A Single Dose of The Mango Leaf Extract Zynamite® in Combination with Quercetin Enhances Peak Power Output During Repeated Sprint Exercise in Men and Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
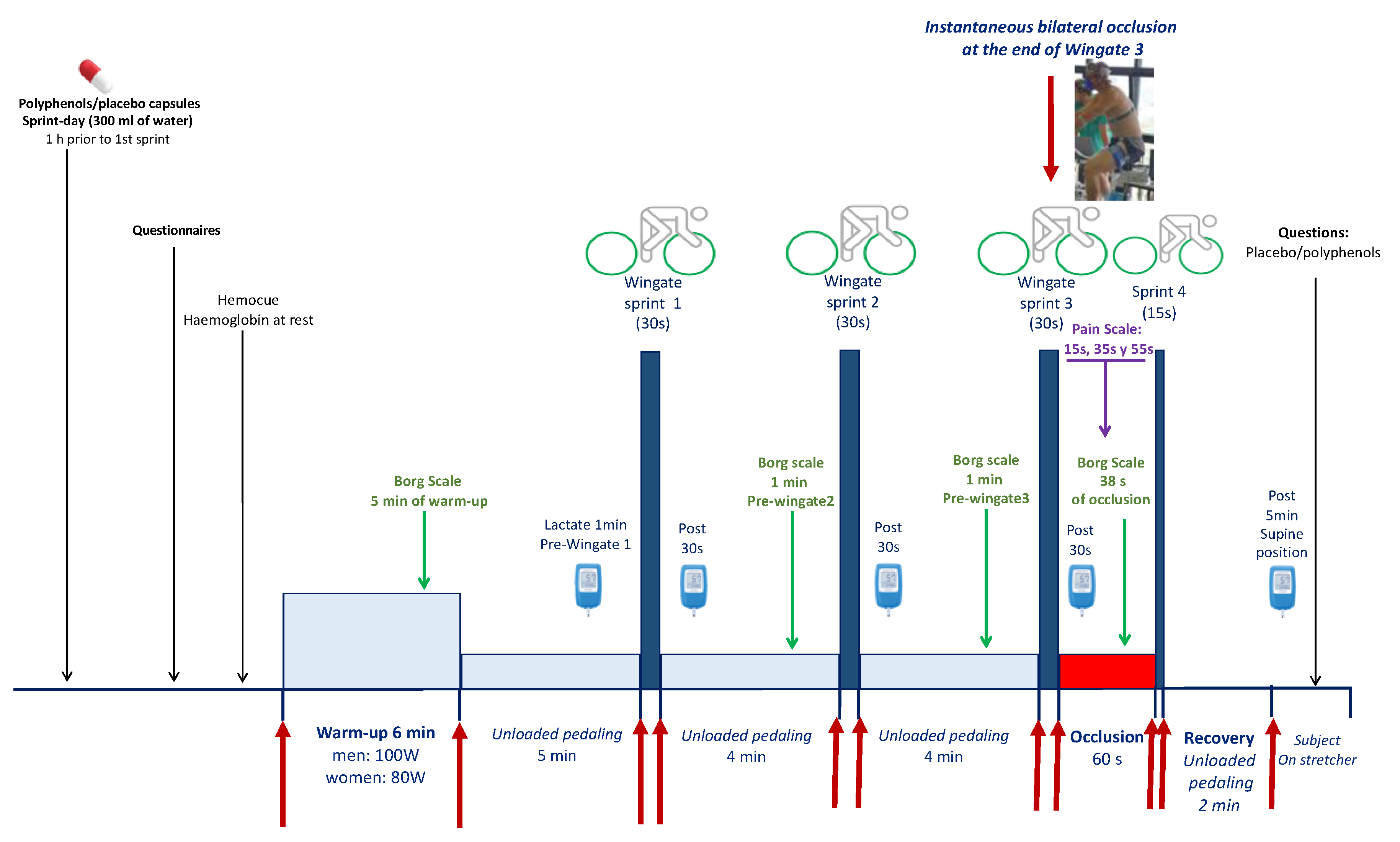
2.2. General Overview
2.3. Pre-Tests
2.4. Main Sprint Experiments
2.5. Power Output
2.6. Oxygen Demand and Deficit
2.7. Vastus Lateralis Muscle and Cerebral Oxygenation
2.8. Assessment of Pain and Effectiveness of Concealment
2.9. Statistical Analysis
3. Results
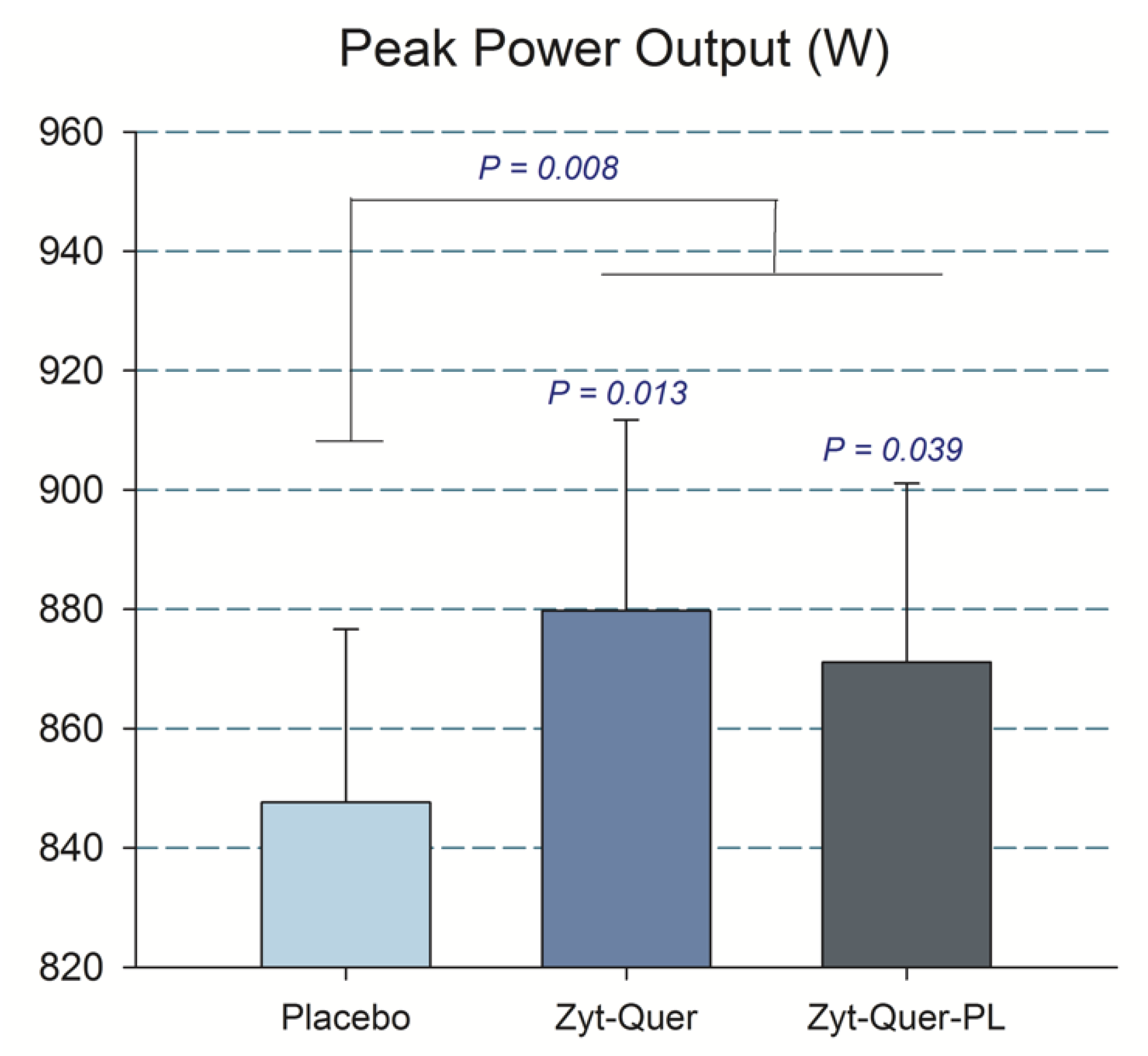
3.1. Effects on Sprint Performance
3.2. Efficiency of Concealment
4. Discussion
4.1. The Combination of Zynamite® with Quercetin Improves Peak Power Output During Repeated Maximal Sprints
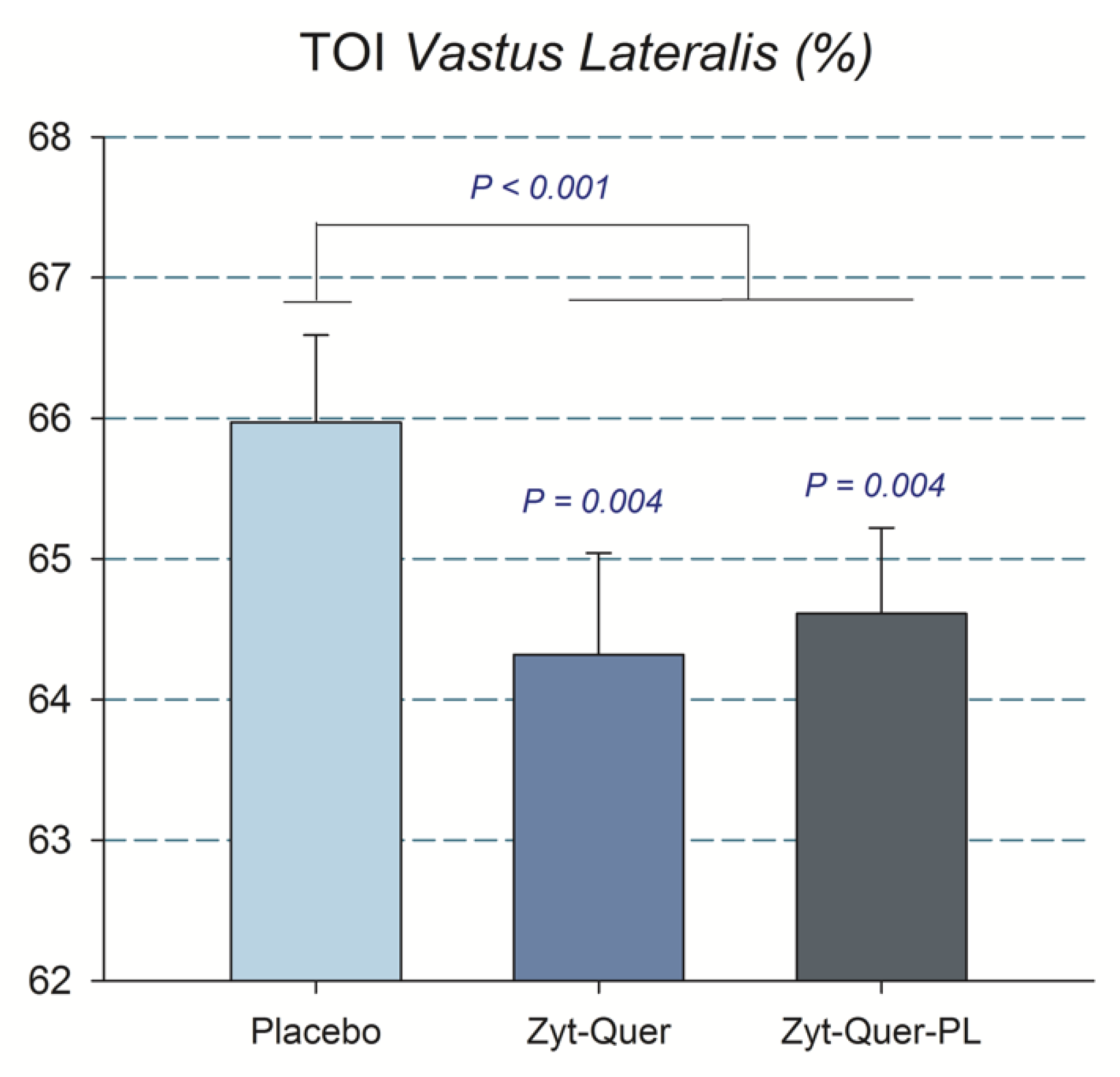
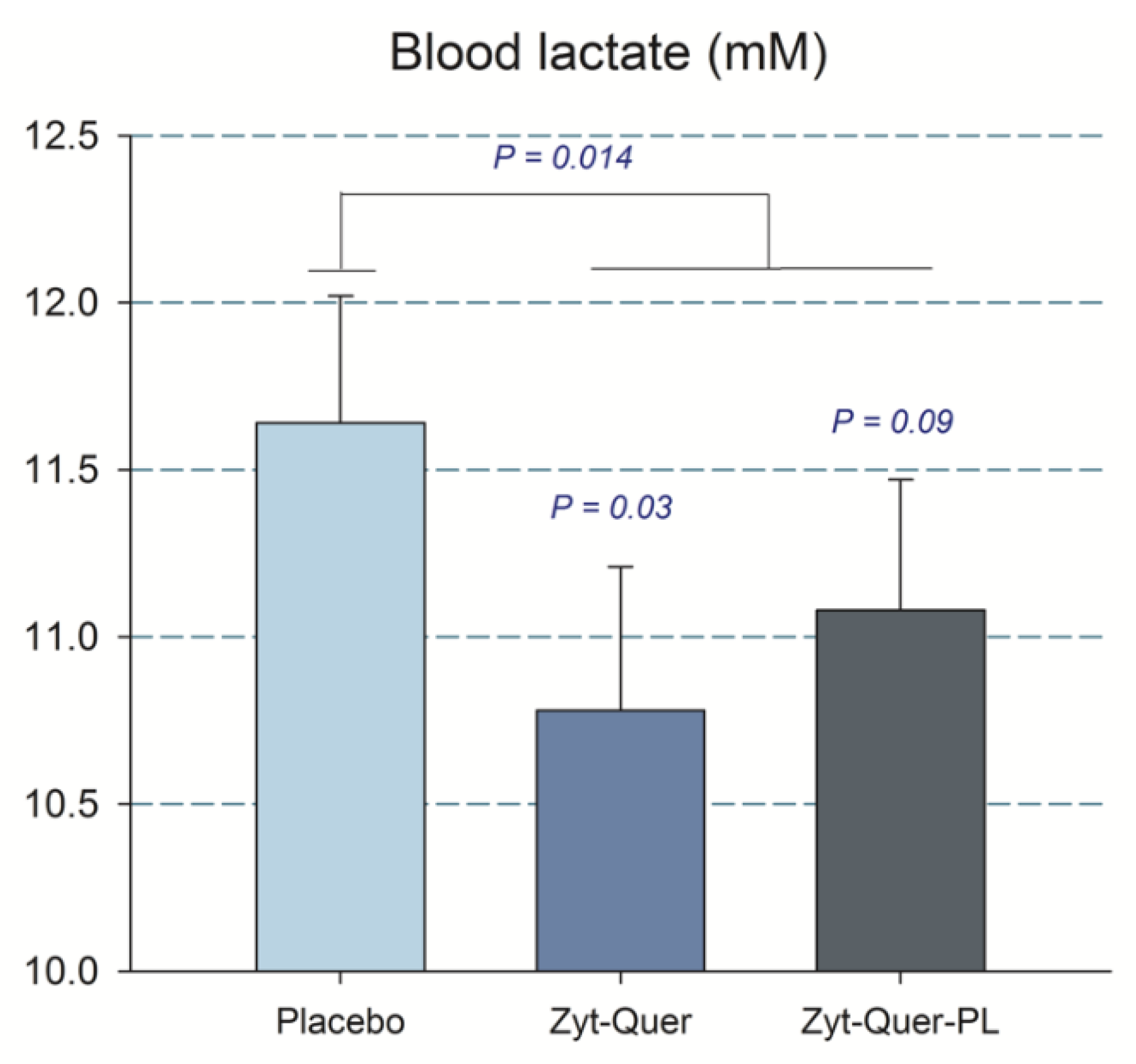
4.2. The Combination of Zynamite® with Quercetin Improves O2 Extraction and Reduces Peak Blood Lactate Concentration During Repeated Sprint Exercise
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Gericke, N.; Perez-Valera, M.; Curtelin, D.; Galvan-Alvarez, V.; Lopez-Rios, L.; Morales-Alamo, D.; Calbet, J.A.L. Mangifera indica L. Leaf extract in combination with luteolin or quercetin enhances vo2peak and peak power output, and preserves skeletal muscle function during ischemia-reperfusion in humans. Front. Physiol. 2018, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Galvan-Alvarez, V.; Curtelin, D.; Perez-Valera, M.; Habib, J.J.; Pérez-López, A.; Vega, T.; Morales-Alamo, D.; et al. Enhancement of exercise performance by 48 hours, and 15-day supplementation with mangiferin and luteolin in men. Nutrients 2019, 11, 344. [Google Scholar] [CrossRef]
- Amann, M.; Calbet, J.A. Convective oxygen transport and fatigue. J. Appl. Physiol. 2008, 104, 861–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitts, R.H. Cellular mechanisms of muscle fatigue. Physiol. Rev. 1994, 74, 49–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Bruton, J.D.; Katz, A.; Westerblad, H. Limited oxygen diffusion accelerates fatigue development in mouse skeletal muscle. J. Physiol. 2006, 572, 551–559. [Google Scholar] [CrossRef]
- Cheng, A.J.; Bruton, J.D.; Lanner, J.T.; Westerblad, H. Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres. J. Physiol. 2015, 593, 457–472. [Google Scholar] [CrossRef]
- Bruton, J.D.; Place, N.; Yamada, T.; Silva, J.P.; Andrade, F.H.; Dahlstedt, A.J.; Zhang, S.J.; Katz, A.; Larsson, N.G.; Westerblad, H. Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and sod2 overexpressing mice. J. Physiol. 2008, 586, 175–184. [Google Scholar] [CrossRef]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Translating fatigue to human performance. Med. Sci. Sports Exerc. 2016, 48, 2228–2238. [Google Scholar] [CrossRef]
