Inhibitory Effects of Osthole on Human Breast Cancer Cell Progression via Induction of Cell Cycle Arrest, Mitochondrial Dysfunction, and ER Stress
Abstract
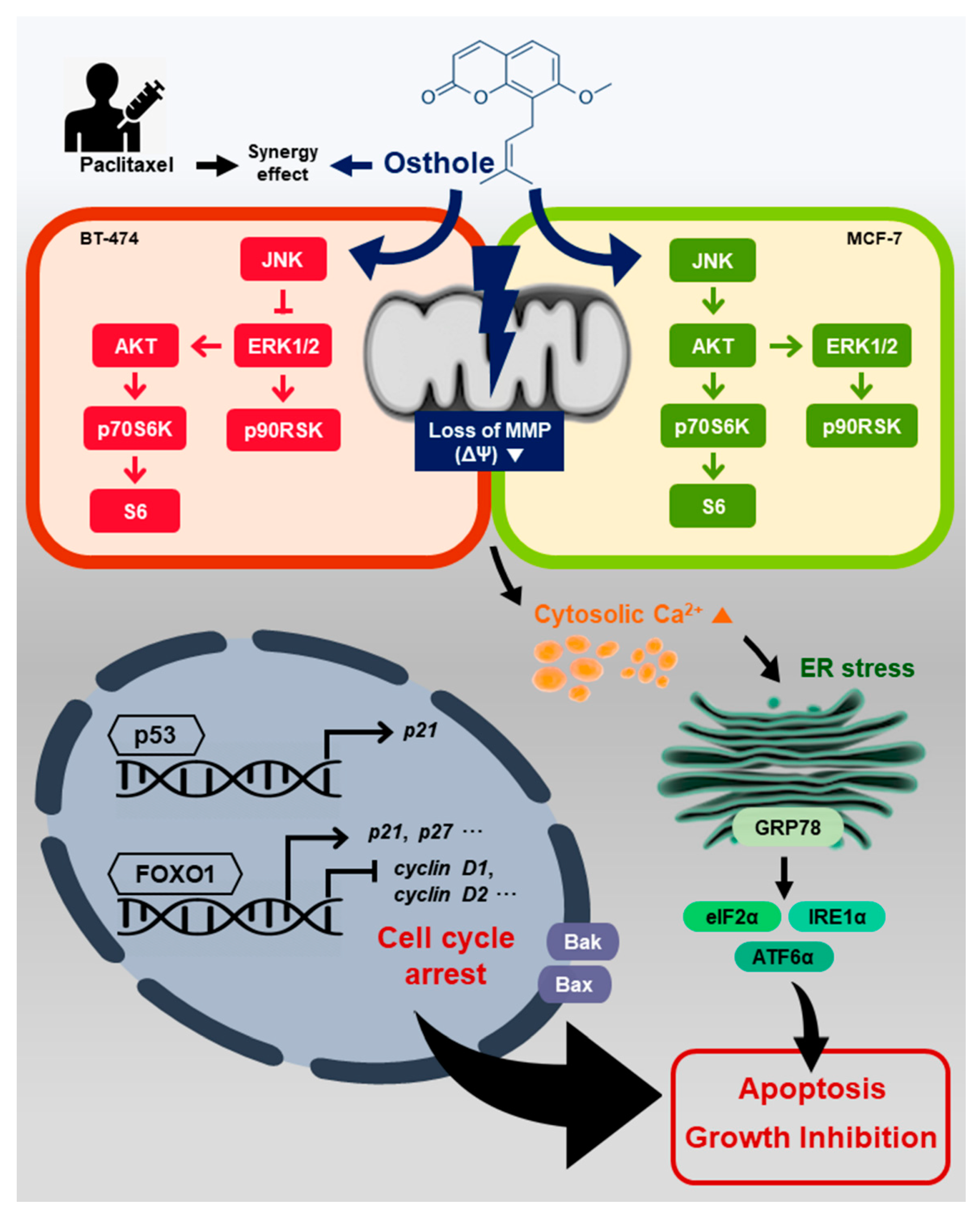
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Cell Culture
2.3. Proliferation Assay
2.4. Cell Cycle Analysis
2.5. Immunofluorescence Microscopy
2.6. Annexin V and PI Staining
2.7. TUNEL Assay
2.8. JC-1 Mitochondrial Membrane Potential Assay
2.9. Measurement of Cytosolic Calcium Influx
2.10. Quantitative RT-PCR Analysis
2.11. Western Blot Analyses
2.12. Statistical Analysis
3. Results
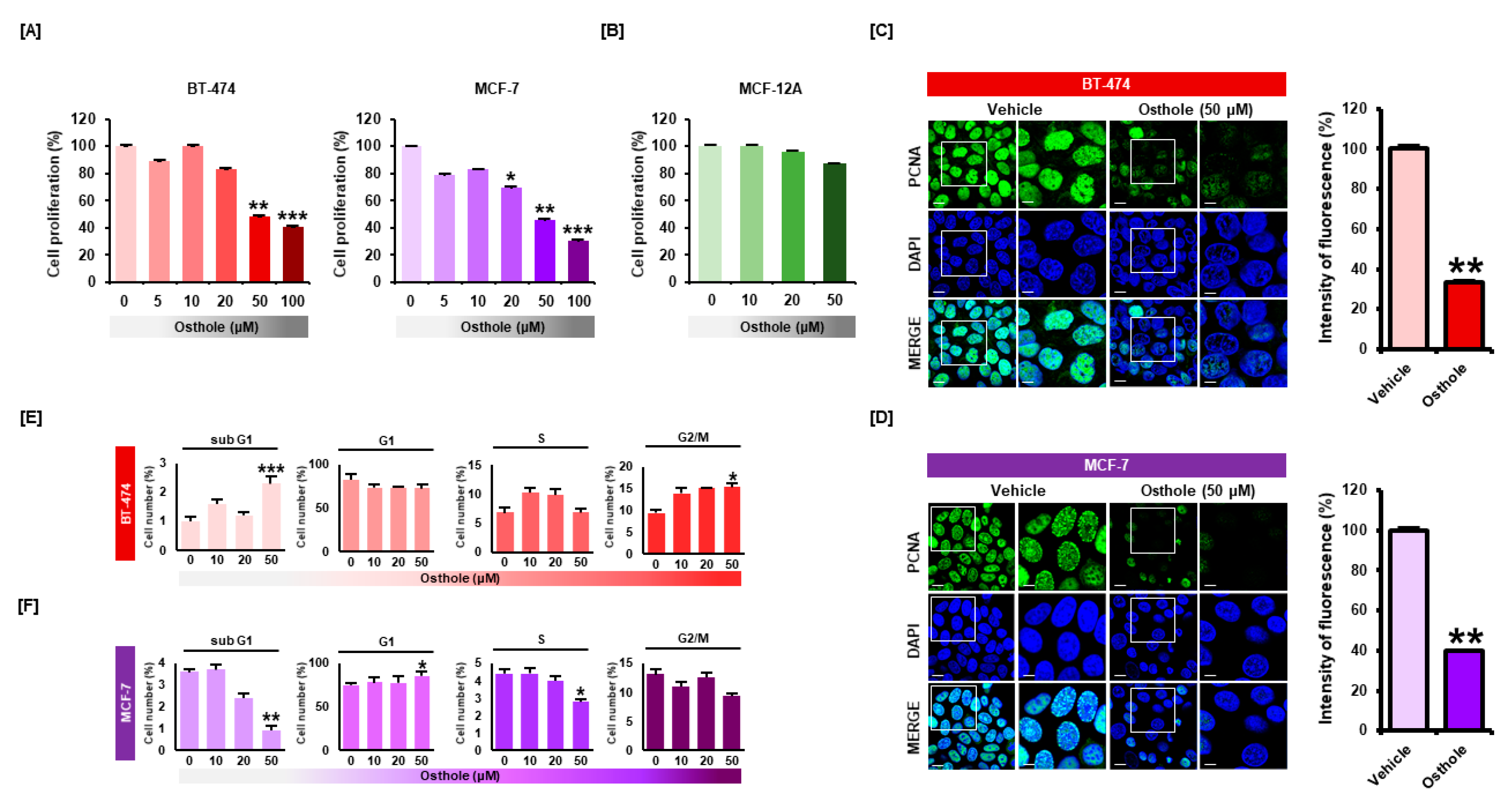
3.1. Osthole Selectively Inhibited Cellular Proliferation of Human Breast Cancer Cells
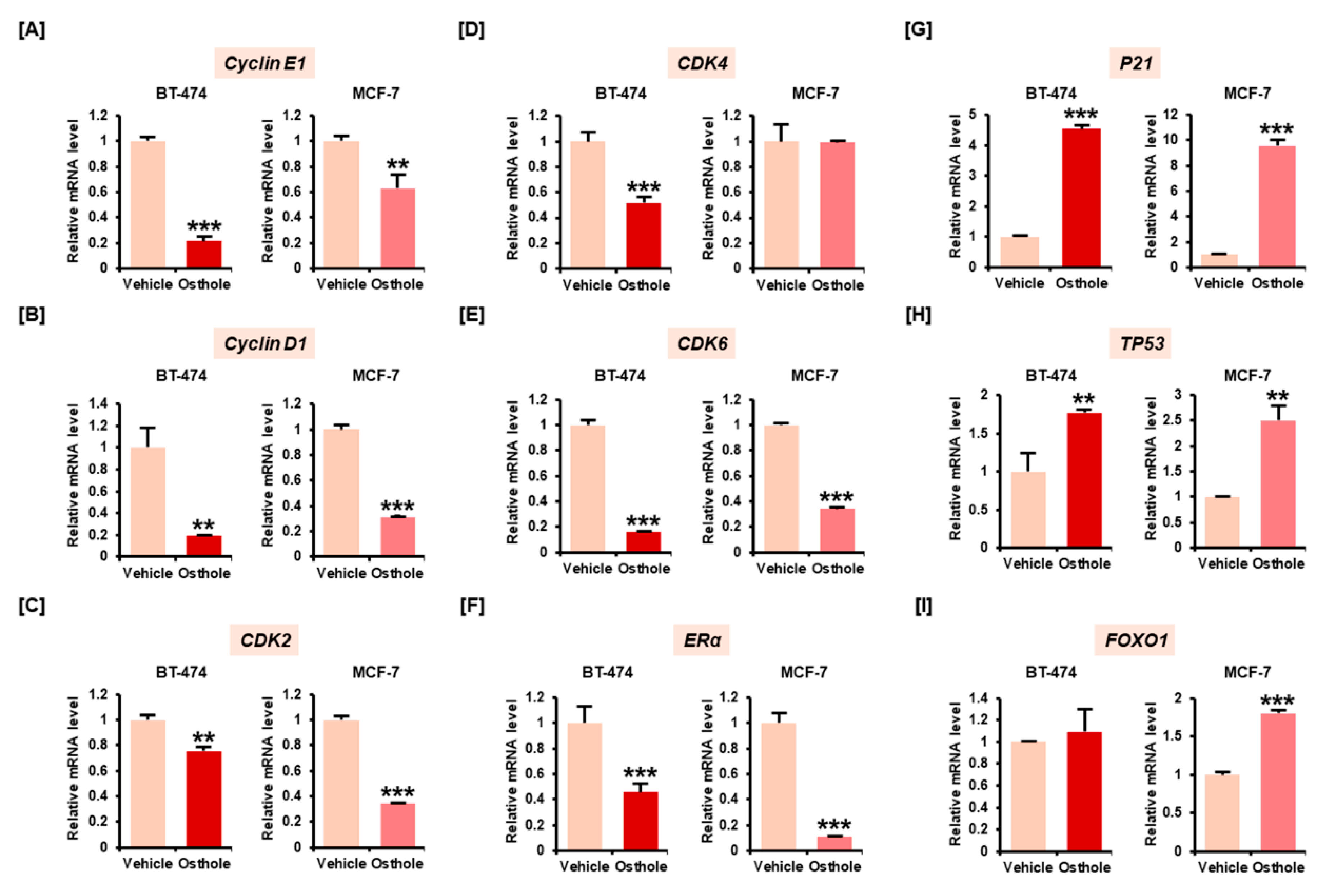
3.2. Osthole Downregulated Gene Expression Associated with Cell Cycle Progression in Breast Cancer Cells
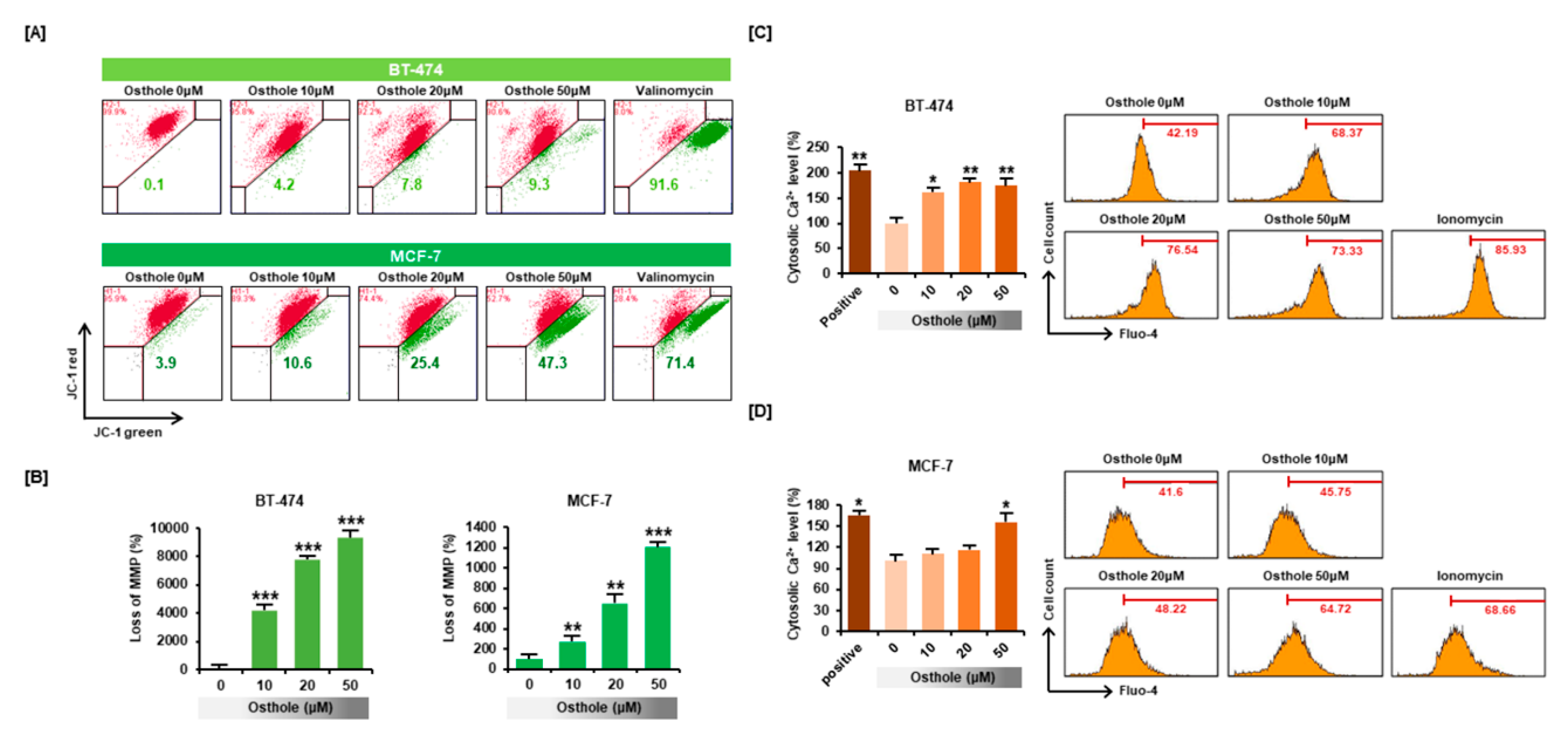
3.3. Osthole Disrupted the Mitochondrial Membrane Potential and Intracellular Calcium Flux in Human Breast Cancer Cells
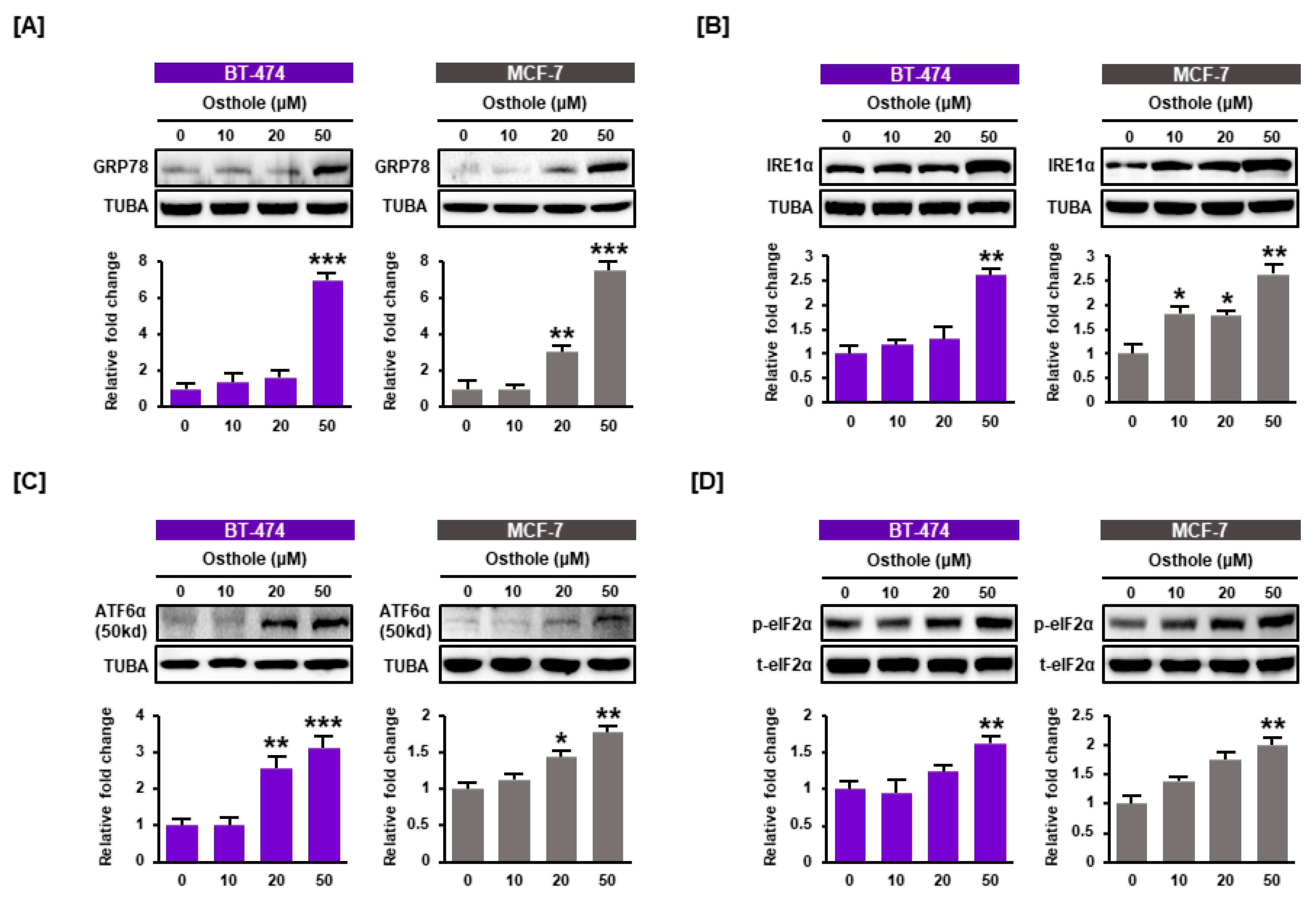
3.4. Osthole Activated ER Stress Pathways in Human Breast Cancer Cells
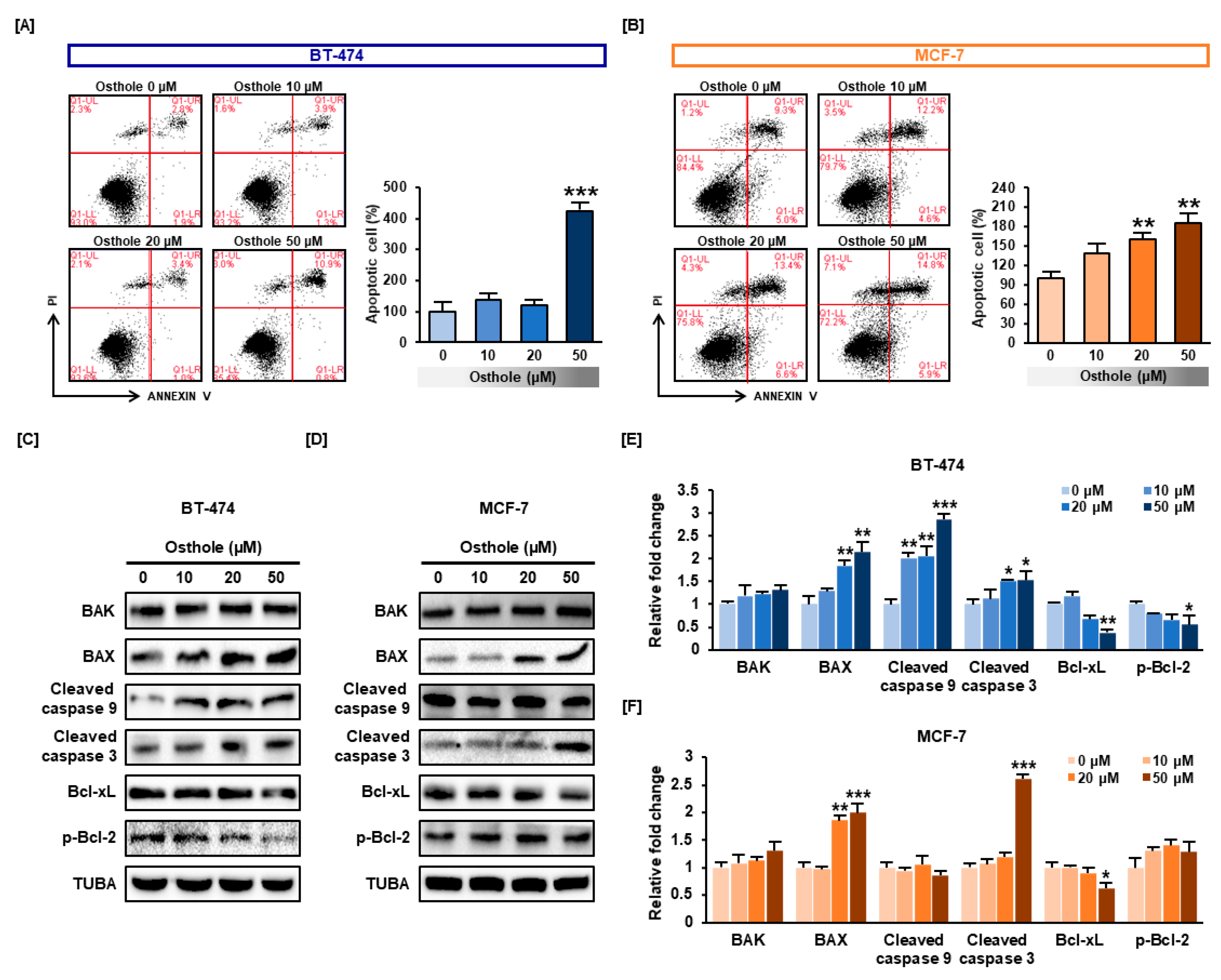
3.5. Osthole Induced Apoptosis in Human Breast Cancer Cells
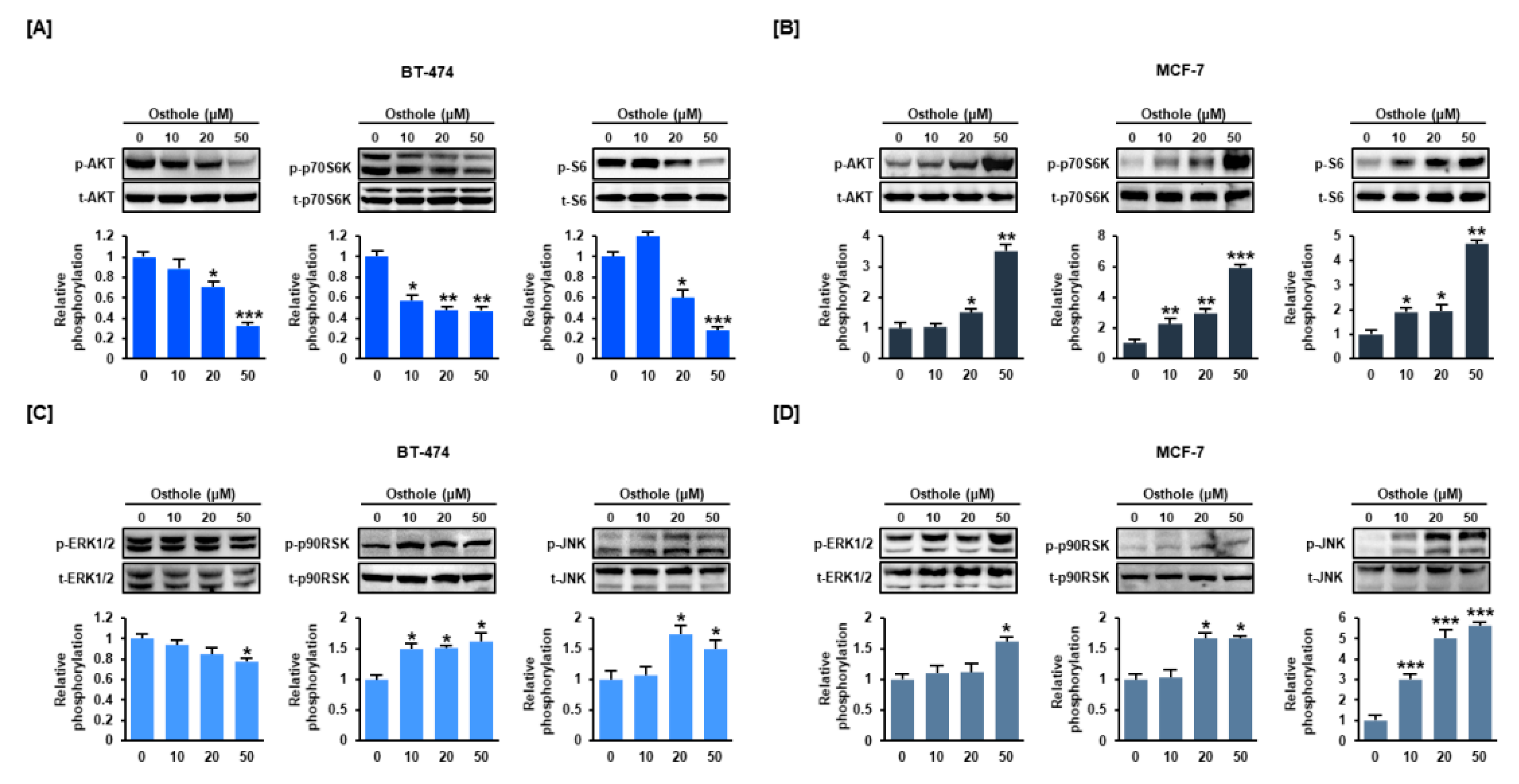
3.6. Osthole Regulated the PI3K/Akt and MAPK Signaling Pathways in Human Breast Cancer Cells
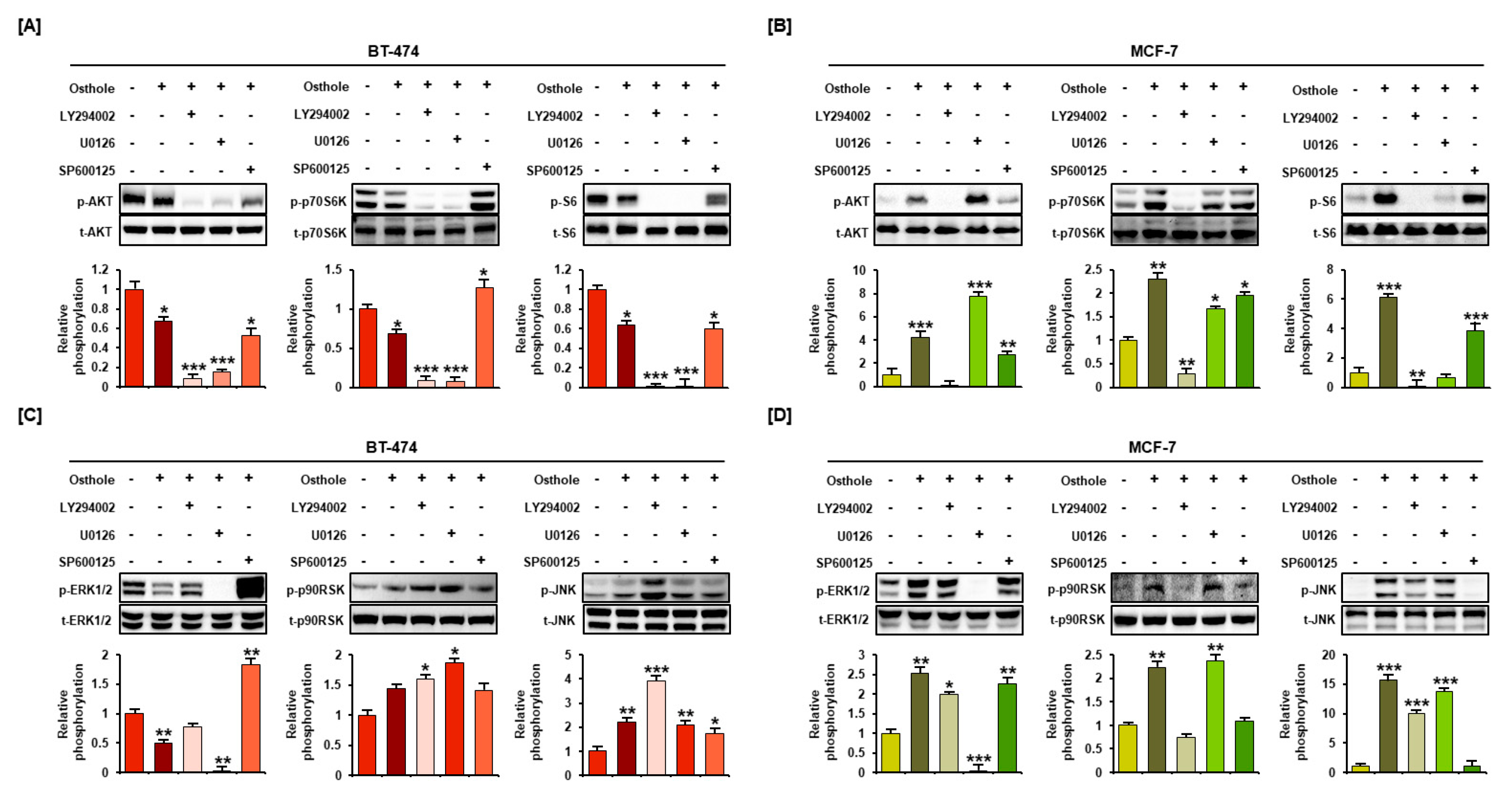
3.7. Interaction between Osthole-Mediated Modulation of the PI3K/Akt and MAPK Pathways
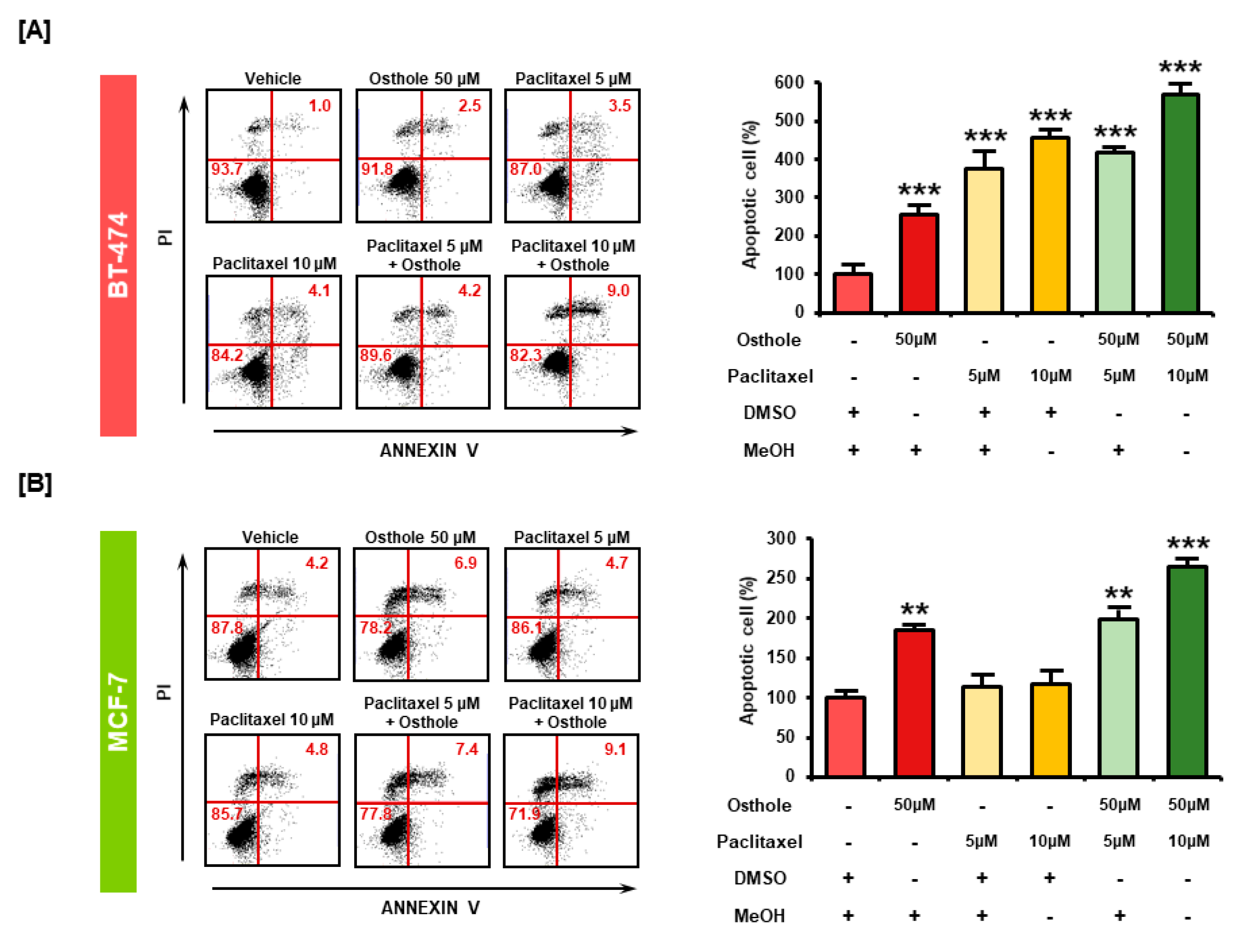
3.8. Combinational Effects of Osthole with Commercial Chemotherapeutic Drugs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Winters, S.; Martin, C.; Murphy, D.; Shokar, N.K. Breast Cancer Epidemiology, Prevention, and Screening. Prog. Mol. Biol. Transl. Sci. 2017, 151, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Winters, S.; Martin, C.; Murphy, D.; Shokar, N.K. Chapter One—Breast Cancer Epidemiology, Prevention, and Screening. In Progress in Molecular Biology and Translational Science; Lakshmanaswamy, R., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 151, pp. 1–32. [Google Scholar]
