Basal Metabolic Rate and Body Composition Predict Habitual Food and Macronutrient Intakes: Gender Differences
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Macronutrient Intake
2.3. Anthropometry
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pelchat, M.L. Of human bondage: Food craving, obsession, compulsion, and addiction. Physiol. Behav. 2002, 76, 347–352. [Google Scholar] [CrossRef]
- White, M.A.; Whisenhunt, B.L.; Williamson, D.A.; Greenway, F.L.; Netemeyer, R.G. Development and validation of the food-craving inventory. Obes. Res. 2002, 10, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mela, D.J. Determinants of food choice: relationships with obesity and weight control. Obes. Res. 2001, 249S–255S. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Hu, F.B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef]
- Davis, C.; Curtis, C.; Levitan, R.D.; Carter, J.C.; Kaplan, A.S.; Kennedy, J.L. Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite 2011, 57, 711–717. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef]
- Gardner, C.D.; Kiazand, A.; Alhassan, S.; Kim, S.; Stafford, R.S.; Balise, R.R.; Kraemer, H.C.; King, A.C. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: The A to Z Weight Loss Study: A randomized trial. JAMA 2007, 297, 969–977. [Google Scholar] [CrossRef]
- Samaha, F.F.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, T.; Williams, M.; Gracely, E.J.; Stern, L. A low-carbohydrate as compared with a low-fat diet in severe obesity. N. Engl. J. Med. 2003, 348, 2074–2081. [Google Scholar] [CrossRef]
- Noakes, M.; Keogh, J.B.; Foster, P.R.; Clifton, P.M. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional low-fat, high-carbohydrate diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am. J. Clin. Nutr. 2005, 81, 1298–1306. [Google Scholar] [CrossRef]
- McLaughlin, T.; Carter, S.; Lamendola, C.; Abbasi, F.; Yee, G.; Schaaf, P.; Basina, M.; Reaven, G. Effects of moderate variations in macronutrient composition on weight loss and reduction in cardiovascular disease risk in obese, insulin-resistant adults. Am. J. Clin. Nutr. 2006, 84, 813–821. [Google Scholar] [CrossRef]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; McGuckin, B.G.; Brill, C.; Mohammed, B.S.; Szapary, P.O.; Rader, D.J.; Edman, J.S.; Klein, S. A randomized trial of a low-carbohydrate diet for obesity. N. Engl. J. Med. 2003, 348, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Gilhooly, C.H.; Golden, J.K.; Pittas, A.G.; Fuss, P.J.; Cheatham, R.A.; Tyler, S.; Tsay, M.; McCrory, M.A.; Lichtenstein, A.H.; et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: A 1-y randomized controlled trial. Am. J. Clin. Nutr. 2007, 85, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.B.; Luscombe-Marsh, N.D.; Noakes, M.; Wittert, G.A.; Clifton, P.M. Long-term weight maintenance and cardiovascular risk factors are not different following weight loss on carbohydrate-restricted diets high in either monounsaturated fat or protein in obese hyperinsulinemic men and women. Br. J. Nutr. 2007, 97, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Goss, A.M.; Goree, L.L.; Ellis, A.C.; Chandler-Laney, P.C.; Casazza, K.; Lockhart, M.E.; Gower, B.A. Effects of diet macronutrient composition on body composition and fat distribution during weight maintenance and weight loss. Obesity 2013, 21, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Halkjar, J.; Tjonneland, A.; Thomsen, B.L.; Overvad, K.; Sorensen, T.I.A. Intake of macronutrients as predictors of 5-y changes in waist circumference. Am. J. Clin. Nutr. 2006, 84, 789–797. [Google Scholar] [CrossRef]
- Bessesen, D.H. Regulation of body weight: What is the regulated parameter? Physiol. Behav. 2011, 104, 599–607. [Google Scholar] [CrossRef]
- Blundell, J.E.; Caudwell, P.; Gibbons, C.; Hopkins, M.; Näslund, E.; King, N.A.; Finlayson, G. Body composition and appetite: Fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. Br. J. Nutr. 2011, 107, 445–449. [Google Scholar] [CrossRef]
- Caudwell, P.; Finlayson, G.; Gibbons, C.; Hopkins, M.; King, N.A.; Näslund, E.; Blundell, J.E. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am. J. Clin. Nutr. 2013, 97, 7–14. [Google Scholar] [CrossRef]
- Weise, C.M.; Hohenadel, M.G.; Krakoff, J.; Votruba, S.B. Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. Int. J. Obes. 2013, 38, 243–251. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. Obesity: The protein leverage hypothesis. Obes. Rev. 2005, 6, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.B.; Baskin, M. Handbook of Assessment Methods for Eating Behaviours and Weight Related Problems: Measures, Theory, and Research, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2009. [Google Scholar]
- Health Promotion Board. National Nutrition Survey. 2010. Available online: www.hpb.org.sg (accessed on 04 November 2019).
