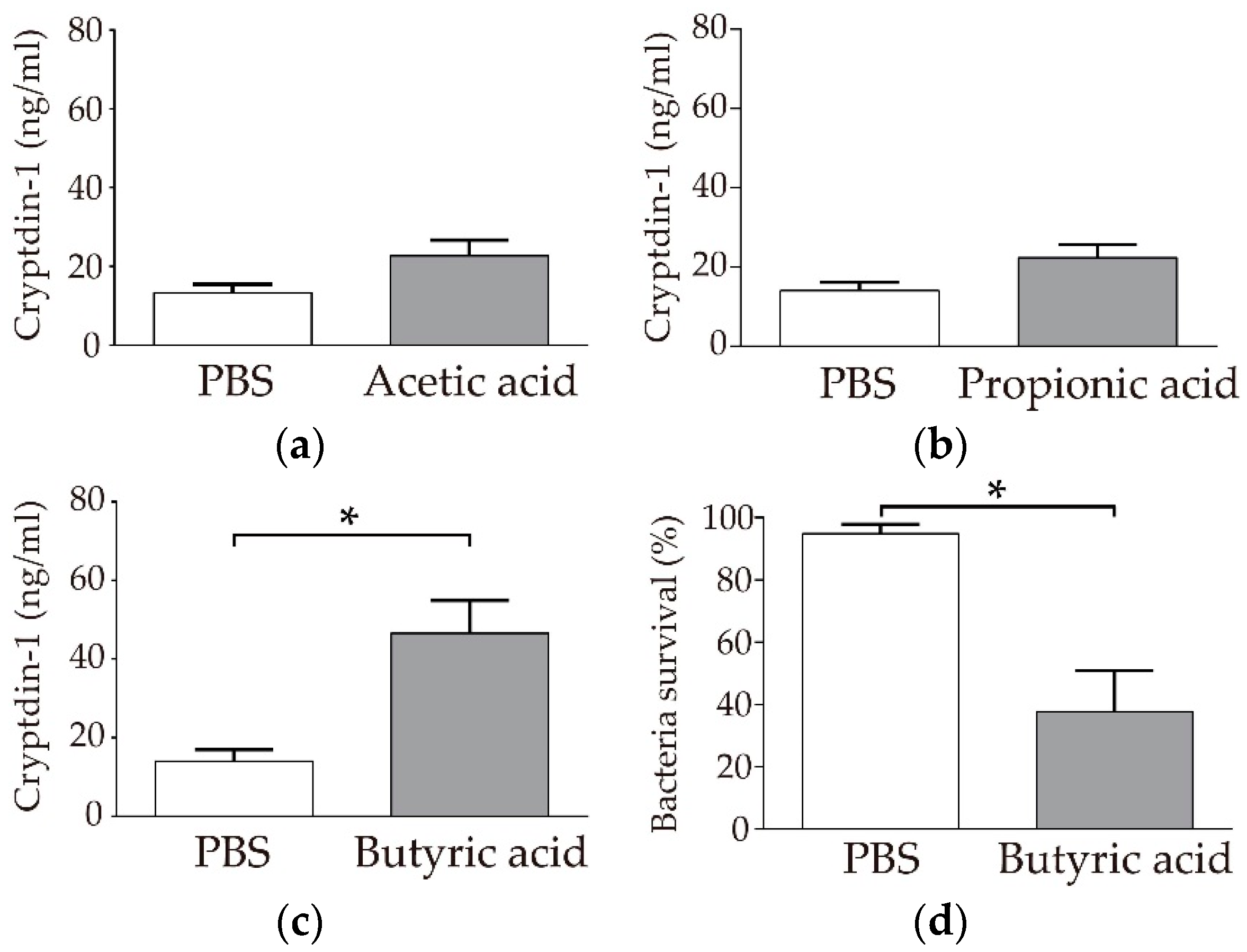
3.1. Paneth Cells Secrete α-Defensin in Response to Butyric Acid among SCFAs
3.2. Paneth Cells Secrete α-Defensin in Response to Leucine among 20 Amino Acids
3.3. Paneth Cells Express Genes and Proteins for Butyric Acid Receptors and Amino Acid Transporters
3.4. Butyric Acid and Leucine Induce Crp1 Secretion in Enteroids
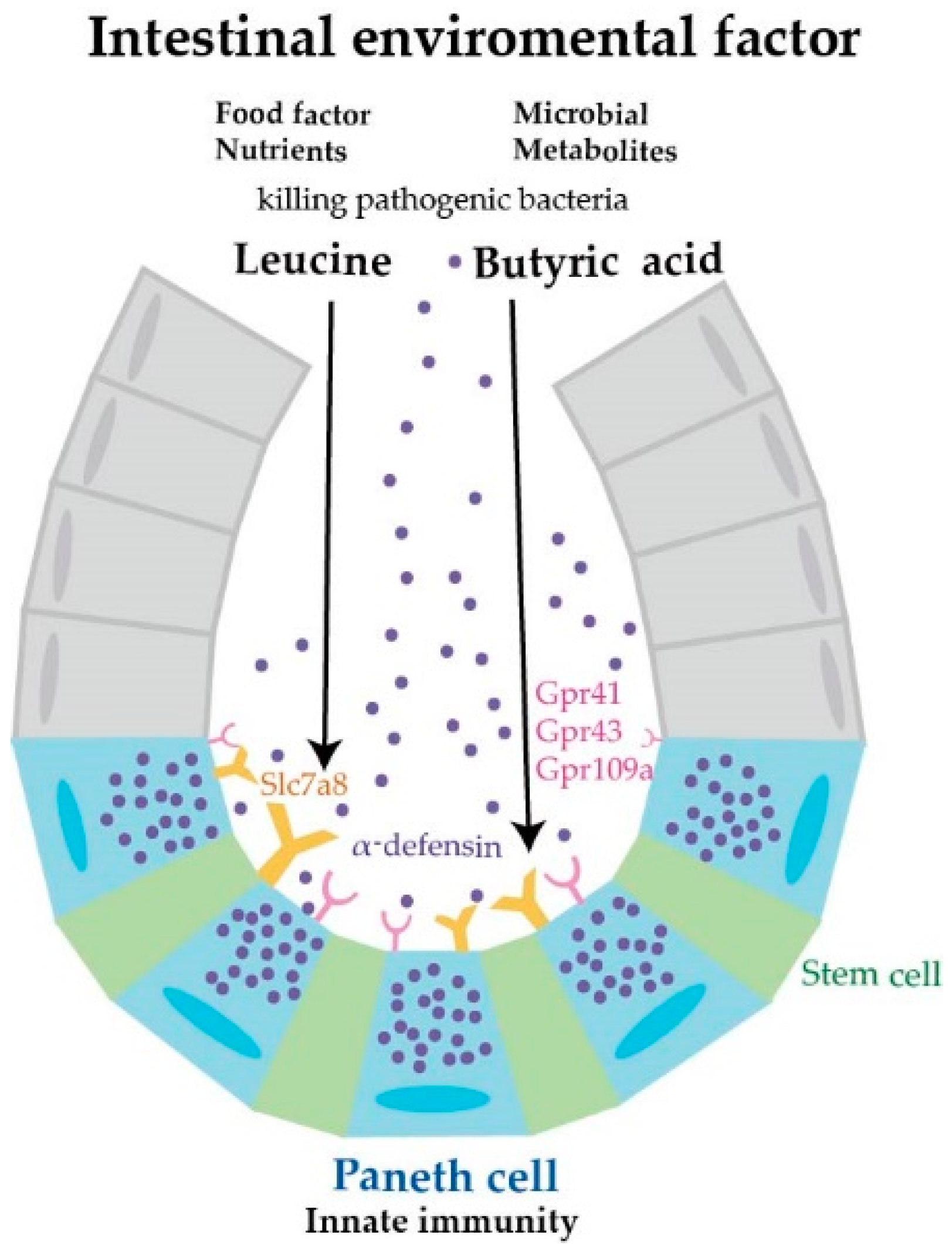
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayabe, T.; Satchell, D.P.; Wilson, C.L.; Parks, W.C.; Selsted, M.E.; Ouellette, A.J. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 2000, 1, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Satchell, D.P.; Pesendorfer, P.; Tanabe, H.; Wilson, C.L.; Hagen, S.J.; Ouellette, A.J. Activation of Paneth cell alpha-defensins in mouse small intestine. J. Biol. Chem. 2002, 277, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Selsted, M.E.; Ouellette, A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005, 6, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Sakai, N.; Nakamura, K.; Yoshioka, S.; Ayabe, T. Bactericidal activity of mouse alpha-defensin cryptdin-4 predominantly affects noncommensal bacteria. J. Innate Immun. 2011, 3, 315–326. [Google Scholar] [CrossRef]
- Salzman, N.H.; Hung, K.; Haribhai, D.; Chu, H.; Karlsson-Sjoberg, J.; Amir, E.; Teggatz, P.; Barman, M.; Hayward, M.; Eastwood, D.; et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 2010, 11, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, J.R.; Ouellette, A.J. Alpha-defensins in enteric innate immunity: Functional Paneth cell alpha-defensins in mouse colonic lumen. J. Biol. Chem. 2009, 284, 27848–27856. [Google Scholar] [CrossRef]
- Mastroianni, J.R.; Costales, J.K.; Zaksheske, J.; Selsted, M.E.; Salzman, N.H.; Ouellette, A.J. Alternative luminal activation mechanisms for Paneth cell alpha-defensins. J. Biol. Chem. 2012, 287, 11205–11212. [Google Scholar] [CrossRef]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef]
- Salzman, N.H.; Bevins, C.L. Dysbiosis—A consequence of Paneth cell dysfunction. Semin. Immunol. 2013, 25, 334–341. [Google Scholar] [CrossRef]
- Nakamura, K.; Sakuragi, N.; Takakuwa, A.; Ayabe, T. Paneth cell alpha-defensins and enteric microbiota in health and disease. Biosci. Microbiota Food Health 2016, 35, 57–67. [Google Scholar] [CrossRef]
