Cuscutae Japonicae Semen Ameliorates Memory Dysfunction by Rescuing Synaptic Damage in Alzheimer’s Disease Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the CJS Extract (CJSE)
2.3. Animals and Administration
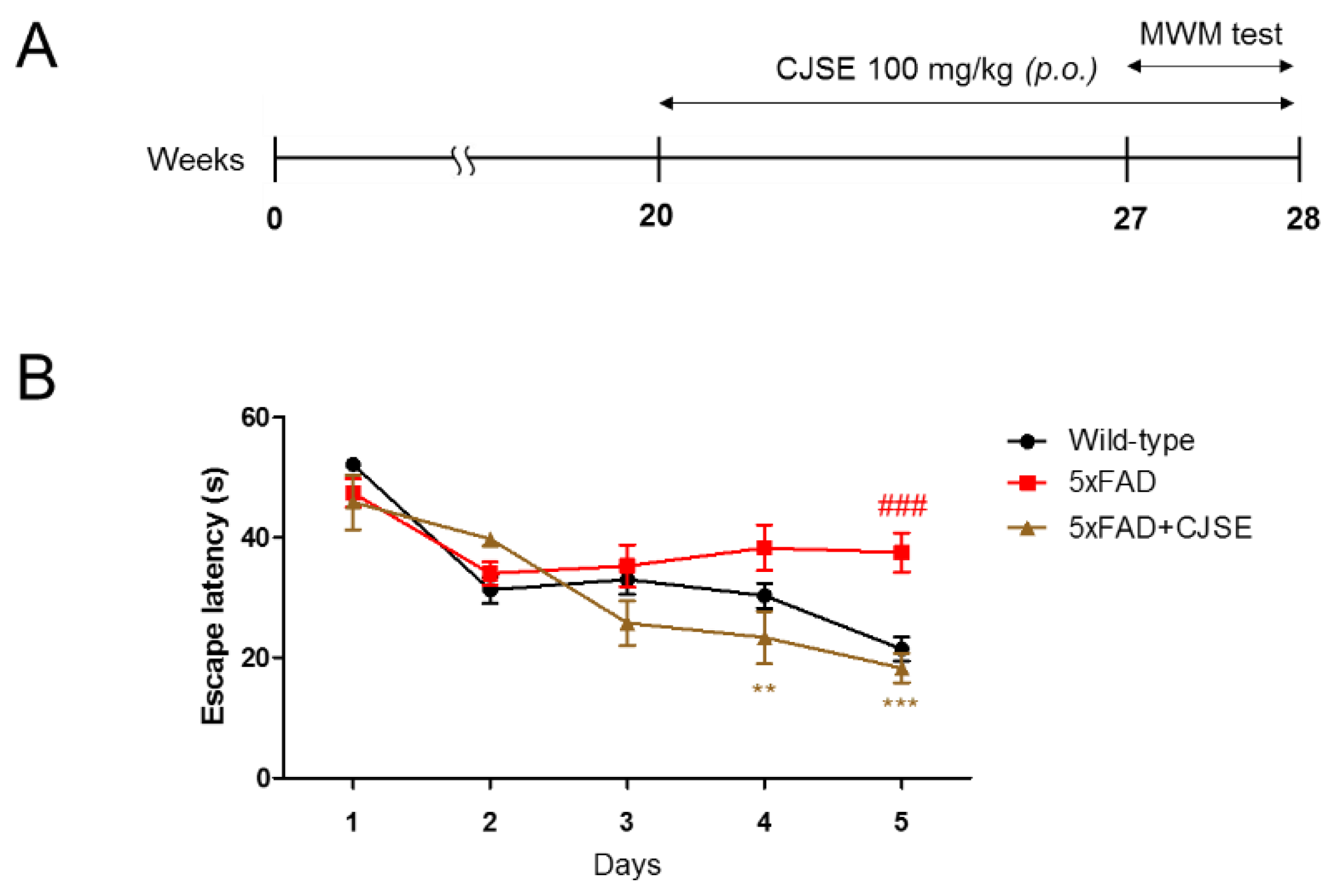
2.4. Morris Water Maze Test
2.5. Brain Tissue Preparation
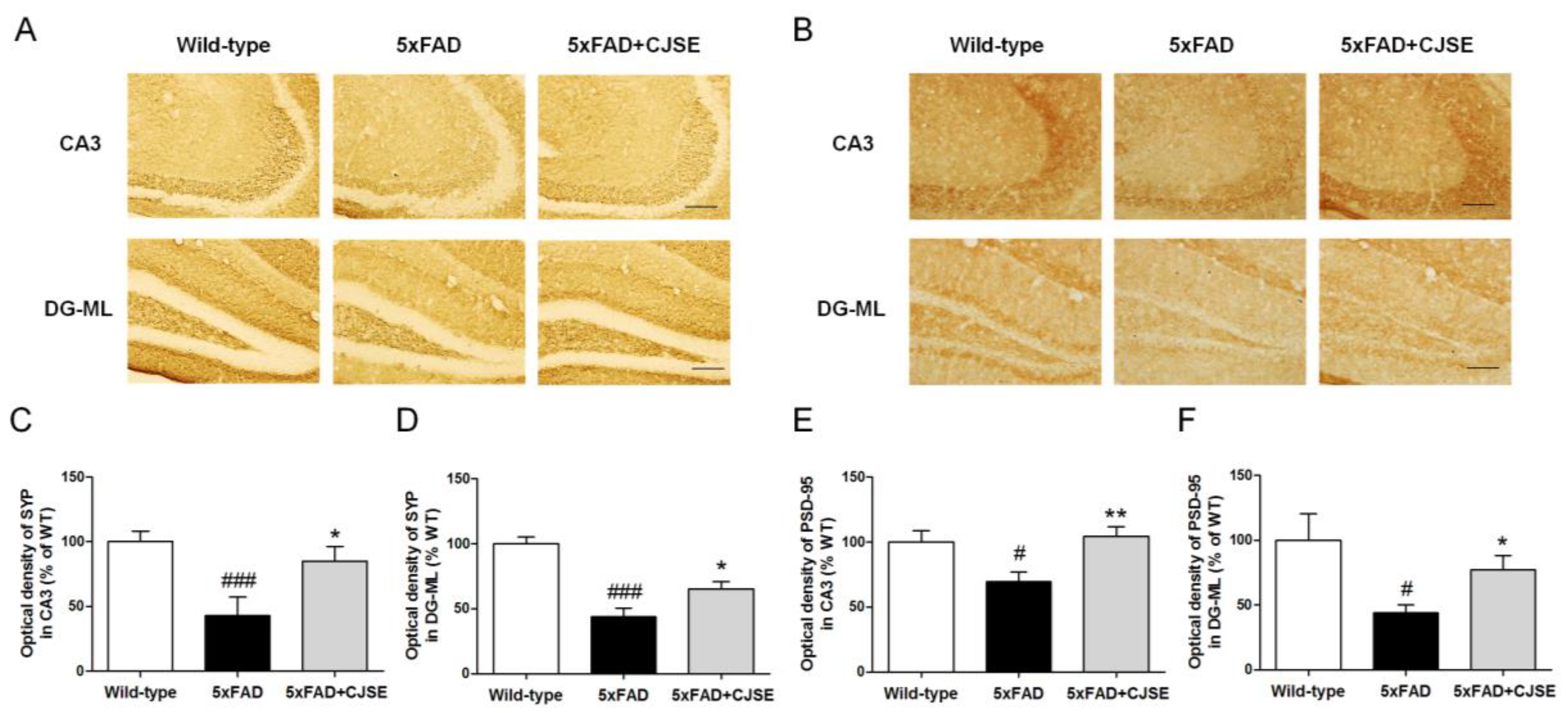
2.6. Immunohistochemistry
2.7. Primary Cultures of Hippocampal Neuronal Cells
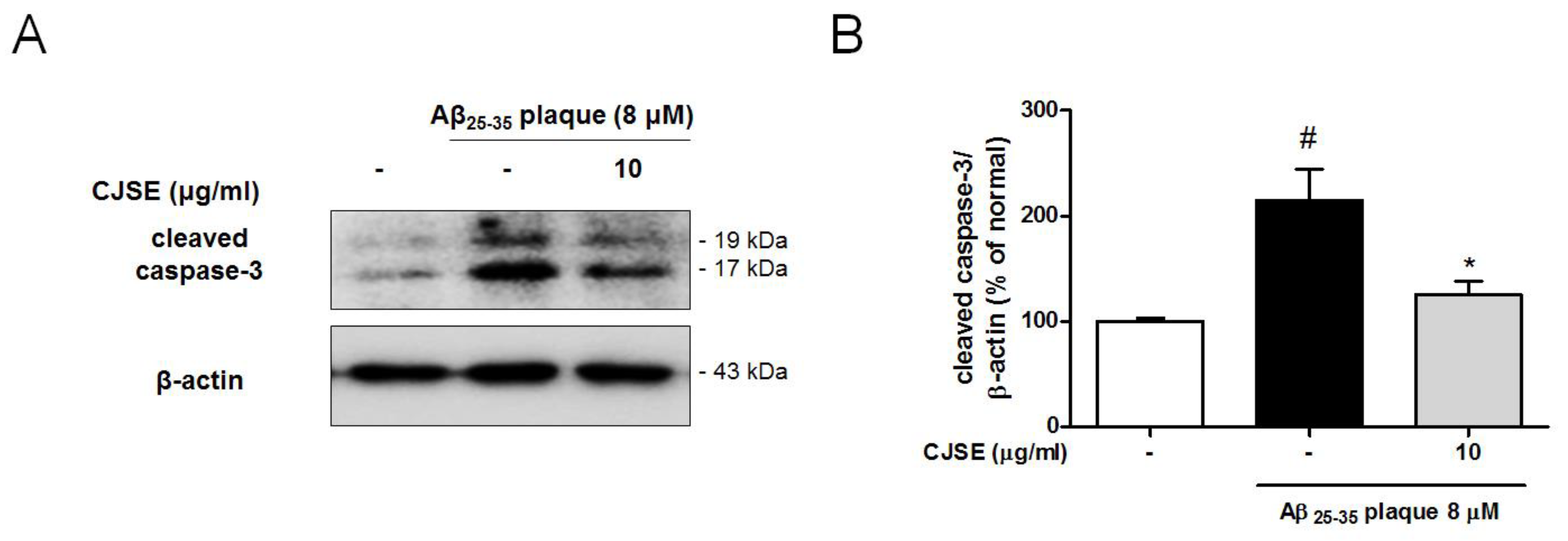
2.8. Western Blotting
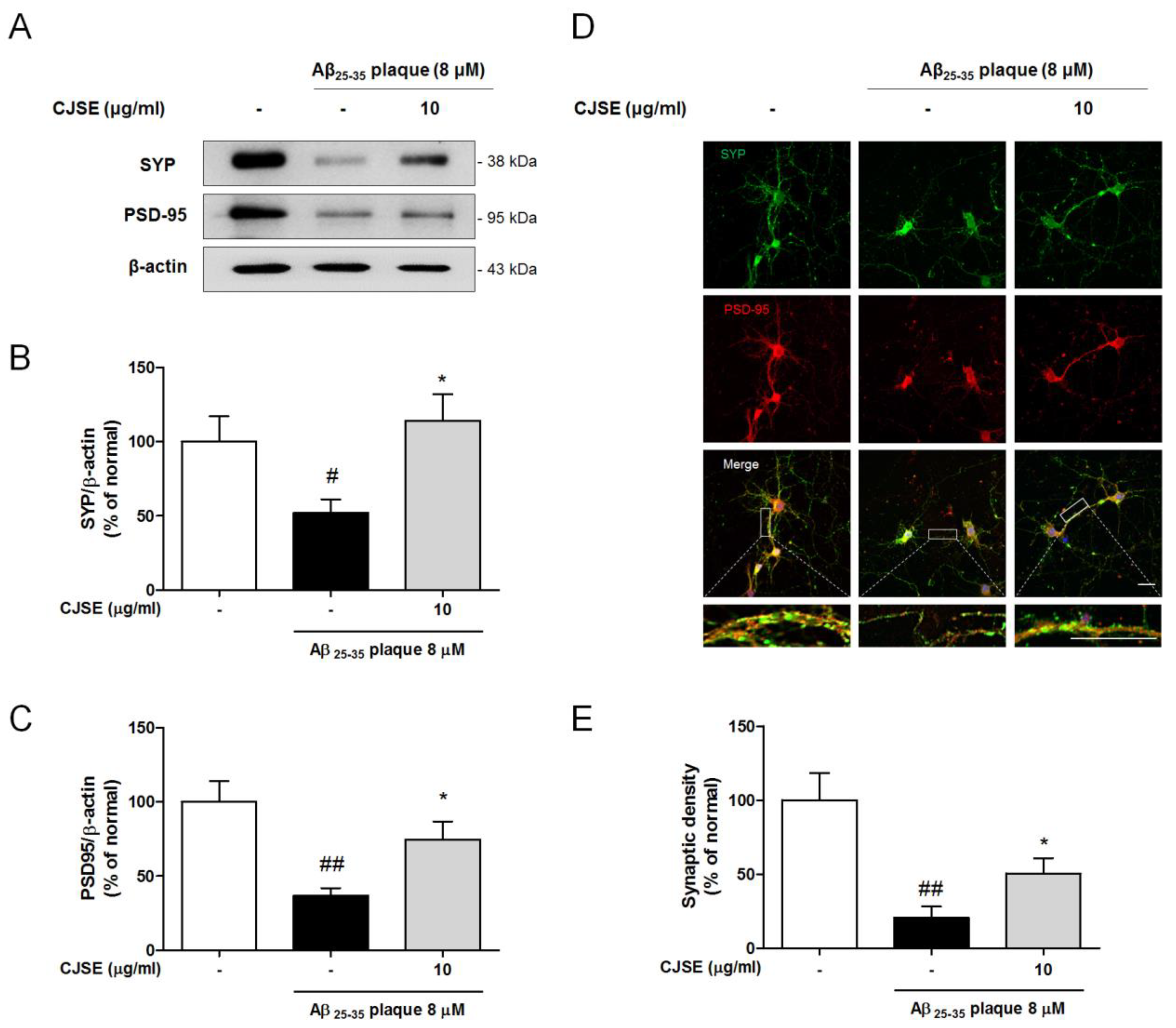
2.9. Immunocytochemistry and Synaptic Density Analysis
2.10. Statistical Analysis
3. Results
3.1. CJSE Attenuated Memory Impairment in 5xFAD Mice
3.2. CJSE Rescued Synaptic Loss in 5xFAD Mice
3.3. CJSE Protected Synaptic Loss Induced by Aβ in Mouse Primary Hippocampal Neurons
3.4. CJSE Suppressed the Activation of Caspase-3
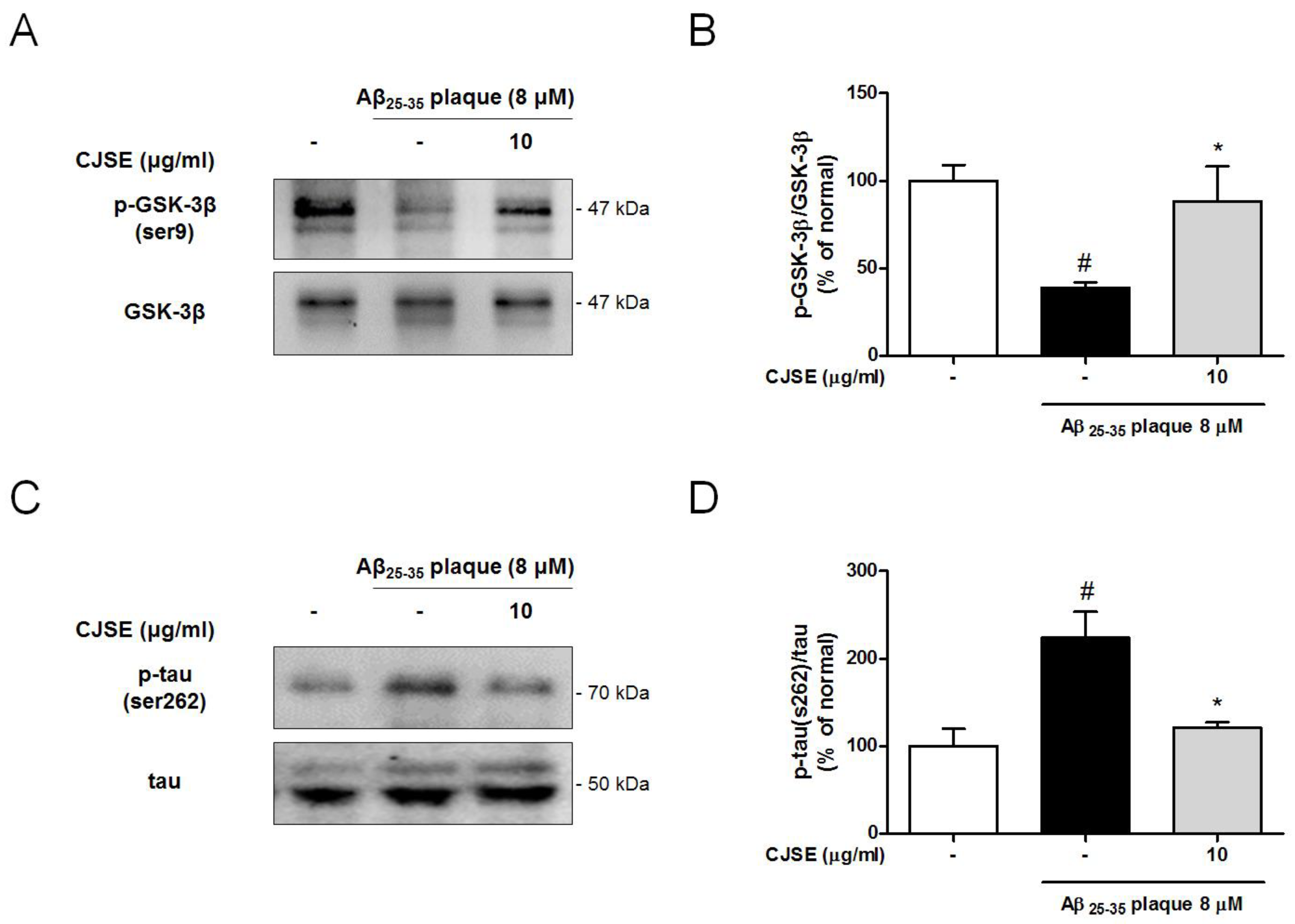
3.5. CJSE Regulated GSK-3β Phosphorylation and Tau Phosphorylation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers. 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.V.; Bonito-Oliva, A.; Sakmar, T.P. Update on Alzheimer’s Disease Therapy and Prevention Strategies. Annu. Rev. Med. 2017, 68, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Aaseth, J.; Dadar, M.; Chirumbolo, S. Molecular Targets in Alzheimer’s Disease. Mol. Neurobiol. 2019. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, Y.; Ma, Q.H.; Liu, Y. Alzheimer’s disease: Amyloid-based pathogenesis and potential therapies. Cell Stress 2018, 2, 150–161. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef]