- Eisenstein, J.; Roberts, S.B.; Dallal, G.; Saltzman, E. High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr. Rev. 2002, 60, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, D.A.; Buchholz, A.C. Energetics of obesity and weight control: Does diet composition matter? J. Am. Diet. Assoc. 2005, 105, S24–S28. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.; Bonomi, A.G.; Lemmens, S.G.; Scholte, J.; Thijssen, M.A.; van Berkum, F.; Westerterp-Plantenga, M.S. Relatively high-protein or ’low-carb’ energy-restricted diets for body weight loss and body weight maintenance? Physiol. Behav. 2012, 107, 374–380. [Google Scholar] [CrossRef]
- Leidy, H.J.; Carnell, N.S.; Mattes, R.D.; Campbell, W.W. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity 2007, 15, 421–429. [Google Scholar] [CrossRef]
- Weigle, D.S.; Breen, P.A.; Matthys, C.C.; Callahan, H.S.; Meeuws, K.E.; Burden, V.R.; Purnell, J.Q. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 2005, 82, 41–48. [Google Scholar] [CrossRef]
- Layman, D.K.; Evans, E.M.; Erickson, D.; Seyler, J.; Weber, J.; Bagshaw, D.; Griel, A.; Psota, T.; Kris-Etherton, P. A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. J. Nutr. 2009, 139, 514–521. [Google Scholar] [CrossRef]
- Layman, D.K.; Evans, E.; Baum, J.I.; Seyler, J.; Erickson, D.J.; Boileau, R.A. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J. Nutr. 2005, 135, 1903–1910. [Google Scholar] [CrossRef]
- Laerseb, T.M.; Dalskov, S.M.; van Baak, M.; Jebb, S.A.; Papadaki, A.; Pfeiffer, A.F.H.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunesova, M.; Pihlsgard, M.; et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N. Engl. J. Med. 2010, 363, 2102–2113. [Google Scholar]
