Altilix® Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study
Abstract
1. Introduction
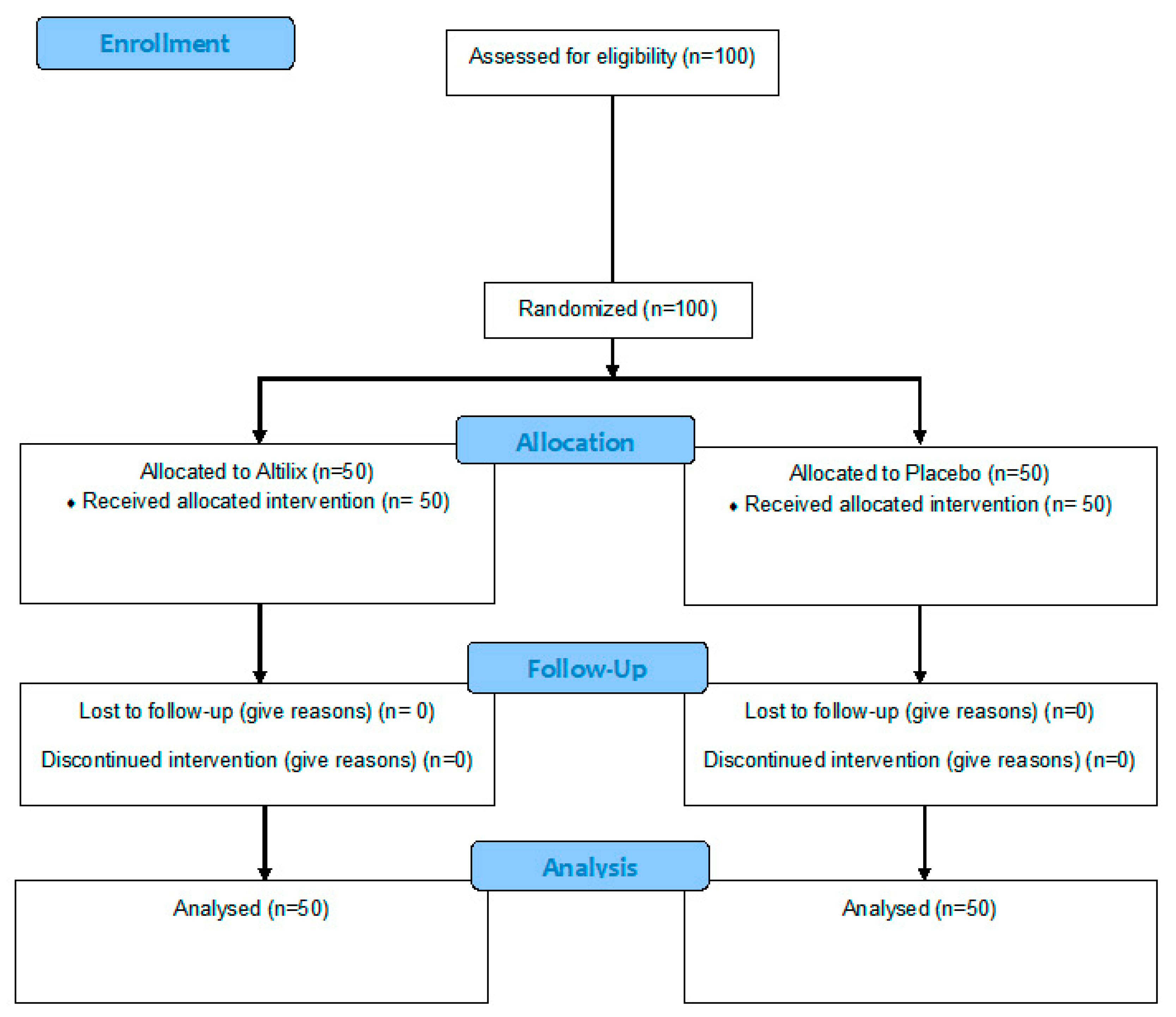
2. Materials and Methods
2.1. Design of the Study
2.2. Clinical Variables
2.3. Biochemical Analyses
2.4. Serum Cytokine and Adipokine Quantification
2.5. Color Doppler Ultrasound of Carotid Arteries
2.6. Ultrasound Assessment of Endothelial Function
2.7. Statistical Analysis
3. Results
3.1. Baseline Subjects Characteristics
3.2. Cardiometabolic and Liver Parameters
3.3. Clinical and Biochemical Parameters
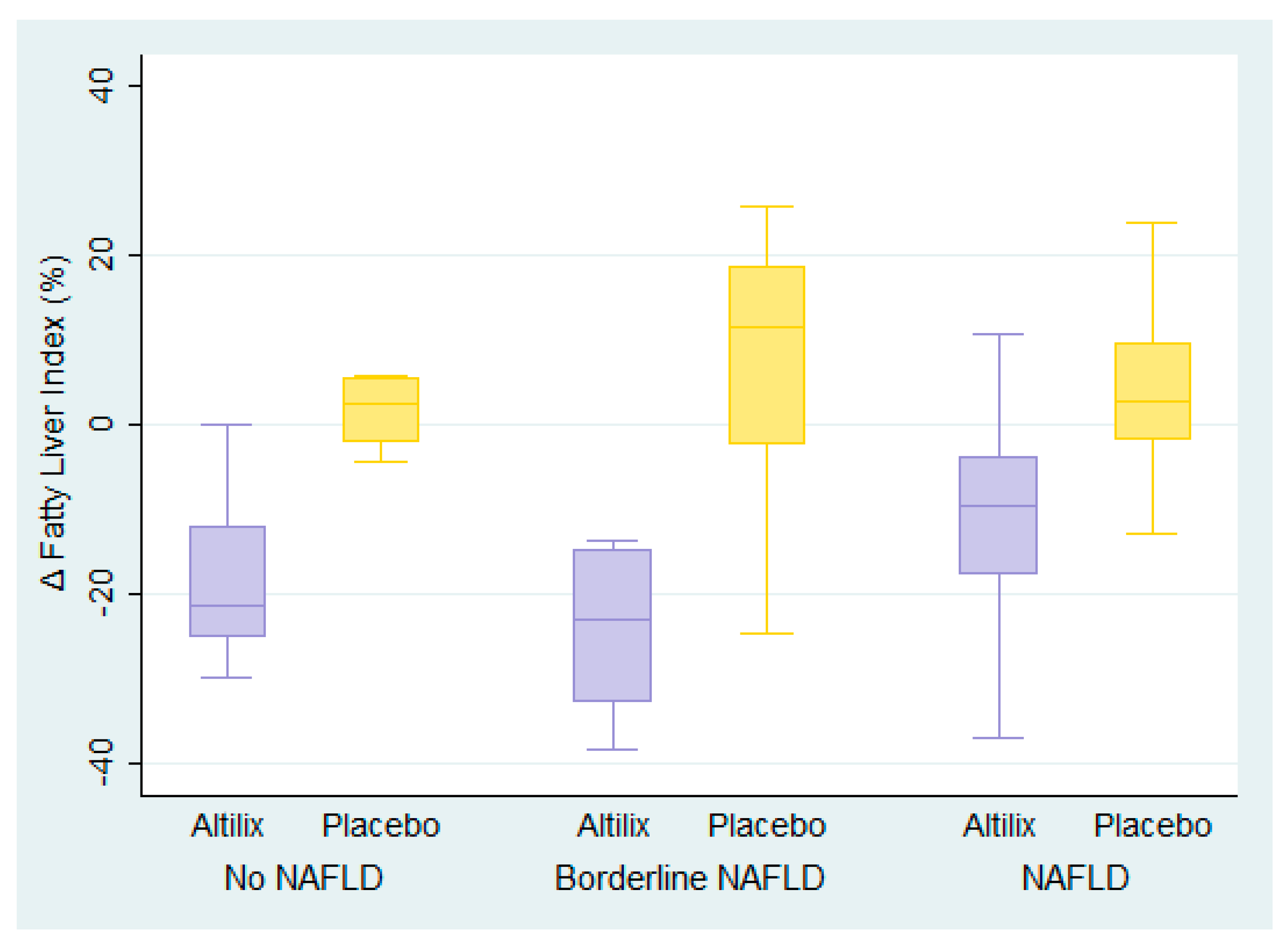
3.4. Fatty Liver Index
3.5. Cytokine Analysis
3.6. Pearson’s Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mikhailidis, D.P.; Elisaf, M.; Rizzo, M.; Berneis, K.; Griffin, B.; Zambon, A.; Athyros, V.; de Graaf, J.; Marz, W.; Parhofer, K.G.; et al. “European panel on low density lipoprotein (ldl) subclasses”: A statement on the pathophysiology, atherogenicity and clinical significance of ldl subclasses. Curr. Vasc. Pharmacol. 2011, 9, 533–571. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martinez, P.; Mikhailidis, D.P.; Athyros, V.G.; Bullo, M.; Couture, P.; Covas, M.I.; de Koning, L.; Delgado-Lista, J.; Diaz-Lopez, A.; Drevon, C.A.; et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: An international panel recommendation. Nutr. Rev. 2017, 75, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Levene, A.P.; Goldin, R.D. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology 2012, 61, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Fazel, Y.; Koenig, A.B.; Sayiner, M.; Goodman, Z.D.; Younossi, Z.M. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 2016, 65, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Burgess, M.I.; Sprung, V.S.; Irwin, A.; Hamer, M.; Jones, J.; Daousi, C.; Adams, V.; Kemp, G.J.; Shojaee-Moradie, F.; et al. Metabolically healthy and unhealthy obesity: Differential effects on myocardial function according to metabolic syndrome, rather than obesity. Int. J. Obes. 2016, 40, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Wilman, H.R.; Kelly, M.; Garratt, S.; Matthews, P.M.; Milanesi, M.; Herlihy, A.; Gyngell, M.; Neubauer, S.; Bell, J.D.; Banerjee, R.; et al. Characterisation of liver fat in the uk biobank cohort. PLoS ONE 2017, 12, e0172921. [Google Scholar]
- Patti, A.M.; Al-Rasadi, K.; Giglio, R.V.; Nikolic, D.; Mannina, C.; Castellino, G.; Chianetta, R.; Banach, M.; Cicero, A.F.G.; Lippi, G.; et al. Natural approaches in metabolic syndrome management. Arch. Med. Sci. 2018, 14, 422–441. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Veronesi, M.; Rizzo, M.; Giovannini, M.; Borghi, C. Short-term effects of a combined nutraceutical on lipid level, fatty liver biomarkers, hemodynamic parameters, and estimated cardiovascular disease risk: A double-blind, placebo-controlled randomized clinical trial. Adv. Ther. 2017, 34, 1966–1975. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 european guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the european society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts)developed with the special contribution of the european association for cardiovascular prevention & rehabilitation (eacpr). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar]
- Cicero, A.F.G.; Colletti, A.; Bellentani, S. Nutraceutical approach to non-alcoholic fatty liver disease (nafld): The available clinical evidence. Nutrients 2018, 10, 1153. [Google Scholar] [CrossRef]
- Ben Salem, M.; Affes, H.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Pharmacological studies of artichoke leaf extract and their health benefits. Plant Foods Hum. Nutr. 2015, 70, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, K.; Aliashrafi, S.; Asghari-Jafarabadi, M.; Ebrahimi-Mameghani, M. Antioxidant response to artichoke leaf extract supplementation in metabolic syndrome: A double-blind placebo-controlled randomized clinical trial. Clin. Nutr. 2018, 37, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Muller, M.; Naumann, J.; Schenk, T.; Ludtke, R. Artichoke leave extract for chronic hepatitis c—A pilot study. Phytomedicine 2009, 16, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Skarpanska-Stejnborn, A.; Pilaczynska-Szczesniak, L.; Basta, P.; Deskur-Smielcka, E.; Horoszkiewicz-Hassan, M. The influence of supplementation with artichoke (cynara scolymus l.) extract on selected redox parameters in rowers. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Rangboo, V.; Noroozi, M.; Zavoshy, R.; Rezadoost, S.A.; Mohammadpoorasl, A. The effect of artichoke leaf extract on alanine aminotransferase and aspartate aminotransferase in the patients with nonalcoholic steatohepatitis. Int. J. Hepatol. 2016, 2016, 4030476. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Y.; Liu, C.; Xu, D.; Zhang, R.; Cheng, Y.; Pan, Y.; Huang, C.; Chen, Y. Luteolin alleviates alcoholic liver disease induced by chronic and binge ethanol feeding in mice. J. Nutr. 2014, 144, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Sa, C.; Oliveira, A.R.; Machado, C.; Azevedo, M.; Pereira-Wilson, C. Effects on liver lipid metabolism of the naturally occurring dietary flavone luteolin-7-glucoside. Evid. Based Complement. Altern. Med. 2015, 2015, 647832. [Google Scholar] [CrossRef]