- Redig, A.J.; McAllister, S.S. Breast cancer as a systemic disease: A view of metastasis. J. Intern. Med. 2013, 274, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2016; Volume 40, pp. 209–232. [Google Scholar] [CrossRef]
- Rattani, N.S.; Swift-Scanlan, T. Deconstructing breast cancer heterogeneity: Clinical implications for women with Basal-like tumors. Oncol. Nurs. Forum 2014, 41, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef]
- Subik, K.; Lee, J.-F.; Baxter, L.; Strzepek, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.-C.; Bonfiglio, T.; Hicks, D.G.; et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer (Auckl.) 2010, 4, 35–41. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Semin. Cancer Biol. 2016, 40, 192–208. [Google Scholar] [CrossRef]
- Kanematsu, S.; Uehara, N.; Miki, H.; Yoshizawa, K.; Kawanaka, A.; Yuri, T.; Tsubura, A. Autophagy inhibition enhances sulforaphane-induced apoptosis in human breast cancer cells. Anticancer Res. 2010, 30, 3381–3390. [Google Scholar]
- Garvin, S.; Öllinger, K.; Dabrosin, C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006, 231, 113–122. [Google Scholar] [CrossRef]
- Koohpar, Z.K.; Entezari, M.; Movafagh, A.; Hashemi, M. Anticancer activity of curcumin on human breast adenocarcinoma: Role of Mcl-1 gene. Iran. J. Cancer Prev. 2015, 8, e2331. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Sehrawat, A.; Singh, S.V. Dietary chemopreventative benzyl isothiocyanate inhibits breast cancer stem cells in vitro and in vivo. Cancer Prev. Res. 2013, 6, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Hoult, J.R.; Paya, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Orhan, I.E.; Cordell, G.A.; Nabavi, S.M.; Budzyńska, B. Implication of coumarins towards central nervous system disorders. Pharmacol. Res. 2016, 103, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, A.M.; Pereira, J.A.; Labiris, R.; McDonald, H.; Douketis, J.D.; Crowther, M.; Wells, P.S. Systematic overview of warfarin and its drug and food interactions. Arch. Intern. Med. 2005, 165, 1095–1106. [Google Scholar] [CrossRef]
- Yu, D.; Suzuki, M.; Xie, L.; Morris-Natschke, S.L.; Lee, K.H. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med. Res. Rev. 2003, 23, 322–345. [Google Scholar] [CrossRef]