- Forner, S.; Baglietto-Vargas, D.; Martini, A.C.; Trujillo-Estrada, L.; LaFerla, F.M. Synaptic Impairment in Alzheimer’s Disease: A Dysregulated Symphony. Trends. Neurosci. 2017, 40, 347–357. [Google Scholar] [CrossRef]
- Llorens-Martin, M.; Jurado, J.; Hernandez, F.; Avila, J. GSK-3beta, a pivotal kinase in Alzheimer disease. Front. Mol. Neurosci. 2014, 7, 46. [Google Scholar] [CrossRef]
- Bradley, C.A.; Peineau, S.; Taghibiglou, C.; Nicolas, C.S.; Whitcomb, D.J.; Bortolotto, Z.A.; Kaang, B.K.; Cho, K.; Wang, Y.T.; Collingridge, G.L. A pivotal role of GSK-3 in synaptic plasticity. Front. Mol. Neurosci. 2012, 5, 13. [Google Scholar] [CrossRef]
- Choi, J.G.; Kim, S.Y.; Jeong, M.; Oh, M.S. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol. Ther. 2018, 182, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Moon, M.; Oh, H.; Kim, H.G.; Kim, S.Y.; Oh, M.S. Ginger improves cognitive function via NGF-induced ERK/CREB activation in the hippocampus of the mouse. J. Nutr. Biochem. 2014, 25, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- da Costa, I.M.; Freire, M.A.M.; de Paiva Cavalcanti, J.R.L.; de Araujo, D.P.; Norrara, B.; Moreira Rosa, I.M.M.; de Azevedo, E.P.; do Rego, A.C.M.; Filho, I.A.; Guzen, F.P. Supplementation with Curcuma longa Reverses Neurotoxic and Behavioral Damage in Models of Alzheimer’s Disease: A Systematic Review. Curr. Neuropharmacol. 