- Martin, P.G.; Weerakkody, N.; Gandevia, S.C.; Taylor, J.L. Group iii and iv muscle afferents differentially affect the motor cortex and motoneurones in humans. J. Physiol. 2008, 586, 1277–1289. [Google Scholar] [CrossRef]
- Curtelin, D.; Morales-Alamo, D.; Torres-Peralta, R.; Rasmussen, P.; Martin-Rincon, M.; Perez-Valera, M.; Siebenmann, C.; Perez-Suarez, I.; Cherouveim, E.; Sheel, A.W.; et al. Cerebral blood flow, frontal lobe oxygenation and intra-arterial blood pressure during sprint exercise in normoxia and severe acute hypoxia in humans. J. Cereb. Blood Flow Metab. 2018, 38, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Sidhu, S.K.; Weavil, J.C.; Mangum, T.S.; Venturelli, M. Autonomic responses to exercise: Group iii/iv muscle afferents and fatigue. Auton. Neurosci. 2015, 188, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Masibo, M.; He, Q. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Calbet, J.A. Free radicals and sprint exercise in humans. Free Radic. Res. 2014, 48, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Liu, J.; Liu, H.Y.; Gao, L.H.; Feng, G.H.; Liu, X.; Li, L. Hypouricaemic action of mangiferin results from metabolite norathyriol via inhibiting xanthine oxidase activity. Pharm. Biol. 2016, 54, 1680–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kressler, J.; Millard-Stafford, M.; Warren, G.L. Quercetin and endurance exercise capacity: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2011, 43, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, K.H. Polyphenol supplementation: Benefits for exercise performance or oxidative stress? Sports Med. 2014, 44 (Suppl. 1), 57–70. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Hopkins, W.G. Impact of dietary antioxidants on sport performance: A review. Sports Med. 2015, 45, 939–955. [Google Scholar] [CrossRef]
- Ekinci Akdemir, F.N.; Gulcin, I.; Karagoz, B.; Soslu, R. Quercetin protects rat skeletal muscle from ischemia reperfusion injury. J. Enzym. Inhib. Med. Chem. 2016, 31, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Ozyurek, M.; Bektasoglu, B.; Guclu, K.; Apak, R. Measurement of xanthine oxidase inhibition activity of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (cuprac) method. Anal. Chim. Acta 2009, 636, 42–50. [Google Scholar] [CrossRef]
- Holland, J.A.; O′Donnell, R.W.; Chang, M.M.; Johnson, D.K.; Ziegler, L.M. Endothelial cell oxidant production: Effect of NADPH oxidase inhibitors. Endothelium 2000, 7, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Guo, Y.; Song, D.; Bruno, R.S.; Lu, X. Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci. 2014, 103, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.; Moysi, J.S.; Dorado, C.; Rodriguez, L.P. Bone mineral content and density in professional tennis players. Calcif. Tissue Int. 1998, 62, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Jones, A.M. Measurement of the maximum oxygen uptake vo2max: Vo2peak is no longer acceptable. J. Appl. Physiol. 2017, 122, 997–1002. [Google Scholar] [CrossRef]