- Haaf, D.S.M.; Dongen, E.J.I.; Nuijten, M.A.H.; Eijsvogels, T.M.H.; Groot, L.C.P.G.M.; Hopman, M.T.E. Protein intake and distribution in relation to physical functioning and quality of life in community-dwelling elderly people: Acknowledging the role of physical activity. Nutrients 2018, 10, 506. [Google Scholar] [CrossRef]
- Shiferaw, B.; Verrill, L.; Booth, H.; Zansky, S.M.; Norton, D.M.; Crim, S.; Henao, O.L. Sex-based differences in food consumption: Foodborne Diseases Active Surveillance Network (FoodNet) Population Survey, 2006–2007. Clin. Infect. Dis. 2012, 54, S453–S457. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.; Finlayson, G.; Duarte, C.; Whybrow, S.; Ritz, P.; Horgan, G.W.; Blundell, J.E.; Stubbs, R.J. Modelling the associations between fat-free mass, resting metabolic rate and energy intake in the context of total energy balance. Int. J. Obes. 2016, 40, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Jacquet, J.; Girardier, L. Poststarvation hyperphagia and body fat overshooting in humans: A role for feedback signals from lean and fat tissues. Am. J. Clin. Nutr. 1997, 65, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Jacquet, J.; Montani, J.P. How dieting makes some fatter: From a perspective of human body composition autoregulation. Proc. Nutr. Soc. 2012, 71, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Millward, D.J. A protein-stat mechanism for regulation of growth and maintenance of the lean body mass. Nutr. Res. Rev. 1995, 8, 93–120. [Google Scholar] [CrossRef] [PubMed]
- Mellinkoff, S.M.; Frankland, M.; Boyle, D.; Greipel, M. Relationship between serum amino acid concentration and fluctuations in appetite. J. Appl. Physiol. 1956, 8, 535. [Google Scholar] [CrossRef] [PubMed]