2019, 17, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Gilani, A.H. Therapeutic potential of turmeric in Alzheimer’s disease: Curcumin or curcuminoids? Phytother. Res. 2014, 28, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yao, L.; Zhou, H.; Qu, S.; Zeng, X.; Zhou, D.; Zhou, Y.; Li, X.; Liu, Z. Neuroprotection against Abeta25-35-induced apoptosis by Salvia miltiorrhiza extract in SH-SY5Y cells. Neurochem. Int. 2014, 75, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Pang, M.; Rong, L.; Zhou, W.; Wang, J.; Liu, C.; Wang, X. Effects of Salvia miltiorrhiza on neural differentiation of induced pluripotent stem cells. J. Ethnopharmacol. 2014, 153, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Rehman, K.; Hussain, I.; Farooq, T.; Ali, B.; Majeed, I.; Akash, M.S.H. Ethnopharmacological Investigations of Phytochemical Constituents Isolated from the Genus Cuscuta. Crit. Rev. Eukaryot. Gene. Expr. 2017, 27, 113–150. [Google Scholar] [CrossRef]
- Oh, H.; Kang, D.G.; Lee, S.; Lee, H.S. Angiotensin converting enzyme inhibitors from Cuscuta japonica Choisy. J. Ethnopharmacol. 2002, 83, 105–108. [Google Scholar] [CrossRef]