- Torres-Peralta, R.; Morales-Alamo, D.; Gonzalez-Izal, M.; Losa-Reyna, J.; Perez-Suarez, I.; Izquierdo, M.; Calbet, J.A. Task failure during exercise to exhaustion in normoxia and hypoxia is due to reduced muscle activation caused by central mechanisms while muscle metaboreflex does not limit performance. Front. Physiol. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Losa-Reyna, J.; Torres-Peralta, R.; Martin-Rincon, M.; Perez-Valera, M.; Curtelin, D.; Ponce-Gonzalez, J.G.; Santana, A.; Calbet, J.A. What limits performance during whole-body incremental exercise to exhaustion in humans? J. Physiol. 2015, 593, 4631–4648. [Google Scholar] [CrossRef]
- Calbet, J.A.; Chavarren, J.; Dorado, C. Fractional use of anaerobic capacity during a 30-and a 45-s wingate test. Eur. J. Appl. Physiol. 1997, 76, 308–313. [Google Scholar] [CrossRef]
- Van der Zee, P.; Cope, M.; Arridge, S.R.; Essenpreis, M.; Potter, L.A.; Edwards, A.D.; Wyatt, J.S.; McCormick, D.C.; Roth, S.C.; Reynolds, E.O.; et al. Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. Adv. Exp. Med. Biol. 1992, 316, 143–153. [Google Scholar]
- Abbey, E.L.; Rankin, J.W. Effect of quercetin supplementation on repeated-sprint performance, xanthine oxidase activity, and inflammation. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 91–96. [Google Scholar] [CrossRef]
- Bogdanis, G.C.; Nevill, M.E.; Boobis, L.H.; Lakomy, H.K.; Nevill, A.M. Recovery of power output and muscle metabolites following 30 s of maximal sprint cycling in man. J. Physiol. 1995, 482, 467–480. [Google Scholar] [CrossRef]
- Ying, W.; Han, S.K.; Miller, J.W.; Swanson, R.A. Acidosis potentiates oxidative neuronal death by multiple mechanisms. J. Neurochem. 1999, 73, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintac, D.; Majkic, T.; Bekvalac, K.; Orcic, D.; Mimica-Dukic, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Legault, J.; Perron, T.; Mshvildadze, V.; Girard-Lalancette, K.; Perron, S.; Laprise, C.; Sirois, P.; Pichette, A. Antioxidant and anti-inflammatory activities of quercetin 7-O-β-D-glucopyranoside from the leaves of brasenia schreberi. J. Med. Food 2011, 14, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Amic, A.; Lucic, B.; Stepanic, V.; Markovic, Z.; Markovic, S.; Dimitric Markovic, J.M.; Amic, D. Free radical scavenging potency of quercetin catecholic colonic metabolites: Thermodynamics of 2h(+)/2e(-) processes. Food Chem. 2017, 218, 144–151. [Google Scholar] [CrossRef]
- Samadarsi, R.; Dutta, D. Design and characterization of mangiferin nanoparticles for oral delivery. J. Food Eng. 2019, 247, 80–94. [Google Scholar] [CrossRef]