- Okamoto, T.; Yoshida, S.; Kobayashi, T.; Okabe, S. Inhibition of concanavalin A-induced mice hepatitis by coumarin derivatives. Jpn. J. Pharmacol. 2001, 85, 95–97. [Google Scholar] [CrossRef]
- Matsuda, H.; Tomohiro, N.; Ido, Y.; Kubo, M. Anti-allergic effects of cnidii monnieri fructus (dried fruits of Cnidium monnieri) and its major component, osthol. Biol. Pharm. Bull. 2002, 25, 809–812. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, G.; Yao, F.; He, Y.; Liang, G.; Zhang, Y.; Hu, B.; Wu, Y.; Li, Y.; Liu, H. Growth inhibition and apoptosis induced by osthole, a natural coumarin, in hepatocellular carcinoma. PLoS ONE 2012, 7, e37865. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Qu, D.; Jiang, T.; Li, S. Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells by modulating PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2011, 30, 33. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, J.; Ren, B.; Tang, Y.; Owusu, L.; Li, M.; Zhang, J.; Liu, L.; Li, W. Anti-tumor effects of osthole on ovarian cancer cells in vitro. J. Ethnopharmacol. 2016, 193, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Gu, T.; Wang, T.; Tang, Q.; Ma, C. Effects of osthole on migration and invasion in breast cancer cells. Biosci. Biotechnol. Biochem. 2010, 74, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yin, C.; Zhang, Y.; Guo, G.; Zhao, C.; Wang, O.; Xiang, Y.; Zhang, X.; Liang, G. Osthole inhibits triple negative breast cancer cells by suppressing STAT3. J. Exp. Clin. Cancer Res. 2018, 37, 322. [Google Scholar] [CrossRef] [PubMed]
- Santa-Maria, C.A.; Gradishar, W.J. Changing treatment paradigms in metastatic breast cancer: Lessons learned. JAMA Oncol. 2015, 1, 528–534. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Diamandis, E.P. Breast and prostate cancer: An analysis of common epidemiological, genetic, and biochemical features. Endocr. Rev. 1998, 19, 365–396. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A. Breast cancer management: Opportunities and barriers to an individualized approach. Oncologist 2011, 16, 20–22. [Google Scholar] [CrossRef]