- Kim, H.; Song, M.J. Analysis and recordings of orally transmitted knowledge about medicinal plants in the southern mountainous region of Korea. J. Ethnopharmacol. 2011, 134, 676–696. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, H.N.; Kim, Y.R.; Choi, Y.H.; Kim, B.W.; Shin, H.K.; Choi, B.T. Aqueous fraction from Cuscuta japonica seed suppresses melanin synthesis through inhibition of the p38 mitogen-activated protein kinase signaling pathway in B16F10 cells. J. Ethnopharmacol. 2012, 141, 338–344. [Google Scholar] [CrossRef]
- Yang, X.; Ding, C.F.; Zhang, Y.H.; Yan, Z.Z.; Du, J. Protection of extract from Cuscuta japonica on human sperm acrosome and ultrastructure. Zhongguo Zhong Yao Za Zhi 2006, 31, 422–425. [Google Scholar] [PubMed]
- Moon, M.; Jeong, H.U.; Choi, J.G.; Jeon, S.G.; Song, E.J.; Hong, S.P.; Oh, M.S. Memory-enhancing effects of Cuscuta japonica Choisy via enhancement of adult hippocampal neurogenesis in mice. Behav. Brain Res. 2016, 311, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Choi, J.G.; Park, S.; Lee, J.K.; Oh, M.S. Butterbur Leaves Attenuate Memory Impairment and Neuronal Cell Damage in Amyloid Beta-Induced Alzheimer’s Disease Models. Int. J. Mol. Sci. 2018, 19, 1644. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Franklin, K.B.J.; Paxinos, G. Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Ju, I.G.; Choi, J.G.; Kim, N.; Kwak, C.; Lee, J.K.; Oh, M.S. Peucedani Japonici Radix ameliorates lipopolysaccharide-induced neuroinflammation by regulating microglial responses. Neurosci. Lett. 2018, 686, 161–167. [Google Scholar] [CrossRef]