- Gu, C.Z.; Yang, M.L.; Zhou, Z.H.; Khan, A.; Cao, J.X.; Cheng, G.G. Purification and characterization of four benzophenone derivatives from Mangifera indica L. Leaves and their antioxidant, immunosuppressive and alpha-glucosidase inhibitory activities. J. Funct. Foods 2019, 52, 709–714. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Total phenolics, antioxidant and xanthine oxidase inhibitory activity of three colored onions (Allium cepa L.). Front. Life Sci. 2013, 7, 224–228. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Hou, F.; Wang, X.; Liu, B. Mangiferin inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/NLRP3 inflammasome activation with regulation of AMPK in endothelial cells. Metabolism 2015, 64, 428–437. [Google Scholar] [CrossRef]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [PubMed]
- Reid, M.B.; Khawli, F.A.; Moody, M.R. Reactive oxygen in skeletal muscle. Iii. Contractility of unfatigued muscle. J. Appl. Physiol. 1993, 75, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Shanely, R.A.; Knab, A.M.; Nieman, D.C.; Jin, F.; McAnulty, S.R.; Landram, M.J. Quercetin supplementation does not alter antioxidant status in humans. Free Radic. Res. 2010, 44, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Morales-Alamo, D.; Ponce-Gonzalez, J.G.; Guadalupe-Grau, A.; Rodriguez-Garcia, L.; Santana, A.; Cusso, R.; Guerrero, M.; Dorado, C.; Guerra, B.; Calbet, J.A. Critical role for free radicals on sprint exercise-induced CaMKII and AMPKα phosphorylation in human skeletal muscle. J. Appl. Physiol. 2013, 114, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imranb, M.; Patel, S. Mangiferin: A phytochemical with panacea potential. Biomed. Pharmacother. 2017, 96, 1562–1564. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Agusti, A.G.; Alonso, A.; Poole, D.C.; Viegas, C.; Barbera, J.A.; Rodriguez-Roisin, R.; Ferrer, A.; Wagner, P.D. Effects of training on muscle o2 transport at vo2max. J. Appl. Physiol. 1992, 73, 1067–1076. [Google Scholar] [CrossRef]
- Calbet, J.A.; Losa-Reyna, J.; Torres-Peralta, R.; Rasmussen, P.; Ponce-Gonzalez, J.G.; Sheel, A.W.; de la Calle-Herrero, J.; Guadalupe-Grau, A.; Morales-Alamo, D.; Fuentes, T.; et al. Limitations to oxygen transport and utilization during sprint exercise in humans: Evidence for a functional reserve in muscle O2 diffusing capacity. J. Physiol. 2015, 593, 4649–4664. [Google Scholar] [CrossRef]
- Calbet, J.A.; Joyner, M.J. Disparity in regional and systemic circulatory capacities: Do they affect the regulation of the circulation? Acta Physiol. 2010, 199, 393–406. [Google Scholar] [CrossRef]
- Calbet, J.A.; Lundby, C.; Sander, M.; Robach, P.; Saltin, B.; Boushel, R. Effects of atp-induced leg vasodilation on VO2peak and leg O2 extraction during maximal exercise in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R447–R453. [Google Scholar] [CrossRef]
- Cardinale, D.A.; Larsen, F.J.; Jensen-Urstad, M.; Rullman, E.; Sondergaard, H.; Morales-Alamo, D.; Ekblom, B.; Calbet, J.A.L.; Boushel, R. Muscle mass and inspired oxygen influence oxygen extraction at maximal exercise: Role of mitochondrial oxygen affinity. Acta Physiol. 2019, 225, e13110. [Google Scholar] [CrossRef]