- He, W.; An, X.; Li, L.; Shao, X.; Li, Q.; Yao, Q.; Zhang, J.A. Relationship between hypothyroidism and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front. Endocrinol. 2017, 8, 335. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; Group, C. Consort 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, A.; Rahmoune, H.; Leedjarv, K.; Knorpp, T.; Joos, T.; Stocki, P.; Guest, P.C.; Bahn, S. Development of a novel assay for proprotein converting enzyme activity on a multiplex bead-based array system. Proteomics 2013, 13, 2976–2979. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Rizvi, A.A.; Patti, A.M.; Nikolic, D.; Giglio, R.V.; Castellino, G.; Li Volti, G.; Caprio, M.; Montalto, G.; Provenzano, V.; et al. Liraglutide improves metabolic parameters and carotid intima-media thickness in diabetic patients with the metabolic syndrome: An 18-month prospective study. Cardiovasc. Diabetol. 2016, 15, 162. [Google Scholar] [CrossRef] [PubMed]
- Peretz, A.; Leotta, D.F.; Sullivan, J.H.; Trenga, C.A.; Sands, F.N.; Aulet, M.R.; Paun, M.; Gill, E.A.; Kaufman, J.D. Flow mediated dilation of the brachial artery: An investigation of methods requiring further standardization. BMC Cardiovasc. Disord. 2007, 7, 11. [Google Scholar] [CrossRef]
- Nakagawa, S. A farewell to bonferroni: The problems of low statistical power and publication bias. Behav. Ecol. 2004, 15, 1044–1045. [Google Scholar] [CrossRef]
- Guruvaiah, P.; Guo, H.; Li, D.; Xie, Z. Preventive effect of flavonol derivatives abundant sanglan tea on long-term high-fat-diet-induced obesity complications in c57bl/6 mice. Nutrients 2018, 10, 1276. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Atkin, S.L.; Butler, A.E.; Jafari, R.; Badeli, R.; Sahebkar, A. Efficacy of artichoke leaf extract in non-alcoholic fatty liver disease: A pilot double-blind randomized controlled trial. Phytother. Res. 2018, 32, 1382–1387. [Google Scholar] [CrossRef]
- Lupattelli, G.; Marchesi, S.; Lombardini, R.; Roscini, A.R.; Trinca, F.; Gemelli, F.; Vaudo, G.; Mannarino, E. Artichoke juice improves endothelial function in hyperlipemia. Life Sci. 2004, 76, 775–782. [Google Scholar] [CrossRef]
- Rizzo, M.; Berneis, K.; Koulouris, S.; Pastromas, S.; Rini, G.B.; Sakellariou, D.; Manolis, A.S. Should we measure routinely oxidised and atherogenic dense low-density lipoproteins in subjects with type 2 diabetes? Int. J. Clin. Pract. 2010, 64, 1632–1642. [Google Scholar] [CrossRef]
- Samochowiec, L. The action of herbs and roots of artichokes (cynara scolymnus) and cardoon (cynara cardunculus) on the development of experimental atherosclerosis in white rats. Diss Pharm 1962, 14, 115. [Google Scholar]
- Brown, J.E.; Rice-Evans, C.A. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic. Res. 1998, 29, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R. Inhibition of hepatic cholesterol biosynthesis by artichoke leaf extracts is mainly due to luteolin. Cell Biol. Toxicol. 1996, 10, 89–150. [Google Scholar]