- Kim, D.H.; Lim, H.; Lee, D.; Choi, S.J.; Oh, W.; Yang, Y.S.; Oh, J.S.; Hwang, H.H.; Jeon, H.B. Thrombospondin-1 secreted by human umbilical cord blood-derived mesenchymal stem cells rescues neurons from synaptic dysfunction in Alzheimer’s disease model. Sci. Rep. 2018, 8, 354. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Diniz, L.P.; Almeida, J.C.; Tortelli, V.; Vargas Lopes, C.; Setti-Perdigao, P.; Stipursky, J.; Kahn, S.A.; Romao, L.F.; de Miranda, J.; Alves-Leon, S.V.; et al. Astrocyte-induced synaptogenesis is mediated by transforming growth factor beta signaling through modulation of D-serine levels in cerebral cortex neurons. J. Biol. Chem. 2012, 287, 41432–41445. [Google Scholar] [CrossRef]
- Wang, J.Y.; Luo, Z.G. Non-apoptotic role of caspase-3 in synapse refinement. Neurosci. Bull. 2014, 30, 667–670. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Roselli, F.; Almeida, O.F.; Gao, X.; Wang, X.; Yew, D.T.; Wu, Y. Amyloid-beta induces caspase-dependent loss of PSD-95 and synaptophysin through NMDA receptors. J. Alzheimers Dis. 2010, 22, 541–556. [Google Scholar] [CrossRef]
- Medina, M.; Avila, J. Understanding the relationship between GSK-3 and Alzheimer’s disease: A focus on how GSK-3 can modulate synaptic plasticity processes. Expert Rev. Neurother. 