- Gnaiger, E. Bioenergetics at low oxygen: Dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir. Physiol. 2001, 128, 277–297. [Google Scholar] [CrossRef]
- Denton, R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 2009, 1787, 1309–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink, B.D.; Bai, F.; Yu, L.; Sivitz, W.I. Regulation of atp production: Dependence on calcium concentration and respiratory state. Am. J. Physiol. Cell Physiol. 2017, 313, C146–C153. [Google Scholar] [CrossRef] [PubMed]
- Montero, M.; Lobaton, C.D.; Hernandez-Sanmiguel, E.; Santodomingo, J.; Vay, L.; Moreno, A.; Alvarez, J. Direct activation of the mitochondrial calcium uniporter by natural plant flavonoids. Biochem. J. 2004, 384, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Apontes, P.; Fomenko, E.V.; Chi, N.; Schuster, V.L.; Kurland, I.J.; Pessin, J.E.; Chi, Y. Mangiferin accelerates glycolysis and enhances mitochondrial bioenergetics. Int. J. Mol. Sci. 2018, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Y.; Song, J.; Hou, F.; Liu, B.; Li, A. Mangiferin protects mitochondrial function by preserving mitochondrial hexokinase-ii in vessel endothelial cells. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.S.; Noyszewski, E.A.; Kendrick, K.F.; Leigh, J.S.; Wagner, P.D. Myoglobin o2 desaturation during exercise. Evidence of limited O2 transport. J. Clin. Invest. 1995, 96, 1916–1926. [Google Scholar] [CrossRef]
- Hendgen-Cotta, U.B.; Merx, M.W.; Shiva, S.; Schmitz, J.; Becher, S.; Klare, J.P.; Steinhoff, H.J.; Goedecke, A.; Schrader, J.; Gladwin, M.T.; et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2008, 105, 10256–10261. [Google Scholar] [CrossRef] [Green Version]
- Shiva, S.; Huang, Z.; Grubina, R.; Sun, J.; Ringwood, L.A.; MacArthur, P.H.; Xu, X.; Murphy, E.; Darley-Usmar, V.M.; Gladwin, M.T. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007, 100, 654–661. [Google Scholar] [CrossRef]
- Tedesco, I.; Russo, M.; Russo, P.; Iacomino, G.; Russo, G.L.; Carraturo, A.; Faruolo, C.; Moio, L.; Palumbo, R. Antioxidant effect of red wine polyphenols on red blood cells. J. Nutr. Biochem. 2000, 11, 114–119. [Google Scholar] [CrossRef]
- Clerc, P.; Rigoulet, M.; Leverve, X.; Fontaine, E. Nitric oxide increases oxidative phosphorylation efficiency. J. Bioenerg. Biomembr. 2007, 39, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Jubrias, S.A.; Crowther, G.J.; Shankland, E.G.; Gronka, R.K.; Conley, K.E. Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J. Physiol. 2003, 553, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, E.; Sanchez-Gomez, M.V.; Ruiz, A.; Cavaliere, F.; Ortiz-Sanz, C.; Quintela-Lopez, T.; Capetillo-Zarate, E.; Sole-Domenech, S.; Matute, C. Mangiferin and morin attenuate oxidative stress, mitochondrial dysfunction, and neurocytotoxicity, induced by amyloid beta oligomers. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Lemus-Molina, Y.; Sanchez-Gomez, M.V.; Delgado-Hernandez, R.; Matute, C. Mangifera indica l. Extract attenuates glutamate-induced neurotoxicity on rat cortical neurons. Neurotoxicology 2009, 30, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Fornai, M.; Pellegrini, C.; Colucci, R.; Benvenuti, L.; Duranti, E.; Masi, S.; Carpi, S.; Nieri, P.; Nericcio, A.; et al. Luteolin prevents cardiometabolic alterations and vascular dysfunction in mice with HFD-induced obesity. Front. Pharmacol. 2018, 9, 1094. [Google Scholar] [CrossRef]
| Men | Women | p | |||||
|---|---|---|---|---|---|---|---|
| Age (Years) | 23.1 | ± | 2.2 | 23.5 | ± | 2.9 | 0.64 |
| Height (cm) | 174.4 | ± | 5.6 | 165.1 | ± | 6.5 | 0.000 |
| Weight (kg) | 73.4 | ± | 9.1 | 59.5 | ± | 8.0 | 0.000 |
| Body fat (%) | 18.5 | ± | 3.7 | 27.7 | ± | 4.1 | 0.000 |
| Fat mass (kg) | 13.7 | ± | 3.8 | 16.7 | ± | 4.4 | 0.03 |
| Lean mass (kg) | 56.6 | ± | 6.3 | 40.3 | ± | 4.2 | 0.000 |
| Legs lean mass (kg) | 20.2 | ± | 2.7 | 14.1 | ± | 1.8 | 0.000 |
| VO2max (mL min−1) | 3189.7 | ± | 525.4 | 2167.4 | ± | 279.2 | 0.000 |
| VO2max (mL kg−1 min−1) | 43.5 | ± | 5.4 | 36.6 | ± | 3.9 | 0.000 |
| LLM VO2max (mL kg−1 min−1) | 158.7 | ± | 19.3 | 154.4 | ± | 17.1 | 0.47 |
| Hemoglobin (g dL−1) | 15.4 | ± | 0.7 | 13.3 | ± | 0.7 | 0.000 |
| Systolic BP (mmHg) | 122.4 | ± | 9.4 | 111.3 | ± | 5.4 | 0.000 |
| Diastolic BP (mmHg) | 66.2 | ± | 7.3 | 67.9 | ± | 4.2 | 0.365 |
| A | B | C | Treatment | Treat × Sex | Treat × Wing | Treat × Wing × Sex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPO (W) | All | 871 | ± | 193 | 880 | ± | 203 | 848 | ± | 184 | 0.01 | 0.048 | 0.96 | 0.79 |
| M | 1025 | ± | 133 | 1015 | ± | 171 | 981 | ± | 152 | |||||
| W | 717 | ± | 93 | 738 | ± | 120 | 714 | ± | 95 | |||||
| MPO (W) | All | 482 | ± | 107 | 479 | ± | 109 | 478 | ± | 109 | 0.80 | 0.39 | 0.70 | 0.84 |
| M | 570 | ± | 75 | 564 | ± | 81 | 567 | ± | 80 | |||||
| W | 394 | ± | 39 | 390 | ± | 41 | 389 | ± | 34 | |||||
| VO2 (mL) | All | 1069 | ± | 245 | 1054 | ± | 228 | 1064 | ± | 225 | 0.69 | 0.24 | 0.38 | 0.60 |
| M | 1269 | ± | 177 | 1226 | ± | 175 | 1246 | ± | 153 | |||||
| W | 870 | ± | 88 | 874 | ± | 103 | 882 | ± | 104 | |||||
| VCO2 (mL) | All | 1102 | ± | 278 | 1093 | ± | 273 | 1108 | ± | 276 | 0.43 | 0.61 | 0.28 | 0.84 |
| M | 1336 | ± | 181 | 1310 | ± | 177 | 1340 | ± | 166 | |||||
| W | 869 | ± | 104 | 864 | ± | 129 | 876 | ± | 128 | |||||
| RER | All | 1.03 | ± | 0.06 | 1.02 | ± | 0.07 | 1.03 | ± | 0.08 | 0.20 | 0.81 | 0.49 | 0.45 |
| M | 1.05 | ± | 0.06 | 1.05 | ± | 0.06 | 1.08 | ± | 0.06 | |||||
| W | 1.00 | ± | 0.06 | 0.99 | ± | 0.07 | 0.99 | ± | 0.07 | |||||