- Rondanelli, M.; Monteferrario, F.; Perna, S.; Faliva, M.A.; Opizzi, A. Health-promoting properties of artichoke in preventing cardiovascular disease by its lipidic and glycemic-reducing action. Monaldi Arch. Chest Dis. 2013, 80, 17–26. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Kuczmannova, A.; Balazova, A.; Racanska, E.; Kamenikova, M.; Fialova, S.; Majernik, J.; Nagy, M.; Gal, P.; Mucaji, P. Agrimonia eupatoria l. And cynara cardunculus l. Water infusions: Comparison of anti-diabetic activities. Molecules 2016, 21, 564. [Google Scholar] [CrossRef]
- Isokuortti, E.; Zhou, Y.; Peltonen, M.; Bugianesi, E.; Clement, K.; Bonnefont-Rousselot, D.; Lacorte, J.M.; Gastaldelli, A.; Schuppan, D.; Schattenberg, J.M.; et al. Use of homa-ir to diagnose non-alcoholic fatty liver disease: A population-based and inter-laboratory study. Diabetologia 2017, 60, 1873–1882. [Google Scholar] [CrossRef]
- Santos, H.O.; Bueno, A.A.; Mota, J.F. The effect of artichoke on lipid profile: A review of possible mechanisms of action. Pharmacol. Res. 2018, 137, 170–178. [Google Scholar] [CrossRef]
- Englisch, W.; Beckers, C.; Unkauf, M.; Ruepp, M.; Zinserling, V. Efficacy of artichoke dry extract in patients with hyperlipoproteinemia. Arzneimittelforschung 2000, 50, 260–265. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid lowering nutraceuticals in clinical practice: Position paper from an international lipid expert panel. Arch. Med. Sci. 2017, 13, 965–1005. [Google Scholar] [CrossRef]
- Chatrath, H.; Vuppalanchi, R.; Chalasani, N. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin. Liver Dis. 2012, 32, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Flamm, S.L.; Di Bisceglie, A.M.; Bodenheimer, H.C. Serum activity of alanine aminotransferase (alt) as an indicator of health and disease. Hepatology 2008, 47, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Caldara, G.F.; Nuzzo, D.; Baldassano, S.; Picone, P.; Rizzo, M.; Mule, F.; Di Carlo, M. Nafld and atherosclerosis are prevented by a natural dietary supplement containing curcumin, silymarin, guggul, chlorogenic acid and inulin in mice fed a high-fat diet. Nutrients 2017, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Wittemer, S.M.; Ploch, M.; Windeck, T.; Muller, S.C.; Drewelow, B.; Derendorf, H.; Veit, M. Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of artichoke leaf extracts in humans. Phytomedicine 2005, 12, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, E.; Rafieian-Kopaei, M. Protective effect of artichoke (cynara scolymus) leaf extract against lead toxicity in rat. Pharm. Biol. 2013, 51, 1104–1109. [Google Scholar] [CrossRef]