2013, 13, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Pooler, A.M.; Noble, W.; Hanger, D.P. A role for tau at the synapse in Alzheimer’s disease pathogenesis. Neuropharmacology 2014, 76, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arendt, T. Synaptic degeneration in Alzheimer’s disease. Acta. Neuropathol. 2009, 118, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.Y.; Mirra, S.S.; Sait, H.B.; Sacktor, T.C.; Sigurdsson, E.M. Postsynaptic degeneration as revealed by PSD-95 reduction occurs after advanced Abeta and tau pathology in transgenic mouse models of Alzheimer’s disease. Acta. Neuropathol. 2011, 122, 285–292. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, J.; Zhu, Y.; Zhang, J.; Shen, H.; Lu, J.; Pan, X.; Lin, N.; Dai, X.; Zhou, M.; et al. Tripchlorolide improves cognitive deficits by reducing amyloid beta and upregulating synapse-related proteins in a transgenic model of Alzheimer’s Disease. J. Neurochem. 2015, 133, 38–52. [Google Scholar] [CrossRef]
- Devi, L.; Ohno, M. PERK mediates eIF2alpha phosphorylation responsible for BACE1 elevation, CREB dysfunction and neurodegeneration in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 2272–2281. [Google Scholar] [CrossRef]
- Mattson, M.P.; Partin, J.; Begley, J.G. Amyloid beta-peptide induces apoptosis-related events in synapses and dendrites. Brain Res. 1998, 807, 167–176. [Google Scholar] [CrossRef]