- Nakamura, K.; Sakuragi, N.; Ayabe, T. A monoclonal antibody-based sandwich enzyme-linked immunosorbent assay for detection of secreted alpha-defensin. Anal. Biochem. 2013, 443, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Becerril, A.; Castillo-Robles, G.; Gonzalez-Hernandez, M.; Villanueva, I. Influence of high-calorie (cafeteria) diets on the population of Paneth cells in the small intestine of the rat. Eur. J. Morphol. 2005, 42, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; van den Born, M.; Barker, N.; Shroyer, N.F.; van de Wetering, M.; Clevers, H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Guarente, L. mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 2016, 166, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.H.; Katajisto, P.; Lamming, D.W.; Gultekin, Y.; Bauer-Rowe, K.E.; Sengupta, S.; Birsoy, K.; Dursun, A.; Yilmaz, V.O.; Selig, M.; et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 2012, 486, 490–495. [Google Scholar] [CrossRef]
- Hara, T.; Hirasawa, A.; Ichimura, A.; Kimura, I.; Tsujimoto, G. Free Fatty Acid Receptors FFAR1 and GPR120 as Novel Therapeutic Targets for Metabolic Disorders. J. Pharm. Sci. 2011, 100, 3594–3601. [Google Scholar] [CrossRef]
- Liu, J.; Yu, K.; Zhu, W. Amino acid sensing in the gut and its mediation in gut-brain signal transduction. Anim. Nutr. 2016, 2, 69–73. [Google Scholar] [CrossRef]
- Yokoi, Y.; Nakamura, K.; Yoneda, T.; Kikuchi, M.; Sugimoto, R.; Shimizu, Y.; Ayabe, T. Paneth cell granule dynamics on secretory responses to bacterial stimuli in enteroids. Sci. Rep. 2019, 9, 2710. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Kim, C.S.; Cho, S.H.; Chun, H.S.; Lee, S.Y.; Endou, H.; Kanai, Y.; Kim, D.K. BCH, an inhibitor of system L amino acid transporters, induces apoptosis in cancer cells. Biol. Pharm. Bull. 2008, 31, 1096–1100. [Google Scholar] [CrossRef]
- Wrong, O.M. Definitions and history. In Physiological and Clinical Aspects of Short-Chain Fatty Acids, 1st ed.; Cummings, J.H., Rombeau, J.L., Sakata, T., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 1–14. ISBN 0521440483. [Google Scholar]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D.; Martin, P.M.; Singh, N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr. Opin. Pharmacol. 2013, 13, 869–874. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Laerke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Wu, S.; Yi, J.; Xia, Y.; Jin, D.; Zhou, J.; Sun, J. Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin. Ther. 2017, 39, 322–336. [Google Scholar] [CrossRef]