- Polyak, K. Heterogeneity in breast cancer. J. Clin. Investig. 2011, 121, 3786–3788. [Google Scholar] [CrossRef]
- Hu, H.; Ahn, N.-S.; Yang, X.; Lee, Y.-S.; Kang, K.-S. Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int. J. Cancer 2002, 102, 250–253. [Google Scholar] [CrossRef]
- Kang, J.H.; Song, K.H.; Woo, J.K.; Park, M.H.; Rhee, M.H.; Choi, C.; Oh, S.H. Ginsenoside Rp1 from Panax ginseng exhibits anti-cancer activity by down-regulation of the IGF-1R/Akt pathway in breast cancer cells. Plant Foods Hum. Nutr. 2011, 66, 298–305. [Google Scholar] [CrossRef]
- Park, S.H.; Ham, S.; Kwon, T.H.; Kim, M.S.; Lee, D.H.; Kang, J.W.; Oh, S.R.; Yoon, D.Y. Luteolin induces cell cycle arrest and apoptosis through extrinsic and intrinsic signaling pathways in MCF-7 breast cancer cells. J. Env. Pathol. Toxicol. Oncol. 2014, 33, 219–231. [Google Scholar] [CrossRef]
- Seo, H.S.; Jo, J.K.; Ku, J.M.; Choi, H.S.; Choi, Y.K.; Woo, J.K.; Kim, H.I.; Kang, S.Y.; Lee, K.M.; Nam, K.W.; et al. Induction of caspase-dependent extrinsic apoptosis by apigenin through inhibition of signal transducer and activator of transcription 3 (STAT3) signalling in HER2-overexpressing BT-474 breast cancer cells. Biosci. Rep. 2015, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arber, N.; Doki, Y.; Han, E.K.; Sgambato, A.; Zhou, P.; Kim, N.-H.; Delohery, T.; Klein, M.G.; Holt, P.R.; Weinstein, I.B. Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells. Cancer Res. 1997, 57, 1569–1574. [Google Scholar] [PubMed]
- Zhou, P.; Jiang, W.; Zhang, Y.; Kahn, S.M.; Schieren, I.; Santella, R.M.; Weinstein, I.B. Antisense to cyclin D1 inhibits growth and reverses the transformed phenotype of human esophageal cancer cells. Oncogene 1995, 11, 571–580. [Google Scholar] [PubMed]
- Kornmann, M.; Danenberg, K.D.; Arber, N.; Beger, H.G.; Danenberg, P.V.; Korc, M. Inhibition of cyclin D1 expression in human pancreatic cancer cells is associated with increased chemosensitivity and decreased expression of multiple chemoresistance genes. Cancer Res. 1999, 59, 3505–3511. [Google Scholar]