- Louneva, N.; Cohen, J.W.; Han, L.Y.; Talbot, K.; Wilson, R.S.; Bennett, D.A.; Trojanowski, J.Q.; Arnold, S.E. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s disease. Am. J. Pathol. 2008, 173, 1488–1495. [Google Scholar] [CrossRef]
- Jang, B.G.; In, S.; Choi, B.; Kim, M.J. Beta-amyloid oligomers induce early loss of presynaptic proteins in primary neurons by caspase-dependent and proteasome-dependent mechanisms. Neuroreport 2014, 25, 1281–1288. [Google Scholar] [CrossRef]
- Chu, J.; Lauretti, E.; Pratico, D. Caspase-3-dependent cleavage of Akt modulates tau phosphorylation via GSK3beta kinase: Implications for Alzheimer’s disease. Mol. Psychiatry 2017, 22, 1002–1008. [Google Scholar] [CrossRef]
- Regan, P.; Whitcomb, D.J.; Cho, K. Physiological and Pathophysiological Implications of Synaptic Tau. Neuroscientist 2017, 23, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, L. Extraction and content determination of polysaccharide in Cuscuta japonica Choisy in Changbai mountain area. Med. Plant 2010, 1, 42–44. [Google Scholar]
- Szymanska, R.; Kruk, J. Tocopherol content and isomers’ composition in selected plant species. Plant Physiol. Biochem. 2008, 46, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.W.; Wang, X.M.; Ko, H.; Kwon, H.C.; Cha, J.W.; Yang, H.O. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid beta-protein via the PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur. J. Pharmacol. 2011, 672, 45–55. [Google Scholar] [CrossRef]