| VE (L min−1) | All | 49 | ± | 12 | 50 | ± | 12 | 50 | ± | 13 | 0.41 | 0.64 | 0.14 | 0.15 |
| M | 57 | ± | 10 | 58 | ± | 10 | 58 | ± | 10 | |||||
| W | 41 | ± | 8 | 42 | ± | 9 | 41 | ± | 9 | |||||
| PETO2 (mmHg) | All | 119 | ± | 4 | 119 | ± | 4 | 119 | ± | 4 | 0.59 | 0.53 | 0.17 | 0.26 |
| M | 119 | ± | 3 | 119 | ± | 3 | 119 | ± | 3 | |||||
| W | 119 | ± | 4 | 120 | ± | 4 | 119 | ± | 4 | |||||
| PETCO2 (mmHg) | All | 26 | ± | 3 | 26 | ± | 4 | 26 | ± | 3 | 0.29 | 0.56 | 0.12 | 0.37 |
| M | 27 | ± | 3 | 27 | ± | 3 | 27 | ± | 3 | |||||
| W | 26 | ± | 3 | 24 | ± | 4 | 25 | ± | 3 | |||||
| Heart rate (bpm) | All | 163 | ± | 10 | 165 | ± | 11 | 164 | ± | 10 | 0.21 | 0.31 | 0.32 | 0.17 |
| M | 165 | ± | 8 | 166 | ± | 10 | 164 | ± | 8 | |||||
| W | 162 | ± | 12 | 164 | ± | 12 | 164 | ± | 11 | |||||
| A | B | C | Treatment | Treat × Sex | Treat × Wing | Treat × Wing × Sex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxygen deficit (mL) | All | 1625 | ± | 542 | 1632 | ± | 622 | 1612 | ± | 565 | 0.81 | 0.82 | 0.80 | 0.72 |
| M | 1962 | ± | 556 | 1982 | ± | 660 | 1969 | ± | 576 | |||||
| W | 1289 | ± | 232 | 1263 | ± | 278 | 1256 | ± | 238 | |||||
| Lactate (mM) L | All | 11.1 | ± | 1.9 | 11.3 | ± | 2.0 | 11.5 | ± | 1.9 | 0.46 | 0.059 | 0.83 | 0.16 |
| M | 11.1 | ± | 1.8 & | 10.8 | ± | 1.9 * | 11.6 | ± | 1.7 | |||||
| W | 11.2 | ± | 2.0 | 11.9 | ± | 2.1 | 11.3 | ± | 2.2 | |||||
| TOI Frontal Lobe (%) | All | 66 | ± | 5 | 65 | ± | 7 | 67 | ± | 5 | 0.20 | 0.62 | 0.32 | 0.33 |
| M | 68 | ± | 6 | 67 | ± | 7 | 69 | ± | 5 | |||||
| W | 65 | ± | 3 | 63 | ± | 6 | 64 | ± | 4 | |||||
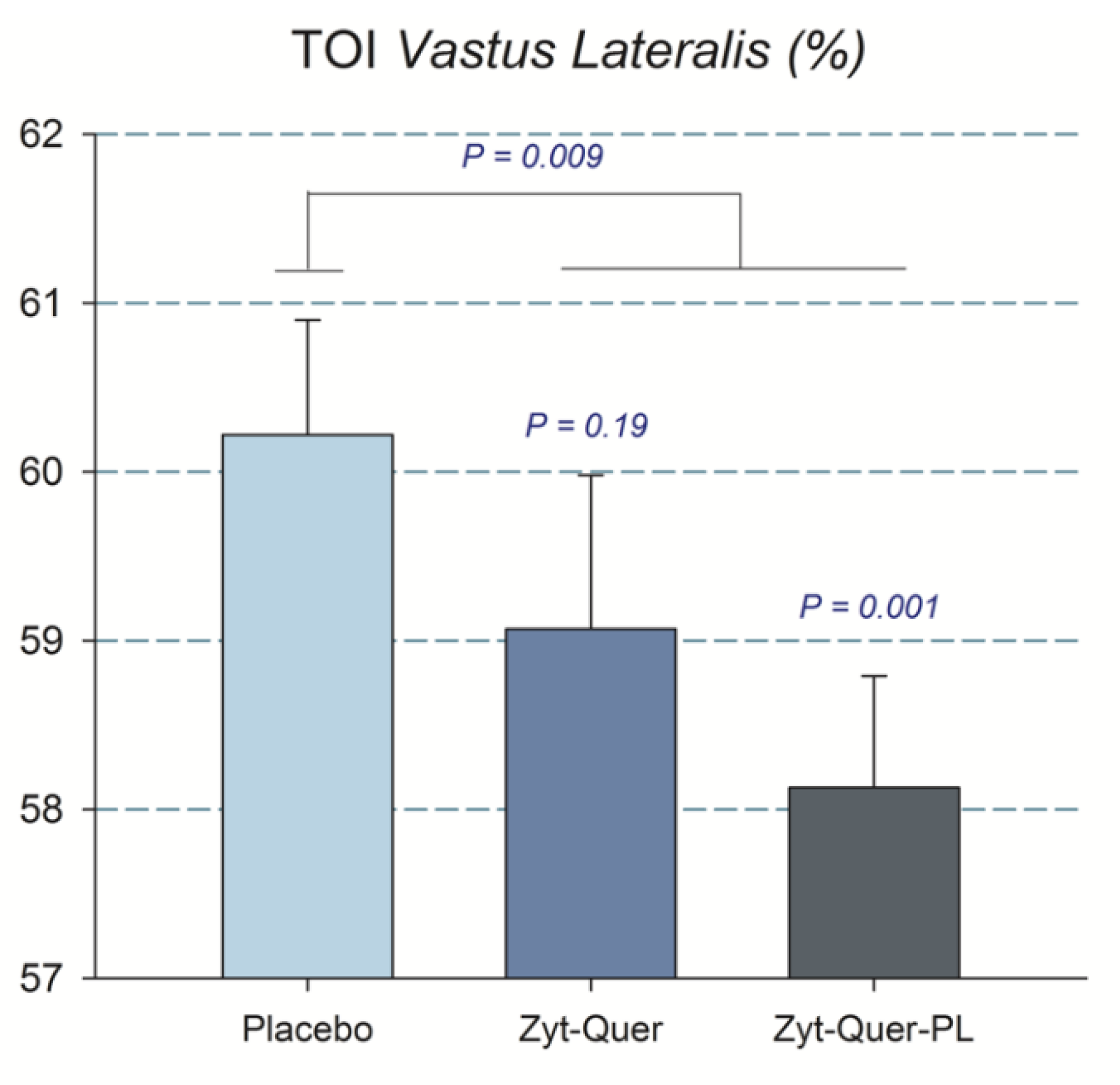
| TOI Vastus Lateralis (%) | All | 64.6 | ± | 3.8 | 64.3 | ± | 4.5 | 66.0 | ± | 3.9 | 0.01 | 0.04 | 0.60 | 0.63 |
| M | 63.4 | ± | 3.7 * | 62.3 | ± | 3.9 * | 65.2 | ± | 4.5 | |||||
| W | 65.8 | ± | 3.7 | 66.5 | ± | 4.1 | 66.7 | ± | 3.3 | |||||
| VO2/W (mL W−1) a, L | All | 2.27 | ± | 0.20 | 2.29 | ± | 0.22 | 2.29 | ± | 0.19 | 0.96 | 0.72 | 0.73 | 0.86 |
| M | 2.28 | ± | 0.25 | 2.28 | ± | 0.26 | 2.26 | ± | 0.23 | |||||
| W | 2.25 | ± | 0.12 | 2.30 | ± | 0.18 | 2.31 | ± | 0.13 | |||||
| VO2/W/kg LLM (mL W−1 kg−1) b | All | 0.139 | ± | 0.034 | 0.140 | ± | 0.038 | 0.141 | ± | 0.035 | 0.93 | 0.50 | 0.42 | 0.72 |
| M | 0.116 | ± | 0.023 | 0.114 | ± | 0.027 | 0.115 | ± | 0.025 | |||||
| W | 0.162 | ± | 0.026 | 0.167 | ± | 0.027 | 0.166 | ± | 0.022 | |||||
| RPE | All | 4.5 | ± | 1.6 | 4.7 | ± | 1.5 | 4.7 | ± | 1.5 | 0.51 | 0.77 | 0.57 | 0.72 |
| M | 4.8 | ± | 1.5 | 5.0 | ± | 1.4 | 5.0 | ± | 1.3 | |||||
| W | 4.2 | ± | 1.7 | 4.3 | ± | 1.6 | 4.4 | ± | 1.6 | |||||
| A | B | C | Treatment | Treat × Sex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sprints VO2peak (mL min−1) | All | 2668 | ± | 597 | 2650 | ± | 581 | 2624 | ± | 566 | 0.290 | 0.110 |
| M | 3160 | ± | 391 | 3099 | ± | 425 | 3046 | ± | 453 | |||
| W | 2177 | ± | 265 | 2178 | ± | 252 | 2202 | ± | 279 | |||