- Carroll, J.S.; Prall, O.W.; Musgrove, E.A.; Sutherland, R.L. A pure estrogen antagonist inhibits cyclin E-Cdk2 activity in MCF-7 breast cancer cells and induces accumulation of p130-E2F4 complexes characteristic of quiescence. J. Biol. Chem. 2000, 275, 38221–38229. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Lin, S.-Y.; Brunicardi, F.C.; Seu, P. Use of RNA Interference to Target Cyclin E-overexpressing Hepatocellular Carcinoma. Cancer Res. 2003, 63, 3593–3597. [Google Scholar]
- Carlson, B.A.; Dubay, M.M.; Sausville, E.A.; Brizuela, L.; Worland, P.J. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 1996, 56, 2973–2978. [Google Scholar]
- McClue, S.J.; Blake, D.; Clarke, R.; Cowan, A.; Cummings, L.; Fischer, P.M.; MacKenzie, M.; Melville, J.; Stewart, K.; Wang, S. In vitro and in vivo antitumor properties of the cyclin dependent kinase inhibitor CYC202 (R-roscovitine). Int. J. Cancer 2002, 102, 463–468. [Google Scholar] [CrossRef]
- Sayeed, A.; Konduri, S.; Liu, W.; Bansal, S.; Li, F.; Das, G.M. Estrogen Receptor Inhibits p53-Mediated Transcriptional Repression: Implications for the Regulation of Apoptosis. Cancer Res. 2007, 67, 7746–7755. [Google Scholar] [CrossRef]
- Bailey, S.T.; Shin, H.; Westerling, T.; Liu, X.S.; Brown, M. Estrogen receptor prevents p53-dependent apoptosis in breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 18060–18065. [Google Scholar] [CrossRef] [Green Version]
- El-Deiry, W.S.; Tokino, T.; Velculescu, V.E.; Levy, D.B.; Parsons, R.; Trent, J.M.; Lin, D.; Mercer, W.E.; Kinzler, K.W.; Vogelstein, B. WAF1, a potential mediator of p53 tumor suppression. Cell 1993, 75, 817–825. [Google Scholar] [CrossRef]
- Li, R.; Waga, S.; Hannon, G.J.; Beach, D.; Stillman, B. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature 1994, 371, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tindall, D.J. Dynamic FoxO transcription factors. J. Cell Sci. 2007, 120, 2479–2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-Y.; Chan, C.-Y.; Chou, I.T.; Lien, C.-H.; Hung, H.-C.; Lee, M.-F. Quercetin induces growth arrest through activation of FOXO1 transcription factor in EGFR-overexpressing oral cancer cells. J. Nutr. Biochem. 