- Rizzo, M.; Rizvi, A.A.; Sudar, E.; Soskic, S.; Obradovic, M.; Montalto, G.; Boutjdir, M.; Mikhailidis, D.P.; Isenovic, E.R. A review of the cardiovascular and anti-atherogenic effects of ghrelin. Curr. Pharm. Des. 2013, 19, 4953–4963. [Google Scholar] [CrossRef]
- Rizzo, M.; Abate, N.; Chandalia, M.; Rizvi, A.A.; Giglio, R.V.; Nikolic, D.; Marino Gammazza, A.; Barbagallo, I.; Isenovic, E.R.; Banach, M.; et al. Liraglutide reduces oxidative stress and restores heme oxygenase-1 and ghrelin levels in patients with type 2 diabetes: A prospective pilot study. J. Clin. Endocrinol. Metab. 2015, 100, 603–606. [Google Scholar] [CrossRef]
- Rahman, H.A.; Sahib, N.G.; Saari, N.; Abas, F.; Ismail, A.; Mumtaz, M.W.; Hamid, A.A. Anti-obesity effect of ethanolic extract from cosmos caudatus kunth leaf in lean rats fed a high fat diet. BMC Complement. Altern. Med. 2017, 17, 122. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 family cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef]
- Dittrich, A.; Hessenkemper, W.; Schaper, F. Systems biology of il-6, il-12 family cytokines. Cytokine Growth Factor Rev. 2015, 26, 595–602. [Google Scholar] [CrossRef]
- Alamri, B.N.; Shin, K.; Chappe, V.; Anini, Y. The role of ghrelin in the regulation of glucose homeostasis. Horm. Mol. Biol. Clin. Investig. 2016, 26, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.M.; Mehanna, O.M.; El Askary, A. The association between ghrelin levels and markers of arterial stiffness and inflammatory markers in saudi subjects with metabolic syndrome. Diabetes Metab. Syndr. 2017, 11 (Suppl. 2), S721–S725. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Weickert, M.O.; Lythgoe, D.; Sprung, V.S.; Dobson, R.; Shoajee-Moradie, F.; Umpleby, M.; Pfeiffer, A.F.; Thomas, E.L.; Bell, J.D.; et al. External validation of the fatty liver index and lipid accumulation product indices, using 1h-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur. J. Endocrinol. 2014, 171, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the american gastroenterological association, american association for the study of liver diseases, and american college of gastroenterology. Gastroenterology 2012, 55, 2005–2023. [Google Scholar]
| Variable | Altilix (n = 50) | Placebo (n = 50) | p-Value |
|---|---|---|---|
| Age (years), mean ± SD | 63.0 ± 9.32 | 60.2 ± 10.3 | 0.161 a |
| Women, n (%) | 24 (48) | 22 (44) | 0.688 b |
| Smoking habit, n (%) | 9 (18) | 15 (30) | 0.160 b |
| Family history of cardiovascular diseases, n (%) | 33 (66) | 31 (62) | 0.677 b |
| Diabetes duration (years), mean ± SD | 8.40 ± 7.05 | 7.24 ± 8.03 | 0.449 a |
| Hypertension, n (%) | 41 (82) | 38 (76) | 0.461 b |
| Dyslipidemia, n (%) | 42 (84) | 39 (78) | 0.444 b |
| Obesity, n (%) | 27 (54) | 32 (64) | 0.309 b |
| Parameters | Altilix (n = 50) | Placebo (n = 50) | % Change (n = 100) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | Altilix | Placebo | Difference (95% CI) | p-Value a (between Groups) | |