- Miyamae, Y.; Kurisu, M.; Murakami, K.; Han, J.; Isoda, H.; Irie, K.; Shigemori, H. Protective effects of caffeoylquinic acids on the aggregation and neurotoxicity of the 42-residue amyloid beta-protein. Bioorg. Med. Chem. 2012, 20, 5844–5849. [Google Scholar] [CrossRef]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience 2010, 169, 1039–1045. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, I.G.; Kim, N.; Choi, J.G.; Lee, J.K.; Oh, M.S. Cuscutae Japonicae Semen Ameliorates Memory Dysfunction by Rescuing Synaptic Damage in Alzheimer’s Disease Models. Nutrients 2019, 11, 2591. https://doi.org/10.3390/nu11112591
Ju IG, Kim N, Choi JG, Lee JK, Oh MS. Cuscutae Japonicae Semen Ameliorates Memory Dysfunction by Rescuing Synaptic Damage in Alzheimer’s Disease Models. Nutrients. 2019; 11(11):2591. https://doi.org/10.3390/nu11112591
Chicago/Turabian StyleJu, In Gyoung, Namkwon Kim, Jin Gyu Choi, Jong Kil Lee, and Myung Sook Oh. 2019. "Cuscutae Japonicae Semen Ameliorates Memory Dysfunction by Rescuing Synaptic Damage in Alzheimer’s Disease Models" Nutrients 11, no. 11: 2591. https://doi.org/10.3390/nu11112591
APA StyleJu, I. G., Kim, N., Choi, J. G., Lee, J. K., & Oh, M. S. (2019). Cuscutae Japonicae Semen Ameliorates Memory Dysfunction by Rescuing Synaptic Damage in Alzheimer’s Disease Models. Nutrients, 11(11), 2591. https://doi.org/10.3390/nu11112591