| Pain 15 s L | All | 7.9 | ± | 1.5 | 8.1 | ± | 1.7 | 7.6 | ± | 2.0 | 0.11 | 0.29 |
| M | 7.6 | ± | 1.6 | 7.9 | ± | 1.8 | 7.0 | ± | 2.4 | |||
| W | 8.3 | ± | 1.3 | 8.3 | ± | 1.6 | 8.2 | ± | 1.5 | |||
| Pain 35 s L | All | 8.6 | ± | 1.2 | 8.6 | ± | 1.9 | 8.4 | ± | 1.7 | 0.12 | 0.35 |
| M | 8.5 | ± | 1.3 | 8.6 | ± | 1.4 | 8.1 | ± | 2.1 | |||
| W | 8.7 | ± | 1.2 | 8.6 | ± | 2.4 | 8.8 | ± | 1.2 | |||
| Pain 55 s L | All | 9.1 | ± | 1.1 | 9.3 | ± | 1.1 | 9.2 | ± | 1.0 | 0.20 | 0.27 |
| M | 9.0 | ± | 1.2 | 9.1 | ± | 1.3 | 9.1 | ± | 1.1 | |||
| W | 9.3 | ± | 0.9 | 9.6 | ± | 0.8 | 9.3 | ± | 0.9 | |||
| Mean Pain L | All | 8.5 | ± | 1.2 | 8.7 | ± | 1.3 | 8.4 | ± | 1.5 | 0.12 | 0.61 |
| M | 8.4 | ± | 1.3 | 8.5 | ± | 1.4 | 8.1 | ± | 1.8 | |||
| W | 8.7 | ± | 1.1 | 9.0 | ± | 1.1 | 8.7 | ± | 1.1 | |||
| Hemoglobin (g dL−1) | All | 14.3 | ± | 1.3 | 14.4 | ± | 1.3 | 14.4 | ± | 1.2 | 0.70 | 0.09 |
| M | 15.4 | ± | 0.7 | 15.5 | ± | 0.7 | 15.3 | ± | 0.6 | |||
| W | 13.2 | ± | 0.7 | 13.3 | ± | 0.8 | 13.4 | ± | 0.7 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelabert-Rebato, M.; Martin-Rincon, M.; Galvan-Alvarez, V.; Gallego-Selles, A.; Martinez-Canton, M.; Vega-Morales, T.; Wiebe, J.C.; Fernandez-del Castillo, C.; Castilla-Hernandez, E.; Diaz-Tiberio, O.; et al. A Single Dose of The Mango Leaf Extract Zynamite® in Combination with Quercetin Enhances Peak Power Output During Repeated Sprint Exercise in Men and Women. Nutrients 2019, 11, 2592. https://doi.org/10.3390/nu11112592
Gelabert-Rebato M, Martin-Rincon M, Galvan-Alvarez V, Gallego-Selles A, Martinez-Canton M, Vega-Morales T, Wiebe JC, Fernandez-del Castillo C, Castilla-Hernandez E, Diaz-Tiberio O, et al. A Single Dose of The Mango Leaf Extract Zynamite® in Combination with Quercetin Enhances Peak Power Output During Repeated Sprint Exercise in Men and Women. Nutrients. 2019; 11(11):2592. https://doi.org/10.3390/nu11112592
Chicago/Turabian StyleGelabert-Rebato, Miriam, Marcos Martin-Rincon, Victor Galvan-Alvarez, Angel Gallego-Selles, Miriam Martinez-Canton, Tanausú Vega-Morales, Julia C. Wiebe, Constanza Fernandez-del Castillo, Elizabeth Castilla-Hernandez, Oriana Diaz-Tiberio, and et al. 2019. "A Single Dose of The Mango Leaf Extract Zynamite® in Combination with Quercetin Enhances Peak Power Output During Repeated Sprint Exercise in Men and Women" Nutrients 11, no. 11: 2592. https://doi.org/10.3390/nu11112592
APA StyleGelabert-Rebato, M., Martin-Rincon, M., Galvan-Alvarez, V., Gallego-Selles, A., Martinez-Canton, M., Vega-Morales, T., Wiebe, J. C., Fernandez-del Castillo, C., Castilla-Hernandez, E., Diaz-Tiberio, O., & Calbet, J. A. L. (2019). A Single Dose of The Mango Leaf Extract Zynamite® in Combination with Quercetin Enhances Peak Power Output During Repeated Sprint Exercise in Men and Women. Nutrients, 11(11), 2592. https://doi.org/10.3390/nu11112592