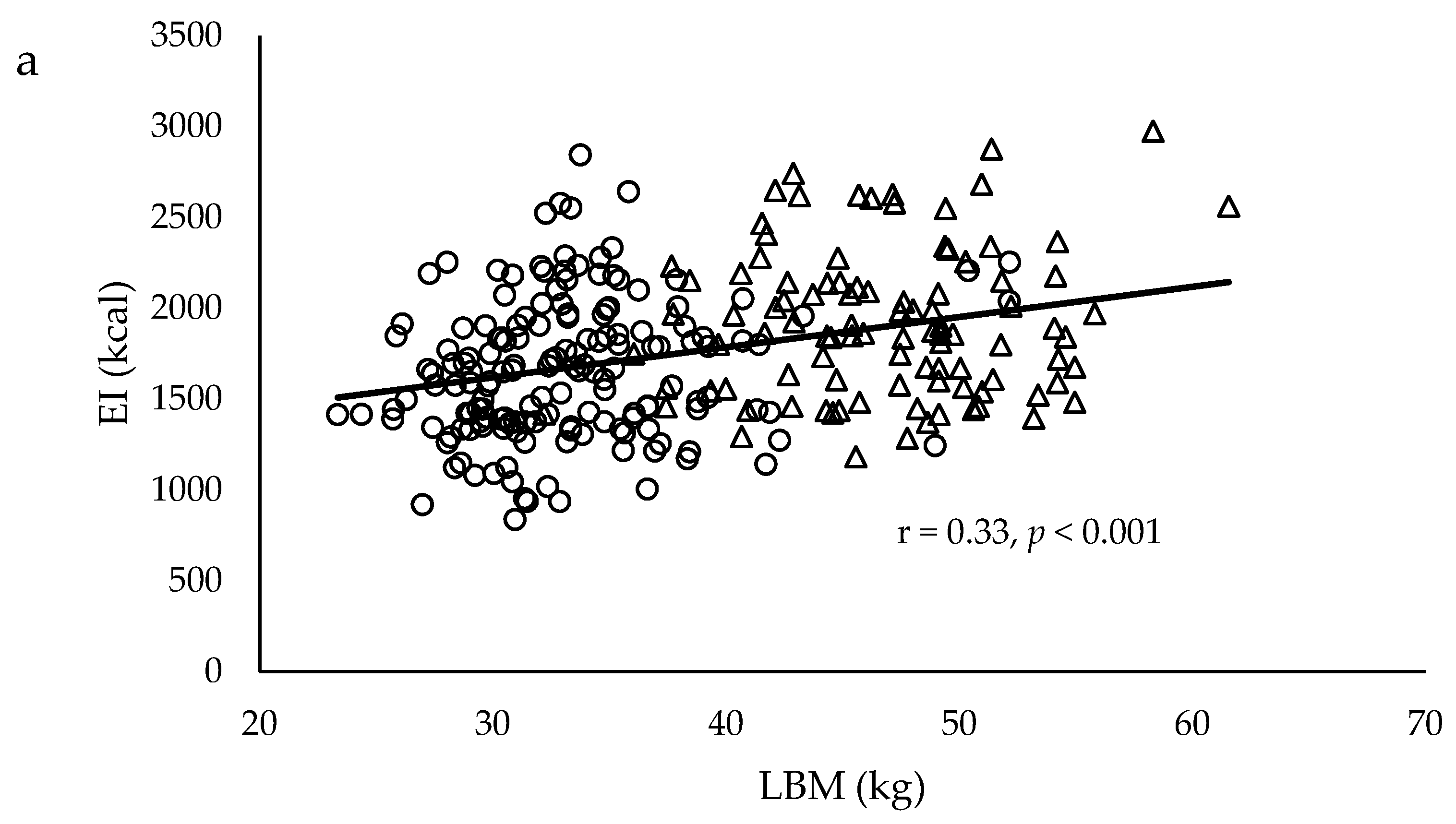
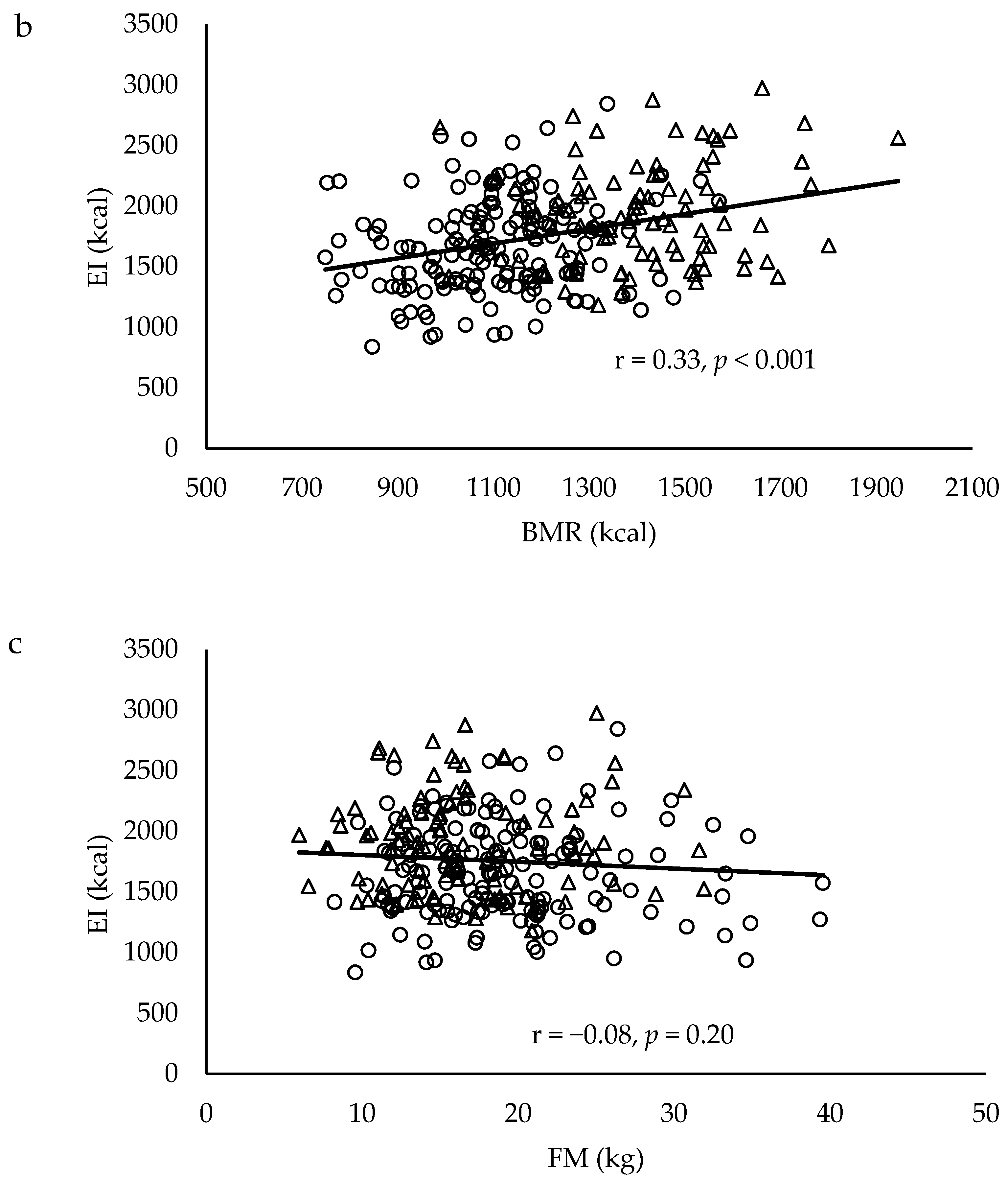
| Variable | Males | Females | p Value a | ||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| n | 99 | 162 | |||
| Age (year) | 41.7 ± 14.6 | 21.0, 69.2 | 39.0 ± 14.6 | 21.0, 68.9 | 0.156 |
| Height (cm) | 170.4 ± 5.5 | 157.6, 184.3 | 159.6 ± 5.9 | 144.2, 174.8 | <0.001 |
| Weight (kg) | 66.8 ± 9.5 | 45.4, 91.4 | 55.1 ± 9.7 | 34.5, 88.3 | <0.001 |
| BMI (kg/m2) | 23.0 ± 3.0 | 16.3, 31.5 | 21.6 ± 3.5 | 16.1, 33.8 | 0.001 |
| WC (cm) | 78.7 ± 8.5 | 62.1, 104.3 | 69.9 ± 7.8 | 56.3, 96.1 | <0.001 |
| FM (kg) b | 16.5 ± 5.6 | 6.0, 31.9 | 19.0 ± 6.0 | 8.2, 39.5 | 0.001 |
| LBM (kg) b | 46.8 ± 5.2 | 32.2, 61.6 | 33.2 ± 4.8 | 23.4, 52.2 | <0.001 |
| PBF (%) b | 24.7 ± 5.5 | 13.1, 37.2 | 34.5 ± 5.9 | 21.9, 51.1 | <0.001 |
| Variable | Males | Females | p Value a | ||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| n | 99 | 162 | |||
| PRO (g) | 81.2 ± 29.1 | 32.2, 172.1 | 70.6 ± 21.5 | 20.2, 134.4 | 0.001 |
| FAT (g) | 61.2 ± 19.3 | 27.4, 121.8 | 54.6 ± 20.1 | 19.3, 155.5 | 0.009 |
| CHO (g) | 251.8 ± 48.7 | 169.5, 412.4 | 212.2 ± 53.8 | 72.0, 451.4 | <0.001 |
| EI (kcal) | 1922.9 ± 405.0 | 1182.7, 2974.7 | 1657.7 ± 386.5 | 837.0, 2843.5 | <0.001 |
| RMR (kcal) | 1403.9 ± 178.5 | 988.0, 1945.0 | 1093.5 ± 173.1 | 749.0, 1571.0 | <0.001 |
| % PRO | 16.7 ± 3.9 | 8.8, 29.0 | 17.1 ± 3.8 | 8.2, 27.6 | 0.460 |
| % FAT | 28.3 ± 5.1 | 16.8, 41.8 | 29.2 ± 6.1 | 14.3, 54.9 | 0.224 |
| % CHO | 53.0 ± 6.5 | 34.2, 69.8 | 51.6 ± 7.9 | 27.5, 71.1 | 0.153 |
| PRO (g/kg) | 1.23 ± 0.46 | 0.48, 2.78 | 1.31 ± 0.42 | 0.43, 2.53 | 0.190 |