2013, 24, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Song, X.; Xie, K.; Zhang, X.; He, W.; Liu, F. Osthole induces apoptosis and suppresses proliferation via the PI3K/Akt pathway in intrahepatic cholangiocarcinoma. Int. J. Mol. Med. 2017, 40, 1143–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, X.; Zhou, X.; Zheng, G.; Dong, C.; Zhang, W.; Song, X.; Jin, T. Osthole induces G2/M cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Pharm. Biol. 2014, 52, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Renault, T.T.; Floros, K.V.; Chipuk, J.E. BAK/BAX activation and cytochrome c release assays using isolated mitochondria. Methods 2013, 61, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Eldeeb, M.A.; Fahlman, R.P.; Esmaili, M.; Ragheb, M.A. Regulating Apoptosis by Degradation: The N-End Rule-Mediated Regulation of Apoptotic Proteolytic Fragments in Mammalian Cells. Int. J. Mol. Sci. 2018, 19, 3414. [Google Scholar] [CrossRef] [Green Version]
- Varshavsky, A. The N-end rule and regulation of apoptosis. Nat. Cell Biol. 2003, 5, 373–376. [Google Scholar] [CrossRef] [Green Version]
- Eldeeb, M.A.; Ragheb, M.A.; Fon, E.A. Cell Death: N-degrons Fine-Tune Pyroptotic Cell Demise. Curr. Biol. 2019, 29, R588–R591. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Calcium: Regulation of cell death: The calcium–apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef]
- Zheng, S.; Ma, Y.; Guo, Y.; Wang, L.; Pan, Y. Osthole promote differentiation and inhibit proliferation of osteoblast by activating wnt signaling and endoplasmic reticulum stress. Pharmacogn. Mag. 2018, 14, 641–646. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Saini, K.S.; Loi, S.; de Azambuja, E.; Metzger-Filho, O.; Saini, M.L.; Ignatiadis, M.; Dancey, J.E.; Piccart-Gebhart, M.J. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat. Rev. 2013, 39, 935–946. [Google Scholar] [CrossRef]
- Steelman, L.; Abrams, S.; Whelan, J.; Bertrand, F.; Ludwig, D.; Bäsecke, J.; Libra, M.; Stivala, F.; Milella, M.; Tafuri, A. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia 2008, 22, 686–707. [Google Scholar] [CrossRef] [Green Version]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Lin, V.C.-H.; Chou, C.-H.; Lin, Y.-C.; Lin, J.-N.; Yu, C.-C.; Tang, C.-H.; Lin, H.-Y.; Way, T.-D. Osthole Suppresses Fatty Acid Synthase Expression in HER2-Overexpressing Breast Cancer Cells through Modulating Akt/mTOR Pathway. J. Agric. Food Chem. 2010, 58, 4786–4793. [Google Scholar] [CrossRef]