- Sugi, Y.; Takahashi, K.; Kurihara, K.; Nakano, K.; Kobayakawa, T.; Nakata, K.; Tsuda, M.; Hanazawa, S.; Hosono, A.; Kaminogawa, S. alpha-Defensin 5 gene expression is regulated by gut microbial metabolites. Biosci. Biotechnol. Biochem. 2017, 81, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.B.; Clausen, M.R. Short-chain fatty acids in the human colon: Relation to gastrointestinal health and disease. Scand. J. Gastroenterol. Suppl. 1996, 216, 132–148. [Google Scholar] [CrossRef] [PubMed]
- Offermanns, S. Free fatty acid (FFA) and hydroxy carboxylic acid (HCA) receptors. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 407–434. [Google Scholar] [CrossRef] [PubMed]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; World Health Organ Technical Report Series; WHO: Geneva, Switzerland, 2007; Volume 935, pp. 1–265. [Google Scholar]
- Marc Rhoads, J.; Wu, G. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 2009, 37, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Coëffier, M.; Claeyssens, S.; Bensifi, M.; Lecleire, S.; Boukhettala, N.; Maurer, B.; Donnadieu, N.; Lavoinne, A.; Cailleux, A.-F.; Déchelotte, P. Influence of leucine on protein metabolism, phosphokinase expression, and cell proliferation in human duodenum. Am. J. Clin. Nutr. 2011, 93, 1255–1262. [Google Scholar] [CrossRef]
- Sener, A.; Malaisse, W.J. The stimulus-secretion coupling of amino acid-induced insulin release: Insulinotropic action of branched-chain amino acids at physiological concentrations of glucose and glutamine. Eur. J. Clin. Investig. 1981, 11, 455–460. [Google Scholar] [CrossRef]
- Liu, Y.J.; Cheng, H.; Drought, H.; MacDonald, M.J.; Sharp, G.W.; Straub, S.G. Activation of the KATP channel-independent signaling pathway by the nonhydrolyzable analog of leucine, BCH. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E380–E389. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef]
- Omata, J.; Pierre, J.F.; Heneghan, A.F.; Tsao, F.H.; Sano, Y.; Jonker, M.A.; Kudsk, K.A. Parenteral nutrition suppresses the bactericidal response of the small intestine. Surgery 2013, 153, 17–24. [Google Scholar] [CrossRef]
- Heneghan, A.F.; Pierre, J.F.; Tandee, K.; Shanmuganayagam, D.; Wang, X.; Reed, J.D.; Steele, J.L.; Kudsk, K.A. Parenteral nutrition decreases paneth cell function and intestinal bactericidal activity while increasing susceptibility to bacterial enteroinvasion. JPEN J. Parenter. Enteral. Nutr. 2014, 38, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evolut. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Nat. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Zeng, B.; Zhi, F.C. Regulation of human enteric alpha-defensins by NOD2 in the Paneth cell lineage. Eur. J. Cell Biol. 2015, 94, 60–66. [Google Scholar] [CrossRef]