| FAT (g/kg) | 0.93 ± 0.31 | 0.43, 1.94 | 1.01 ± 0.37 | 0.35, 2.73 | 0.083 |
| CHO (g/kg) | 3.85 ± 0.94 | 2.00, 6.68 | 3.97 ± 1.18 | 1.22, 7.69 | 0.400 |
| Males (n = 99) | ||
|---|---|---|
| Model 1 | Model 2 | |
| EI (kcal) | FM (kg): 0.04 (−12.77, 17.91) LBM (kg): 0.05 (−12.20, 19.30) | FM (kg): 0.02 (−16.58, 18.86) LBM (kg): 0.04 (−15.24, 21.18) |
| PRO (g) | FM (kg): 0.05 (−0.86, 1.37) LBM (kg): 0.10 (−0.62, 1.66) | FM (kg): 0.001 (−1.28, 1.28) LBM (kg): 0.10 (−0.80, 1.84) |
| FAT (g) | FM (kg): 0.09 (−0.47, 1.07) LBM (kg): 0.07 (−0.54, 1.04) | FM (kg): 0.07 (−0.65, 1.12) LBM (kg): 0.04 (−0.78, 1.05) |
| CHO (g) | FM (kg): −0.10 (−2.70, 0.96) LBM (kg): −0.10 (−2.76, 0.99) | FM (kg): −0.07 (−2.70, 1.52) LBM (kg): −0.07 (−2.76, 1.58) |
| %PRO | FM (kg): 0.04 (−0.13, 0.18) LBM (kg): 0.11 (−0.08, 0.24) | FM (kg): −0.03 (−0.20, 0.16) LBM (kg): 0.13 (−0.09, 0.28) |
| %FAT | FM (kg): 0.11 (−0.10, 0.32) LBM (kg): 0.11 (−0.10, 0.32) | FM (kg): 0.08 (−0.17, 0.31) LBM (kg): 0.07 (−0.17, 0.32) |
| %CHO | FM (kg): −0.20 (−0.49, 0.02) LBM (kg): −0.23 (−0.54, −0.02) * | FM (kg): −0.11 (−0.42, 0.16) LBM (kg): −0.18 (−0.52, 0.08) |
| Females (n = 162) | ||
| EI (kcal) | FM (kg): −0.04 (−14.13, 8.23) LBM (kg): 0.12 (−4.35, 23.10) | FM (kg): −0.19 (−26.57, 1.60) LBM (kg): 0.24 (1.57, 36.33) * |
| PRO (g) | FM (kg): 0.04 (−0.47, 0.77) LBM (kg): 0.26 (0.40, 1.88) ** | FM (kg): −0.19 (−1.46, 0.07) LBM (kg): 0.38 (0.74, 2.61) ** |
| FAT (g) | FM (kg): 0.03 (−0.48, 0.70) LBM (kg): 0.24 (0.29, 1.70) * | FM (kg): −0.18 (−1.36, 0.09) LBM (kg): 0.36 (0.58, 2.37) ** |
| CHO (g) | FM (kg): −0.12 (−2.69, 0.40) LBM (kg): −0.06 (-2.55, 1.28) | FM (kg): −0.14 (−3.32, 0.64) LBM (kg): 0.04 (−2.05, 2.83) |