| Weight (kg) | 82.63 ± 16.4 | 80.06 ± 15.5 | 87.37 ± 16.5 | 86.88 ± 17.0 | −3.00 | −0.60 | −2.40 (−3.79, −1.01) | <0.001 |
| BMI (kg/m2) | 31.04 ± 5.15 | 30.08 ± 4.97 | 32.10 ± 5.80 | 31.89 ± 5.78 | −3.01 | −0.60 | −2.41 (−3.80, −1.03) | <0.001 |
| Waist circumference (cm) | 106.6 ± 12.6 | 103.1 ± 11.7 | 111.2 ± 12.5 | 110.7 ± 14.0 | −3.21 | −0.45 | −2.76 (−4.55, −0.96) | 0.003 |
| HbA1c (%) | 7.28 ± 1.18 | 6.51 ± 0.88 | 7.24 ± 1.44 | 7.42 ± 1.53 | −0.77 | 0.18 | −0.95 (−1.22, −0.67) | <0.001 |
| HOMA-IR | 4.005 ± 2.85 | 3.049 ± 1.99 | 5.157 ± 6.32 | 5.763 ± 6.39 | −21.10 | 22.0 | −43.11 (−51.87, −34.35) | <0.001 |
| HOMA-β | 80.65 ± 52.8 | 96.98 ± 54.7 | 87.37 ± 96.3 | 61.99 ± 60.9 | 16.3 | −25.4 | 41.70 (17.58, 65.83) | 0.001 |
| Total cholesterol (mg/dL) | 191.6 ± 41.1 | 165.2 ± 42.2 | 177.8 ± 36.8 | 188.1 ± 40.7 | −13.6 | 6.03 | −19.59 (−23.71, −15.47) | <0.001 |
| Triglycerides (mg/dL) | 154.1 ± 102 | 115.6 ± 46.2 | 143.6 ± 68.3 | 166.4 ± 79.25 | −17.2 | 17.9 | −35.14 (−44.83, −25.45) | <0.001 |
| LDL-cholesterol (mg/dL) | 116.6 ± 34.4 | 97.79 ± 36.5 | 105.0 ± 32.9 | 112.8 ± 34.0 | −15.9 | 8.89 | −24.79 (−31.43, −18,16) | <0.001 |
| HDL-cholesterol (mg/dL) | 48.82 ± 11.5 | 48.71 ± 11.3 | 47.42 ± 11.1 | 47.31 ± 12.1 | 0.80 | 0.26 | 0.54 (−4.83, 5.91) | 0.842 |
| Fatty Liver Index | 59.55 ± 26.7 | 50.27 ± 27.2 | 62.39 ± 25.4 | 64.50 ± 26.1 | −17.7 | 4.17 | −21.83 (−27.39, −16.27) | <0.001 |
| AST | 20.68 ± 8.35 | 18.56 ± 11.3 | 19.90 ± 5.82 | 21.72 ± 6.11 | −10.3 | 10.3 | −20.54 (−26.87, −14.22) | <0.001 |
| ALT | 26.96 ± 13.7 | 19.84 ± 9.65 | 22.26 ± 11.3 | 26.62 ± 11.9 | −23.3 | 23.7 | −47.06 (−54.97, −39.13) | <0.001 |
| AST/ALT | 0.827 ± 0.22 | 0.978 ± 0.26 | 0.996 ± 0.29 | 0.904 ± 0.31 | 21.1 | −8.66 | 29.71 (19.66, 39.77) | <0.001 |
| Flow-mediated dilation (%) | 21.53 ± 16.1 | 26.26 ± 12.4 | 16.85 ± 14.5 | 10.93 ± 6.85 | 4.64 | −5.91 | 10.56 (5.00, 16.12) | <0.001 |
| Carotid IMT (mm) | 0.922 ± 0.16 | 0.598 ± 0.14 | 0.862 ± 0.17 | 0.892 ± 0.18 | −33.4 | 6.03 | −39.48 (−47.98, −30.97) | <0.001 |
| Parameters | Without NAFLD (n = 22) | With Borderline NAFLD (n = 20) | With NAFLD (n = 58) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altilix (n = 12) | Placebo (n = 10) | Difference (95% CI) | p-Value a (between Groups) | Altilix (n = 10) | Placebo (n = 10) | Difference (95% CI) | p-Value a (between Groups) | Altilix (n = 28) | Placebo (n = 30) | Difference (95% CI) | p-Value a (between Groups) | |
| Weight (kg) | −3.3 | −0.9 | −2.49 (−5.32, 0.34) | 0.082 | −2.7 | 0.4 | −3.02 (−6.32, 0.27) | 0.070 | −3.0 | −0.8 | −2.14 (−4.08, −0.20) | 0.031 |