- Hodin, C.M.; Verdam, F.J.; Grootjans, J.; Rensen, S.S.; Verheyen, F.K.; Dejong, C.H.; Buurman, W.A.; Greve, J.W.; Lenaerts, K. Reduced Paneth cell antimicrobial protein levels correlate with activation of the unfolded protein response in the gut of obese individuals. J. Pathol. 2011, 225, 276–284. [Google Scholar] [CrossRef]
- Eriguchi, Y.; Takashima, S.; Oka, H.; Shimoji, S.; Nakamura, K.; Uryu, H.; Shimoda, S.; Iwasaki, H.; Shimono, N.; Ayabe, T.; et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of alpha-defensins. Blood 2012, 120, 223–231. [Google Scholar] [CrossRef]
- Eriguchi, Y.; Nakamura, K.; Hashimoto, D.; Shimoda, S.; Shimono, N.; Akashi, K.; Ayabe, T.; Teshima, T. Decreased secretion of Paneth cell alpha-defensins in graft-versus-host disease. Transpl. Infect. Dis. 2015, 17, 702–706. [Google Scholar] [CrossRef]
- Hayase, E.; Hashimoto, D.; Nakamura, K.; Noizat, C.; Ogasawara, R.; Takahashi, S.; Ohigashi, H.; Yokoi, Y.; Sugimoto, R.; Matsuoka, S.; et al. R-Spondin1 expands Paneth cells and prevents dysbiosis induced by graft-versus-host disease. J. Exp. Med. 2017, 214, 3507–3518. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takakuwa, A.; Nakamura, K.; Kikuchi, M.; Sugimoto, R.; Ohira, S.; Yokoi, Y.; Ayabe, T. Butyric Acid and Leucine Induce α-Defensin Secretion from Small Intestinal Paneth Cells. Nutrients 2019, 11, 2817. https://doi.org/10.3390/nu11112817
Takakuwa A, Nakamura K, Kikuchi M, Sugimoto R, Ohira S, Yokoi Y, Ayabe T. Butyric Acid and Leucine Induce α-Defensin Secretion from Small Intestinal Paneth Cells. Nutrients. 2019; 11(11):2817. https://doi.org/10.3390/nu11112817
Chicago/Turabian StyleTakakuwa, Akiko, Kiminori Nakamura, Mani Kikuchi, Rina Sugimoto, Shuya Ohira, Yuki Yokoi, and Tokiyoshi Ayabe. 2019. "Butyric Acid and Leucine Induce α-Defensin Secretion from Small Intestinal Paneth Cells" Nutrients 11, no. 11: 2817. https://doi.org/10.3390/nu11112817
APA StyleTakakuwa, A., Nakamura, K., Kikuchi, M., Sugimoto, R., Ohira, S., Yokoi, Y., & Ayabe, T. (2019). Butyric Acid and Leucine Induce α-Defensin Secretion from Small Intestinal Paneth Cells. Nutrients, 11(11), 2817. https://doi.org/10.3390/nu11112817