| %PRO | FM (kg): 0.10 (−0.05, 0.17) LBM (kg): 0.25 (0.06, 0.32) * | FM (kg): −0.08 (−0.19, 0.09) LBM (kg): 0.30 (0.06, 0.40) * |
| %FAT | FM (kg): 0.08 (−0.09, 0.26) LBM (kg): 0.27 (0.12, 0.54) ** | FM (kg): −0.12 (−0.35, 0.09) LBM (kg): 0.35 (−0.17, 0.70) ** |
| %CHO | FM (kg): −0.11 (−0.38, 0.08) LBM (kg): −0.26 (−0.69, −0.14) ** | FM (kg): 0.07 (−0.19, 0.37) LBM (kg): −0.30 (−0.83, −0.13) * |
| Variables | Males (n = 99) | Females (n = 162) | ||
|---|---|---|---|---|
| LBM (kg) | BMR (kcal) | LBM (kg) | BMR (kcal) | |
| PRO (g) | 0.10 | 0.13 | 0.25 ** | 0.19 * |
| FAT (g) | 0.07 | 0.15 | 0.23 * | 0.19 * |
| CHO (g) | −0.10 | −0.06 | −0.05 | 0.07 |
| EI (kcal) | 0.05 | 0.13 | 0.11 | 0.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, X.; Forde, C.G.; Goh, A.T.; Henry, C.J. Basal Metabolic Rate and Body Composition Predict Habitual Food and Macronutrient Intakes: Gender Differences. Nutrients 2019, 11, 2653. https://doi.org/10.3390/nu11112653
Bi X, Forde CG, Goh AT, Henry CJ. Basal Metabolic Rate and Body Composition Predict Habitual Food and Macronutrient Intakes: Gender Differences. Nutrients. 2019; 11(11):2653. https://doi.org/10.3390/nu11112653
Chicago/Turabian StyleBi, Xinyan, Ciarán G. Forde, Ai Ting Goh, and Christiani Jeyakumar Henry. 2019. "Basal Metabolic Rate and Body Composition Predict Habitual Food and Macronutrient Intakes: Gender Differences" Nutrients 11, no. 11: 2653. https://doi.org/10.3390/nu11112653
APA StyleBi, X., Forde, C. G., Goh, A. T., & Henry, C. J. (2019). Basal Metabolic Rate and Body Composition Predict Habitual Food and Macronutrient Intakes: Gender Differences. Nutrients, 11(11), 2653. https://doi.org/10.3390/nu11112653