| BMI (kg/m2) | −3.4 | −0.9 | −2.54 (−5.37, 0.28) | 0.082 | −2.7 | 0.4 | −3.02 (−6.32, 0.27) | 0.070 | −3.0 | −0.8 | −2.14 (−4.08, −0.20) | 0.031 |
| Waist circumference (cm) | −1.9 | −1.2 | −0.70 (−5.51, 4.11) | 0.764 | −4.3 | −0.2 | −4.09 (−7.18, −0.99) | 0.013 | −3.4 | −0.3 | −3.10 (−5.51, −0.68) | 0.013 |
| HbA1c (%) | −0.7 | 0.2 | −0.87 (−1.67, −0.08) | 0.033 | −1.0 | −0.3 | −0.96 (−1.64, −0.27) | 0.009 | −0.7 | 0.2 | −0.96 (−1.28, −0.64) | <0.001 |
| HOMA-IR | −19.7 | 13.2 | −32.94 (−43.77, −22.11) | <0.001 | −24.7 | 38.5 | −63.13 (−87.23, −39,03) | <0.001 | −20.1 | 19.3 | −39.35 (−49.97, −28.72) | <0.001 |
| HOMA-β | 11.1 | −4.0 | 15.02 (8.14, 21.91) | 0.002 | 11.8 | −15.6 | 26.80 (10.58, 43.02) | 0.002 | 19.0 | −31.7 | 50.68 (15.13, 86.23) | 0.006 |
| Total cholesterol (mg/dL) | −16.8 | 3.0 | −19.84 (−30.13, −9.55) | <0.001 | −14.9 | 6.5 | −21.33 (−28.07, −14.59) | <0.001 | −11.7 | 6.8 | −18.48 (−24.25, −12.71) | <0.001 |
| Triglycerides (mg/dL) | −21.5 | 7.0 | −28.52 (−45.40, −11.65) | 0.002 | −12.7 | 13.5 | −26.19 (−38.32, −14.05) | <0.001 | −17.0 | 22.7 | −39.66 (−54.67, −24.65) | <0.001 |
| LDL-cholesterol (mg/dL) | −16.0 | 7.4 | −23.40 (−41.90, −4.90) | 0.016 | −21.1 | 6.8 | −27.90 (−35.98, −19.82) | <0.001 | −14.0 | 10.0 | −24.04 (−33.37, −14.71) | <0.001 |
| HDL-cholesterol (mg/dL) | −4.7 | −2.7 | −2.01 (−15.33, 11.30) | 0.755 | 4.4 | 9.8 | −5.32 (−21.01, 10.36) | 0.485 | 1.7 | −1.9 | 3.59 (−2.26, 9.44) | 0.224 |
| Fatty Liver Index | −21.7 | 5.8 | −27.47 (−41.46, −13.49) | <0.001 | −24.2 | 7.1 | −31.28 (−43.07, −19.49) | <0.001 | −13.6 | 2.7 | −16.26 (−23.38, −9.14) | <0.001 |
| AST | −10.9 | 6.9 | −17.80 (−32.08, −3.53) | 0.017 | −24.3 | 15.3 | −39.63 (−48.81, −30.46) | <0.001 | −4.96 | 9.73 | −14.69 (−23.28, −6.10) | 0.001 |
| ALT | −23.0 | 15.9 | −38.92 (−57.16, −20.67) | <0.001 | −30.7 | 46.4 | −77.11 (−87.77, −66.45) | <0.001 | −20.8 | 18.8 | −39.61 (−49.83, −29.38) | <0.001 |
| AST/ALT | 20.6 | −6.5 | 27.11 (6.23, 48.00) | 0.014 | 9.6 | −21.1 | 30.71 (24.12, 37.31) | <0.001 | 25.3 | −5.2 | 30.56 (15.26, 45.86) | <0.001 |
| Flow-mediated dilation (%) | −0.5 | −8.7 | 8.19 (−10.69, 27.06) | 0.376 | 4.9 | −2.1 | 6.96 (3.23, 10.69) | 0.001 | 6.8 | −6.3 | 13.04 (6.24, 19.83) | <0.001 |
| Carotid IMT (mm) | −28.8 | 16.3 | −45.05 (−66.09, −24.01) | <0.001 | −35.3 | 6.7 | −41.96 (−57.91, −26.01) | <0.001 | −34.8 | 2.4 | −37.19 (−48.74, −25.65) | <0.001 |
| Cytokine | Altilix (n = 25) | Placebo (n = 25) | % Change (n = 50) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | p-Value a (within Group) | Baseline | 6 Months | p-Value a (within Group) | Altilix | Placebo | p-Value b (between Groups) | |
| IL-1 (pg/mL) | 4.80 ± 1.70 | 5.03 ± 2.20 | 0.504 | 3.32 ± 2.23 | 2.69 ± 0.79 | 0.093 | 6.29 | −3.75 | 0.263 |