- Ding, D.; Wei, S.; Song, Y.; Li, L.; Du, G.; Zhan, H.; Cao, Y. Osthole Exhibits Anti-Cancer Property in Rat Glioma Cells Through Inhibiting PI3K/Akt and MAPK Signaling Pathways. Cell. Physiol. Biochem. 2013, 32, 1751–1760. [Google Scholar] [CrossRef]
- Hay, N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 2005, 8, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, V.; Park, Y.; Chen, C.-C.; Xu, P.-Z.; Chen, M.-L.; Tonic, I.; Unterman, T.; Hay, N. Akt Determines Replicative Senescence and Oxidative or Oncogenic Premature Senescence and Sensitizes Cells to Oxidative Apoptosis. Cancer Cell 2008, 14, 458–470. [Google Scholar] [CrossRef] [Green Version]
- Cagnol, S.; Chambard, J.-C. ERK and cell death: Mechanisms of ERK-induced cell death—Apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef]
- Zhou, Q.-M.; Wang, S.; Zhang, H.; Lu, Y.-Y.; Wang, X.-F.; Motoo, Y.; Su, S.-B. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol. Sin. 2009, 30, 1648–1658. [Google Scholar] [CrossRef]
- Yeh, P.Y.; Chuang, S.-E.; Yeh, K.-H.; Song, Y.C.; Chang, L.L.-Y.; Cheng, A.-L. Phosphorylation of p53 on Thr55 by ERK2 is necessary for doxorubicin-induced p53 activation and cell death. Oncogene 2004, 23, 3580–3588. [Google Scholar] [CrossRef] [Green Version]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef] [Green Version]
- Park, S.K.; Sanders, B.G.; Kline, K. Tocotrienols induce apoptosis in breast cancer cell lines via an endoplasmic reticulum stress-dependent increase in extrinsic death receptor signaling. Breast Cancer Res. Treat. 2010, 124, 361–375. [Google Scholar] [CrossRef]
- Deng, Y.T.; Huang, H.C.; Lin, J.K. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol. Carcinog. 2010, 49, 141–151. [Google Scholar] [CrossRef]
- Alanazi, I.O.; Khan, Z. Endocrine and Cell Surface Receptor Signaling in Breast Carcinogenesis. In Breast Cancer and Surgery; IntechOpen: London, UK, 2018. [Google Scholar]