| IL-6 (pg/mL) | 23.13 ± 8.79 | 25.86 ± 10.66 | 0.078 | 13.66 ± 7.98 | 15.30 ± 13.26 | 0.448 | 16.41 | 10.99 | 0.660 |
| GLP-1 (pg/ml) | 38.33 ± 45.25 | 38.11 ± 48.14 | 0.984 | 44.74 ± 47.36 | 61.21 ± 54.20 | 0.093 | −0.22 | 16.47 | 0.253 |
| RANTES (pg/mL) | 1331.51 ± 1074.32 | 1673.34 ± 1137.85 | 0.232 | 1557.48 ± 1140.62 | 1879.08 ± 1233.35 | 0.244 | 112.67 | 74.45 | 0.431 |
| Ghrelin (pg/mL) | 1984.07 ± 917.78 | 2739.52 ± 1840.74 | 0.024 | 2521.72 ± 981.29 | 2481.26 ± 1470.86 | 0.908 | 42.00 | 9.91 | 0.134 |
| Leptin (pg/mL) | 14171 ± 9287.93 | 15363.12 ± 9726.65 | 0.679 | 14529.97 ± 8322.74 | 12360.38 ± 9633.23 | 0.414 | 73.39 | 48.20 | 0.590 |
| Resistin (pg/mL) | 2745.39 ± 847.26 | 2525.14 ± 1312.40 | 0.482 | 2499.69 ± 1068.48 | 2689.30 ± 1017.73 | 0.522 | 1.77 | 30.73 | 0.136 |
| Variable | Correlation Coefficient (r2) | p-Value |
|---|---|---|
| Weight | 0.3276 | <0.001 |
| BMI | 0.3294 | <0.001 |
| Waist circumference | 0.2947 | <0.001 |
| HbA1c | 0.5720 | <0.001 |
| HOMA-IR | 0.7708 | <0.001 |
| HOMA-β | 0.3910 | 0.001 |
| Total cholesterol | 0.6917 | <0.001 |
| Triglycerides | 0.5899 | <0.001 |
| LDL-cholesterol | 0.6034 | <0.001 |
| HDL-cholesterol | −0.0203 | 0.842 |
| Fatty Liver Index | 0.6183 | <0.001 |
| AST | 0.5456 | <0.001 |
| ALT | 0.7659 | <0.001 |
| AST/ALT | −0.5096 | <0.001 |
| Flow-mediated dilation | −0.3558 | <0.001 |
| Carotid IMT | 0.6811 | <0.001 |
| IL-1 | −0.1509 | 0.263 |
| IL-6 | −0.0613 | 0.660 |
| GLP-1 | 0.1632 | 0.253 |
| RANTES | −0.1064 | 0.431 |
| Ghrelin | −0.2104 | 0.134 |
| Leptin | −0.0723 | 0.590 |
| Resistin | 0.2000 | 0.136 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellino, G.; Nikolic, D.; Magán-Fernández, A.; Malfa, G.A.; Chianetta, R.; Patti, A.M.; Amato, A.; Montalto, G.; Toth, P.P.; Banach, M.; et al. Altilix® Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2580. https://doi.org/10.3390/nu11112580
Castellino G, Nikolic D, Magán-Fernández A, Malfa GA, Chianetta R, Patti AM, Amato A, Montalto G, Toth PP, Banach M, et al. Altilix® Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2019; 11(11):2580. https://doi.org/10.3390/nu11112580
Chicago/Turabian StyleCastellino, Giuseppa, Dragana Nikolic, Antonio Magán-Fernández, Giuseppe Antonio Malfa, Roberta Chianetta, Angelo M. Patti, Antonella Amato, Giuseppe Montalto, Peter P. Toth, Maciej Banach, and et al. 2019. "Altilix® Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study" Nutrients 11, no. 11: 2580. https://doi.org/10.3390/nu11112580
APA StyleCastellino, G., Nikolic, D., Magán-Fernández, A., Malfa, G. A., Chianetta, R., Patti, A. M., Amato, A., Montalto, G., Toth, P. P., Banach, M., Cicero, A. F. G., & Rizzo, M. (2019). Altilix® Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients, 11(11), 2580. https://doi.org/10.3390/nu11112580