- Dai, X.; Li, T.; Bai, Z.; Yang, Y.; Liu, X.; Zhan, J.; Shi, B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am. J. Cancer Res. 2015, 5, 2929–2943. [Google Scholar]
| Gene Symbol | GenBank No. | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|---|
| CCND1 | NM_053056.2 | TGCTGGTTTTCTACCCAACG | AGTGCTTGGAAATGGAATGG |
| CCNE1 | NM_001238.3 (Updated as NM_001238.4) | CGGCCTTGTATCATTTCTCG | TCCCCGTCTCCCTTATAACC |
| CDK2 | BT006821.1 | TTTGCTGAGATGGTGACTCG | AAGTAACTCCTGGCCACACC |
| CDK4 | NM_000075.3 (Updated as NM_000075.4) | CCGAAGTTCTTCTGCAGTCC | CCACAGAAGAGAGGCTTTCG |
| CDK6 | AK313491.1 | CATTCAAAATCTGCCCAACC | TGGAAGTATGGGTGAGACAGG |
| ERα | NM_001328100.2 | CAGGCTTTGTGGATTTGACC | ATTTTCCCTGGTTCCTGTCC |
| P21 | NM_000389.4 (Updated as NM_000389.5) | GACTCTCAGGGTCGAAAACG | GGATTAGGGCTTCCTCTTGG |
| TP53 | NM_000546.5 | GTCTTTGAACCCTTGCTTGC | CCACAACAAAACACCAGTGC |
| FOXO1 | NM_002015.3 (Updated as NM_002015.4) | CAGCAAGTTCATTCGTGTGC | CTGTTGTTGTCCATGGATGC |
| GAPDH | NM_001289745.3 | GGCTCTCCAGAACATCATCC | TTTCTAGACGGCAGGTCAGG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, W.; Park, S.; Song, G.; Lim, W. Inhibitory Effects of Osthole on Human Breast Cancer Cell Progression via Induction of Cell Cycle Arrest, Mitochondrial Dysfunction, and ER Stress. Nutrients 2019, 11, 2777. https://doi.org/10.3390/nu11112777
Park W, Park S, Song G, Lim W. Inhibitory Effects of Osthole on Human Breast Cancer Cell Progression via Induction of Cell Cycle Arrest, Mitochondrial Dysfunction, and ER Stress. Nutrients. 2019; 11(11):2777. https://doi.org/10.3390/nu11112777
Chicago/Turabian StylePark, Wonhyoung, Sunwoo Park, Gwonhwa Song, and Whasun Lim. 2019. "Inhibitory Effects of Osthole on Human Breast Cancer Cell Progression via Induction of Cell Cycle Arrest, Mitochondrial Dysfunction, and ER Stress" Nutrients 11, no. 11: 2777. https://doi.org/10.3390/nu11112777
APA StylePark, W., Park, S., Song, G., & Lim, W. (2019). Inhibitory Effects of Osthole on Human Breast Cancer Cell Progression via Induction of Cell Cycle Arrest, Mitochondrial Dysfunction, and ER Stress. Nutrients, 11(11), 2777. https://doi.org/10.3390/nu11112777







